Abstract
Resveratrol and kaempferol are natural chemopreventative agents that are also aryl hydrocarbon receptor (AHR) antagonists and estrogen receptor (ER) agonists. In this study we evaluated the role of ERα in resveratrol- and kaempferol-mediated inhibition of AHR-dependent transcription. Kaempferol or resveratrol inhibited dioxin-induced cytochrome P450 1A1 (CYP1A1) and CYP1B1 expression levels and recruitment of AHR, ERα and co-activators to CYP1A1 and CYP1B1. Both phytochemicals induced the expression and recruitment of ERα to gene amplified in breast cancer 1 (GREB1). RNAi-mediated knockdown of ERα in T-47D cells did not affect the inhibitory action of either phytochemical on AHR activity. Both compounds also inhibited AHR-dependent transcription in ERα-negative MDA-MB-231 and BT-549 breast cancer cells. These data show that ERα does not contribute to the AHR-inhibitory activities of resveratrol and kaempferol.
Keywords: Resveratrol, Kaempferol, Dioxin, Aryl hydrocarbon receptor, Estrogen receptor
1. Introduction
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor and a member of the basic-helix-loop-helix Per (Period) ARNT (aryl hydrocarbon receptor nuclear translocator) SIM (single-minded) (bHLH/PAS) family. The AHR mediates the toxic effects of polycyclic chlorinated and aromatic hydrocarbons such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and benzo[a]-pyrene (B[a]P) [1]. AHR exhibits profound ligand binding diversity, binding dioxins, polycyclic aromatic hydrocarbons (PAHs), coplanar polychlorinated biphenyl congeners and numerous natural compounds [2]. Certain phytochemicals act as AHR agonists [2], whereas polyphenols, such as resveratrol and the flavonoid, kaempferol are AHR antagonists [3,4]. Ligand-dependent activation of AHR causes its translocation to the nucleus where it dimerizes with aryl hydrocarbon receptor nuclear translocator (ARNT). AHR/ARNT complexes bind to its cognate DNA recognition sequence termed AHR response elements (AHREs) located in regulatory regions of phase 1 (cytochrome P4501A1 (CYP1A1), CYP1A2, CYP1B1) and phase II (NQO1, UGT1A6) drug metabolizing enzymes [1].
Crosstalk has been observed between AHR and many different signalling pathways [5,6], including acting has an inhibitor of estrogen-action [7]. Estrogen signalling is mediated by estrogen receptor α (ERα) and ERβ, which are ligand-activated transcription factors and members of the nuclear receptor super-family of transcription factors [8]. ERs regulate gene expression in two ways, via direct DNA-binding to estrogen responsive elements (EREs) in target promoters or via protein–protein interactions with other transcription factors [8]. AHR and ERα physically interact, and activated AHR inhibits many ER-dependent responses through several different mechanisms [7,9]. ER-dependent modulation of AHR-mediated transcription is less clear as it varies from activation, inhibition to having no effect [7,9]. Some of these differences in AHR-ERα interactions may be due, in part, to cell context and the specific response. More recently, it has been shown that a number of AHR agonists directly activate ERα [10]. The ability of some compounds to modulate the activities of more than one receptor system may also contribute to crosstalk between these pathways.
Resveratrol (3,5,4′-trihydroxystilbene) is a polyphenol present in grapes, peanuts and berries [11]. Kaempferol (3,4′,5,7-tetrahydroxyflavone) is found in tea, broccoli, grapefruit and other plant sources, and accounts for approximately 25–33% of the mean dietary flavonol intake in the USA [12]. Resveratrol and kaempferol have received a lot of attention due to their anti-carcinogenic, cardioprotective and anti-aging properties [11,13]. These properties have been attributed to their ability to act as anti-oxidants, anti-inflammatory agents, and pro-apoptotic agents. Resveratrol and kaempferol have also been reported to inhibit the enzymatic activities of some phases I and II drug metabolizing enzymes including CYP1A1, CYP1B1, CYP1A2, CYP3A4, and sulfotransferase 1E1 (SULT1E1) and SULT1A2 [14,15].
Resveratrol and kaempferol also inhibit AHR-dependent transcription by preventing AHR/ARNT binding to the AHRE [16]. This is mediated, in part, by their ability to competitively displace TCDD from the AHR [4,16]. A single dose of 10 μM resveratrol has been reported to inhibit the AHR-dependent activation of a saturating (100 nM) concentration of TCDD even after 48 h [17]. Interestingly, resveratrol was rapidly metabolized in HepG2 human hepatoma cells to several metabolic products within the first 8 h of treatment, including one of its the major phase I metabolites, piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) [18]. Piceatannol is a tyrosine-kinase inhibitor with known anti-cancer and anti-inflammatory properties [19], but its ability to inhibit AHR-dependent transcription has not been determined. In addition to their ability to modulate AHR signalling, resveratrol and kaempferol also activate ERα and ERβ and have been classified as phytoestrogens [20,21]. It remains unclear if the ability of resveratrol and kaempferol to activate ERα influences their ability to inhibit AHR-dependent transcription.
The aims of the present study were to investigate the role of ERα in the inhibitory effects of resveratrol and kaempferol on AHR-dependent transcription and determine the ability of piceatannol to inhibit AHR activity. Our data show that the estrogenic properties of resveratrol and kaempferol and ERα expression levels are independent of their ability to inhibit AHR-dependent transcription.
2. Materials and methods
2.1. Chemicals
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) was purchased from Wellington Laboratories (Guelph, ON, Canada). Resveratrol, kaempferol, piceatannol, 17β-estradiol (E2), ICI 182,780 (ICI) and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). Cell culture media, fetal bovine serum (FBS), and trypsin were all purchased from Wisent (St. Bruno, QC). All other chemicals and biochemicals were of the highest quality available from commercial vendors.
2.2. Cell culture
T-47D, BT-549 and MDA-MB-231 human breast carcinoma cells were purchased from ATCC (Manassas, VA). T-47D cells were cultured in 1:1 mixture of DMEM (Dulbecco’s modified Eagle’s medium) and F12 Ham’s nutrient mixture medium and supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin. BT-549 cells were cultured in low glucose DMEM supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin. MDA-MB-231 cells were cultured in low glucose DMEM supplemented with 10% (v/v) FBS, 1% penicillin/streptomycin and 1X non-essential amino acids. All cell lines were maintained at 37 °C and 5% CO2 and were sub-cultured every 2–3 days or when they reached 80% confluency.
2.3. Chromatin immunoprecipitation (ChIP) assays
T-47D cells were plated in 10 cm dishes in 1:1 mixture of DMEM/F12 supplemented with 10% (v/v) FBS and 1% penicillin/streptomycin. After 48 h, cells were treated with either 1 nM TCDD, 1 nM TCDD + 10 μM resveratrol, 1 nM TCDD + 10 μM kaempferol, 1 nM TCDD + 10 μM piceatannol or DMSO (0.1%) for 1 h. For estrogenic ChIP studies, T-47D cells were plated in 10 cm dishes in phenol red-free DMEM/F12 media supplemented with 5% dextran-coated charcoal (DCC)-treated FBS and 1% penicillin/streptomycin for 72 h. Cells were then treated with 10 nM E2, 10 μMresveratrol, 10 μM kaempferol, 10 μM piceatannol or DMSO (0.1%) for 1 h and ChIP assays were preformed as previously described [22] using 1 μg of anti-AHR; H-211, anti-ARNT; H-172, anti-ERα; HC-20, anti-CBP; A-22, anti-NCoA3; M-397 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-dimethyl-Histone H3 (Lys4); 07-303, anti-acetyl-Histone H3 (Lys9); 07-352 (Upstate, Temecula, CA), anti-trimethyl Histone H3 (Lys4); 39159 (Active Motif, Carlsbad, CA) or anti-Histone H3; ab1791-100 (Abcam, Cambridge, MA). Isolated DNA was quantified by quantitative real-time PCR (qPCR) using Kapa SYBR FAST qPCR Master Mix (KapaBiosystems, Woburn, MA). Primers used to amplify CYP1A1 promoter and enhancer and CYP1B1 enhancer regions have all been previously described [22]. CYP1B1 promoter region primers were designed based on the CYP1B1 gene sequence previously described [23]. Data are reported as percentages relative to 5% total input chromatin. All histone H3 modifications were normalized to total H3 to ensure that any differences in immunoprecipitated DNA were not due to reduced levels of total H3.
2.4. RNA Isolation and qPCR
T-47D, BT-549 and MDA-MB-231 cells were seeded in six-well plates and grown their respective completed media. Cells were treated with DMSO (0.1%), 1 nMTCDD, 10 nM TCDD, 10 μM resveratrol, 10 μM kaempferol or 10 μM piceatannol, alone or each of the phyotchemicals were also treated in combination with TCDD for 1.5 h, 3 h, 6 h and 24 h. RNA was isolated using RNeasy spin columns (Qiagen). For estrogenic studies, T-47D cells were seeded in six-well plates in phenol red-free DMEM/F12 media supplemented with 5% DCC-treated FBS and 1% penicillin/streptomycin for 72 h. Cells were treated with 10 nM E2, 10 μM resveratrol, 10 μM kaempferol, 10 μM piceatannol, 10 nM E2 + 100 μM ICI, 10 μM resveratrol + 100 μM ICI, 10 μM kaempferol + 100 μM ICI, 10 μM piceatannol + 100 μM ICI or DMSO (0.1%) for 24 h. For cDNA synthesis, 500 ng of extracted RNA was reversed transcribed using SuperScriptII (Invitrogen) and random hexamer primers. qPCR was performed with 1 μl of the cDNA synthesis reaction using Kapa SYBR FAST qPCR Master Mix (KapaBiosystems). Primers used to amplify CYP1A1, CYP1B1 and gene amplified in breast cancer 1 (GREB1) mRNAs have been previously described [24]. All target transcripts were normalized to the 18S rRNA and analyzed using the comparative CT (ΔΔCT) method. RNA data are presented as mean and standard error of the mean of at least two independent replicates.
2.5. Transient transfection and RNAi
ERα (siERα-11; J-003401-11-0020 and siERα-14; J-003401-14-0020) ON-TARGETplus siRNAs and Dharma-FECT1 transfection reagent were purchased from Dharmacon (Lafayette, CO). Briefly, T-47D cells were seeded 300,000 per well in six-well plates containing 2 ml of medium. After 24 h, 100 nM of each siRNA against ERα or non-targeting siRNA #2 (D-001810–02-20) (Dharmacon) were transfected using 4 μl of DharmaFECT1. After 48 h, cells were treated with 1 nM TCDD, 1 nM TCDD + 10 μM resveratrol, 1 nM TCDD + 10 μM kaempferol or DMSO (0.1%) for 24 h for mRNA analysis as described above.
2.6. Western blot
T-47D cells were transfected with siERα or NT as described above. Whole cell extracts were prepared 48 h after transfection and proteins were resolved by SDS–PAGE and transferred to nitrocellulose membrane. The membrane was blocked in 2% (w/v) ECL-Advanced blocking agent for 1 h at room temperature and then incubated with anti-ERα (HC-20), anti-AHR (SA-210, Biomol) or anti-β-actin antibody (Sigma) overnight at 4 °C. The membrane was then washed and incubated with appropriate secondary antibody for 1 h at room temperature. After washing, the bands were visualized using ECL-Advanced chemiluminescent substrate (GE Healthcare) according to the manufacturer’s instructions.
2.7. Statistical analysis
All data are presented as means and standard error of the mean. ChIP and siRNA experiments were performed in triplicate and statistically analyzed using the Student’s two-tailed t-test to assess statistical significance (P < 0.05). RNA data represent at least two independent replicates and were statistically analyzed using the Student’s two-tailed t-test.
3. Results
3.1. Resveratrol and kaempferol inhibit TCDD-induced CYP1A1 and CYP1B1 mRNA expression
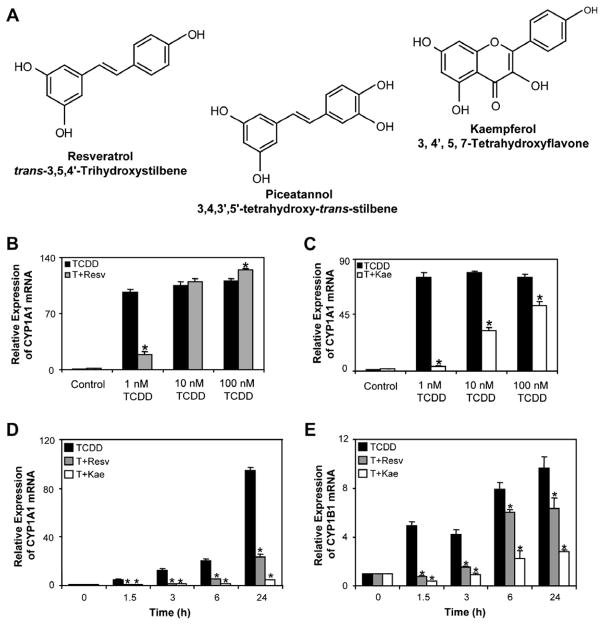
We first performed a series of dose–response experiments to evaluate the ability of resveratrol and kaempferol to inhibit the TCDD-induced CYP1A1 and CYP1B1 mRNA expression in T-47D human breast carcinoma cells. We observed that 10 μM resveratrol inhibited induction of CYP1A1 and CYP1B1 mRNA levels by 1 nM TCDD but not 10 nM TCDD (Fig. 1B). This was in contrast to a recent study demonstrating that 10 μM resveratrol inhibited 100 nM TCDD-induced CYP1A1 and CYP1B1 mRNA expression levels in MCF-7 human breast adenocarcinoma cells and HepG2 human hepatoma cells [17]. The reason for the discrepancy between the two studies is unclear; however, our data are consistent with other reports [4]. Kaempferol at a dose of 10 μM, but not 1 μM, inhibited the induction of CYP1A1 and CYP1B1 mRNA levels by 1, 10 and 100 nM TCDD, revealing it to be a more potent inhibitor of AHR-dependent transcription (Fig. 1C). The ability of kaempferol to inhibit AHR-dependent transcription following treatment with 10 nM TCDD was not observed after 48 h incubation (data not shown). Similar findings were observed in MCF-7 breast cells (data not shown).
Fig. 1.
(A) Chemical structures of resveratrol, kaempferol and piceatannol. (B and C) Concentration-dependent inhibition of TCDD-induced CYP1A1 mRNA expression levels by resveratrol and kaempferol in T-47D cells. T-47D cells were treated with 1–100 nM TCDD alone or in combination with 10 μM resveratrol or kaempferol for 24 h. (D and E) Time-dependent increases in TCDD-induced CYP1A1 and CYP1B1 mRNA expression levels and their inhibition by co-treatment with resveratrol and kaempferol in T-47D cells. T-47D cells were treated with 1 nM TCDD, 1 nM TCDD + 10 μM resveratrol or 1 nM TCDD + 10 μM kaempferol for 1.5–24 h. Results shown are means ± S.E.M for three independent experiments. Expression levels significantly (P < 0.05) different compared to time-matched TCDD treatments are denoted with an asterisk. T; TCDD, Resv; resveratrol, Kae; kaempferol.
We then performed a time course study to determine the kinetics of inhibition of AHR-dependent transcription by both phytochemicals. T-47D cells were treated with 1 nM TCDD alone or in combination with 10 μM resveratrol or 10 μM kaempferol from 1.5 to 24 h. The effects on TCDD-induced CYP1A1 and CYP1B1 mRNA expression were determined using qPCR. We observed inhibition of TCDD-induced CYP1A1 and CYP1B1 mRNA expression by resveratrol and kaempferol as early as 1.5 h of treatment (Fig. 1D and E).
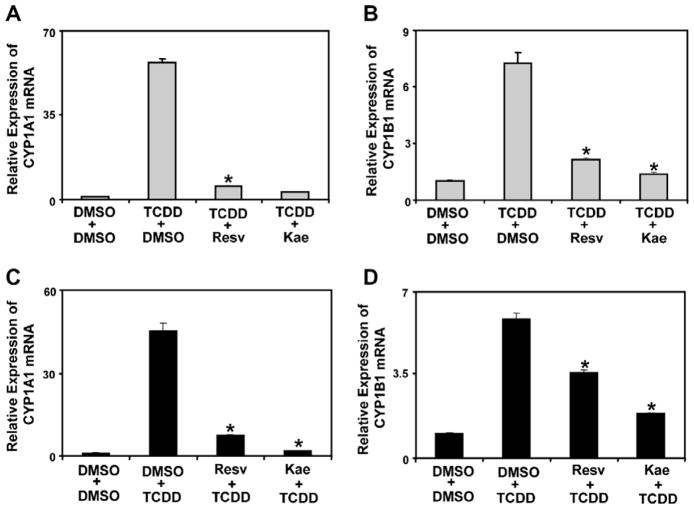
To determine if resveratrol or kaempferol could inhibit TCDD-activated AHR or if TCDD could induce AHR-dependent gene expression in cells that were already exposed to phytochemicals, we performed a series of experiments in T-47D cells which were pre-treated with TCDD or phytochemicals. For these experiments, T-47D cells were pre-treated with either TCDD or phytochemicals for 2 h and then co-treated for 6 h with phytochemicals or TCDD, respectively, prior to determining the effects on CYP1A1 and CYP1B1 mRNA expression levels. Cells pre-treated with TCDD followed by treatment with resveratrol or kaempferol demonstrated significantly lower induction of CYP1A1 and CYP1B1 mRNA compared to TCDD treatment alone (Fig. 2A and B). Similarly, cells pre-treated with resveratrol or kaempferol also displayed significantly lower TCDD-induced CYP1A1 and CYP1B1 mRNA expression levels compared to TCDD treated cells (Fig. 2C and D). These findings show that resveratrol and kaempferol can inhibit activated AHR as well as prevent the induction of AHR-dependent transcription.
Fig. 2.
CYP1A1 and CYP1B1 mRNA expression of T-47D cells pre-treated with 1 nM TCDD, 10 μM resveratrol or 10 μM kaempferol. (A and B) T-47D cells were pre-treated with control (DMSO) or 1 nM TCDD for 2 h and then treated with 10 μM resveratrol or 10 μM kaempferol for 6 h. RNA was isolated and reverse transcribed as described in Section 2. Results are shown as means ± S.E.M for three independent experiments. mRNA expression levels of TCDD + resveratrol or kaempferol co-treatments significantly (P < 0.05) lower than TCDD + DMSO are denoted with an asterisk. (C and D) T-47D cells were pre-treated with either 10 μM resveratrol or 10 μM kaempferol for 2 h and then treated with 1 nM TCDD for 6 h. RNA was isolated and reverse transcribed as described in Section 2. Results shown are as means ± S.E.M for three independent experiments. mRNA expression levels of resveratrol or kaempferol + TCDD co-treatments significantly (P < 0.05) different than DMSO + TCDD are denoted with an asterisk.
3.2. Resveratrol and kaempferol prevented TCDD-induced AHR/ARNT and co-activator recruitment and epigenetic changes at CYP1A1 and CYP1B1
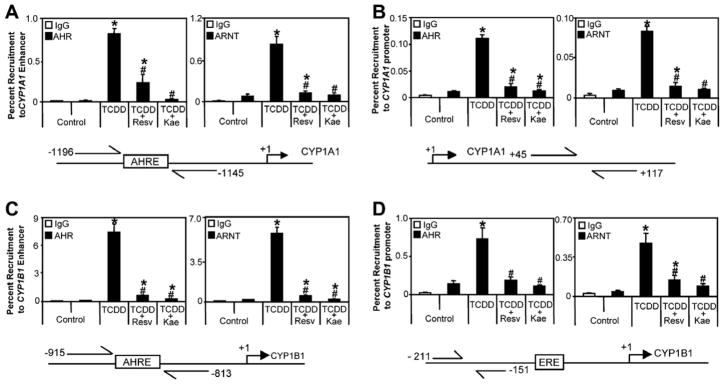
Chromatin immunoprecipitation (ChIP) assays were used to investigate the mechanisms by which resveratrol and kaempferol inhibit TCDD-induced CYP1A1 and CYP1B1 mRNA levels. As expected TCDD-induced recruitment of AHR and ARNT to CYP1A1 and CYP1B1 enhancer and promoter regions was observed following 1 h treatment (Fig. 3). Co-treatment of TCDD with resveratrol or kaempferol resulted in significantly lower recruitment of AHR and ARNT to both regions compared to TCDD alone. Co-treatment of TCDD with either phytochemical also prevented the recruitment of co-activators, Creb binding protein (CBP) and nuclear receptor co-activator 3 (NCoA3/AIB1/SRC-3), and the TCDD-dependent increases in acetylation of histone H3K9 at both CYP1A1 and CYP1B1 (Fig. 4). These data indicate that both phytochemicals interrupt the assembly of an activated AHR/ARNT complex which also prevents subsequent downstream steps that are required for AHR-mediated transcription. Co-treatment with phytochemicals prevented TCDD-dependent increases in histone H3 lysine 9 acetylation (H3K9Ac) and decreases in histone H3 lysine 9 dimethylation (H3K4Me2) at CYP1A1 and increases in H3K9Ac at CYP1B1. No changes in H3K4Me2 status following treatment with TCDD alone or in combination with phytochemicals was observed at CYP1B1.
Fig. 3.
Resveratrol and kaempferol inhibit TCDD-induced recruitment of AHR and ARNT to CYP1A1 (A) enhancer and (B) promoter and CYP1B1 (C) enhancer and (D) promoter. T-47D cells were treated with 1 nM TCDD or co-treated with 1 nM TCDD + 10 μM resveratrol or 1 nM TCDD + 10 μM kaempferol for 1 h and cells were harvested for ChIP assays as described in Section 2. Results shown are means ± S.E.M for three independent experiments. Percent recruitment levels significantly (P < 0.05) different than control are denoted with an asterisk, percent recruitment levels significantly (P < 0.05) different than TCDD treatment are denoted with a pound sign. Resv; resveratrol, Kae; kaempferol.
Fig. 4.
Resveratrol and kaempferol inhibit TCDD-induced recruitment of CBP, NCoA3 and H3K4Me2 and H3K9Ac at (A) CYP1A1 and (B) CYP1B1 enhancer regions. T-47D cells were treated with 1 nM TCDD or co-treated with 1 nM TCDD + 10 μM resveratrol or 1 nM TCDD + 10 μM kaempferol for 1 h and were harvested for ChIP assays as described in Section 2. Results shown are means ± S.E.M for three independent experiments. Percent recruitment levels significantly (P < 0.05) different than control are denoted with an asterisk, percent recruitment levels significantly (P < 0.05) different than TCDD treatment are denoted with a pound sign. Resv; resveratrol, Kae; kaempferol.
3.3. Piceatannol inhibits TCDD-induced CYP1A1 and CYP1B1 expression and recruitment of AHR and ARNT to CYP1A1 and CYP1B1
A single treatment of 10 μM resveratrol has been reported to inhibit TCDD-induced gene expression even after 48 h [17] and (MacPherson and Matthews unpublished data). However, it has also been shown that greater than 90% of resveratrol is metabolized after 8 h treatment in HepG2 cells [18]. Piceatannol is generated during phase I metabolism of resveratrol by CYP1A1, CYP1A2 or CYP1B1 [19], and represents one of its main hydroxylated metabolites. Therefore we were interested in determining the ability of piceatannol to inhibit TCDD-dependent induction of CYP1A1 and CYP1B1 expression levels. We observed that 10 μM piceatannol inhibited 1 nM TCDD-induced CYP1A1 and CYP1B1 mRNA levels, but not those induced by 10 nM TCDD after 24 h of co-treatment (Fig. 5A and B). Moreover, piceatannol also inhibited TCDD-induced recruitment of AHR and ARNT to CYP1A1 and CYP1B1 enhancer regions (Fig. 5C and D). These data show that piceatannol is an inhibitor of AHR-dependent transcription and suggest that piceatannol may contribute to the prolonged inhibition of AHR-dependent gene expression following resveratrol treatment.
Fig. 5.
Piceatannol inhibits TCDD-induced expression of CYP1A1 and CYP1B1 and TCDD-induced recruitment of AHR and ARNT to CYP1A1 and CYP1B1 enhancer regions in T-47D cells. (A and B) Concentration-dependent inhibition on TCDD-induced CYP1A1 and CYP1B1 mRNA expression levels by piceatannol. T-47D cells were treated with 1 nM or 10 nM TCDD alone or in combination of 10 μM piceatannol for 24 h and mRNA was prepared as described in Section 2. (C–F) AHR and ARNT recruitment to CYP1A1 and CYP1B1 enhancer regions was inhibited by piceatannol co-treatment. T-47D cells were treated with 1 nM TCDD alone or in combination with 10 μM piceatannol for 1 h and cells were harvested for ChIP assays. Results shown are means ± S.E.M for three independent experiments. Changes in mRNA expression and percent recruitment levels significantly (P < 0.05) different than TCDD are denoted with an asterisk, percent recruitment levels significantly (P < 0.05) different than control are denoted with a pound sign. Pic; piceatannol.
3.4. Estrogenic activity of resveratrol and kaempferol
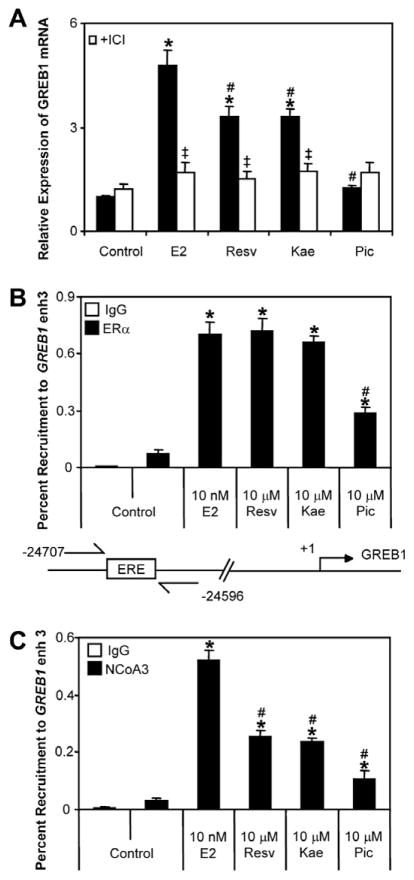
We and others have previously demonstrated the AHR ligand-dependent recruitment of ERα to CYP1A1 and CYP1B1 [10,22] and we have suggested that ERα may influence AHR/ARNT activity through this mechanism [22]. Resveratrol and kaempferol have been described as multifunctional ligands activating multiple signalling pathways, including estrogen signalling pathway [9]. As such, we were interested to determine whether the estrogenic properties of these phytochemicals influenced their ability to inhibit AHR-dependent transcription. Resveratrol and kaempferol significantly induced the expression of the estrogen-responsive, GREB1 mRNA levels in T-47D cells; however, this induction was lower than that induced by E2 (Fig. 6A). Resveratrol- and kaempferol-dependent increases in GREB1 mRNA expression levels were inhibited by co-treatment with the ER antagonist ICI 182,780. Piceatannol did not induce significant increases in GREB1 mRNA expression levels.
Fig. 6.
(A) GREB1 mRNA induction by AHR inhibitors. T-47D cells were plated in media devoid of estrogens and treated with 10 nM E2, 10 μM resveratrol, 10 μM kaempferol or 10 μM piceatannol or in co-treatment with 100 nM ICI for 24 h. mRNA expression levels significantly (P < 0.05) different than control are denoted with an asterisk, mRNA expression levels significantly (P < 0.05) different than E2 treatment are denoted with a pound sign, mRNA expression levels of cells co-treated with ICI significantly (P < 0.05) lower than treatment alone are denoted with a double dagger. (B & C) Recruitment of ERα and NCoA3 to the GREB1 enhancer 3 region by the three phytochemicals. T-47D cells plated in media devoid of estrogens were treated with 10 nM E2, 10 μM resveratrol, 10 μMkaempferol or 10 μMpiceatannol for 1 h and ChIP assays were performed as described in Section 2. Percent recruitment levels significantly (P < 0.05) different than control are denoted with an asterisk, percent recruitment significantly (P < 0.05) different than E2 treatment are denoted with a pound sign. Results shown are means ± S.E.M for three independent experiments. Resv; resveratrol, Kae; kaempferol, Pic; piceatannol, ICI; ICI 182,780.
To determine the ability of both phytochemicals to induce recruitment of ERα and its associated co-activator NCoA3 to the upstream regulatory region of GREB1 (referred to as GREB1 enhancer 3) we performed ChIP assays. The GREB1 enhancer 3 region is an estrogen-responsive genomic region identified by others and important in the ERα-dependent regulation of GREB1 [25]. ChIP assays revealed that 1 h treatment of T-47D cells with resveratrol and kaempferol induced a similar level of recruitment of ERα to the GREB1 enhancer 3 compared to 10 nM E2, whereas piceatannol induced only a modest increase in recruitment of ERα above untreated controls (Fig. 6B). Both phytochemicals also induced recruitment of the co-activator NCoA3 to the GREB1 enhancer 3 region, but the level of recruitment was lower than that induced by E2 (Fig. 6C). Piceatannol induced a slight, but significant recruitment of NCoA3 to GREB1 enhancer 3 region.
3.5. Inhibition of TCDD-induced CYP1A1 and CYP1B1 mRNA expression by resveratrol and kaempferol is independent of ERα
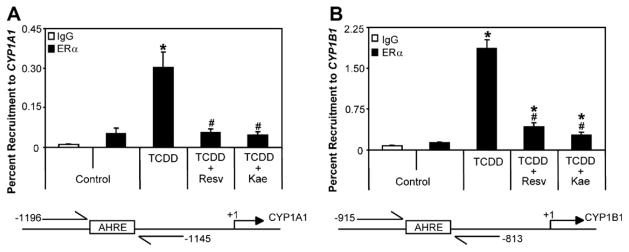
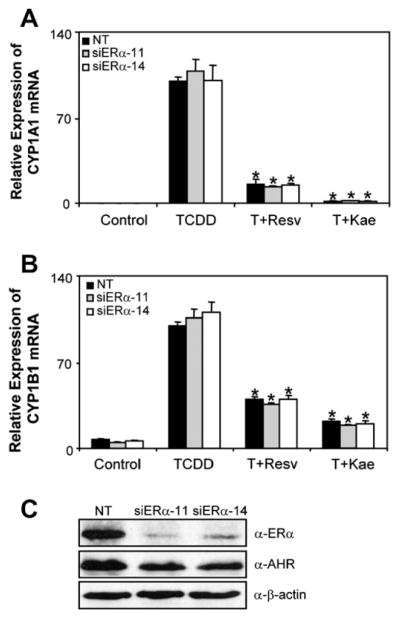
Since resveratrol and kaempferol activate ERs, we were interested to determine the role of ERα in the resveratrol- or the kaempferol-mediated inhibition of AHR-dependent transcription. In agreement with our previous work, we detected ERα at the CYP1A1 and CYP1B1 enhancer regions following treatment with TCDD (Fig. 7) [22]. ChIP assays showed that resveratrol and kaempferol inhibited the TCDD-induced recruitment of ERα to CYP1A1 and CYP1B1, suggesting that ERα may not be directly involved in the inhibition of AHR-transcription mediated by these two phytochemicals. We then used RNAi-mediated knockdown of ERα in T-47D cells and determined the ability of resveratrol and kaempferol to inhibit the TCDD-induced CYP1A1 and CYP1B1 mRNA expression levels. Knockdown of ERα using two independent siRNA sequences did not affect inhibition of TCDD-induced CYP1A1 and CYP1B1 mRNA expression by resveratrol and kaempferol compared to non-targeting control (Fig. 8). Both siRNA sequences resulted in similar knockdown of ERα protein levels but had no effect on AHR protein expression. Moreover, TCDD-dependent gene expression in two ERα-negative breast cancer cell lines; MDA-MB-231 and BT-549, was also significantly inhibited by resveratrol and kaempferol (Fig. 9).
Fig. 7.
Resveratrol and kaempferol inhibit TCDD-induced recruitment of ERα to (A) CYP1A1 and (B) CYP1B1 enhancer regions. T-47D cells were treated with 1 nM TCDD alone or in combination with 10 μM resveratrol or 10 μM kaempferol for 1 h and ChIP assays were performed as described in Section 2. Percent recruitment levels significantly (P < 0.05) different than control are denoted with an asterisk, percent recruitment levels significantly (P < 0.05) different than TCDD treatment alone are denoted with a pound sign. Results shown are means ± S.E.M for three independent experiments. Resv; resveratrol, Kae; kaempferol.
Fig. 8.
RNAi-mediated ERα knockdown in T-47D cells. (A and B) CYP1A1 and CYP1B1 mRNA expression. T-47D cells were transfected with specific siRNA against ERα for 48 h. Cells were then treated with 1 nMTCDD alone or in combination with 10 μM resveratrol or 10 μM kaempferol for 24 h and RNA was isolated and reverse transcribed. Changes in mRNA expression was then determined using qPCR. Data were normalized to non-targeting (NT) DMSO (control) and to ribosomal 18s levels. Results shown are means ± S.E.M for three independent experiments. Changes in mRNA expression levels within similarly transfected cells significantly (P < 0.05) lower than TCDD treated cells are denoted with an asterisk. (C) Western blot analysis of RNAi-mediated ERα knockdown in T-47D cells. Cell extracts were probed with anti-ERα or anti-AHR or anti-β-actin antibodies as described in Section 2. This figure is representative of three independent experiments. NT; non-targeting, Resv; resveratrol, Kae; kaempferol.
Fig. 9.
Inhibition of TCDD-induced CYP1A1 and CYP1B1 mRNA in ERα-negative MDA-MB-231 (A and B) and BT-549 (C and D) cells by resveratrol and kaempferol. MDA-MB-231 and BT-549 cells were treated with 1 nM TCDD alone or in combination with 10 μM resveratrol or 10 μM kaempferol for 24 h and RNA was isolated and reverse transcribed. Changes in mRNA expression levels were determined using qPCR. Data were normalized against DMSO (control) to ribosomal 18s levels. Results shown are means ± S.E.M for three independent experiments. Significant differences (P < 0.05) compared to TCDD treated cells are denoted with an asterisk. Resv; resveratrol, Kae; kaempferol.
4. Discussion
Resveratrol and kaempferol are phytochemicals that have received considerable attention due to their in vitro and in vivo anti-cancer properties. Both compounds are known to modulate the activities of a number of signalling pathways including AHR- and ER-dependent transcription. Previous studies have suggested that ERα expression levels influence the ability of resveratrol to inhibit AHR-dependent transcription [4]; however, this has not been experimentally determined. Although some studies have reported that knockdown of ERα does not affect AHR-responsiveness in human breast cancer cells, we have shown that knockdown of ERα reduces AHR-responsiveness in a gene-specific manner [24]. Moreover, overexpression of ERα in ERα-negative breast cancer cells restores AHR-responsiveness [26]. In this study we evaluated the role of ERα in resveratrol- and kaempferol-mediated inhibition of AHR-dependent transcription. Our data reveal that the ERα expression levels are independent of the ability of resveratrol and kaempferol to inhibit AHR-dependent transcription.
We observed that kaempferol (10 μM) was more effective than resveratrol (10 μM) at inhibiting TCDD (1 nM)-induced AHR-mediated transcription and recruitment of AHR and ARNT to CYP1A1 and CYP1B1 enhancer and proximal promoter regions and at reducing TCDD-dependent increases in CYP1A1 and CYP1B1 mRNA levels. Similarly, kaempferol was found to be the most effective flavonoid at inhibiting the agonistic effect of TCDD and cigarette smoke condensate on AHR-dependent activity [27].We observed that the TCDD-dependent occupancy of CBP and NCoA3, and H3K9Ac levels at CYP1A1 and CYP1B1 was also inhibited by co-treatment with either phytochemical. Recruitment of AHR and ARNT to both the enhancer and proximal promoters regions of CYP1A1 and CYP1B1 is in agreement with our previous studies in MCF-7 human breast cancer cells [22], supporting the notion that the regulation of CYP1A1 and CYP1B1 occurs through enhancer-promoter looping, whereby factors bound to the enhancer sequence interact with the basal transcriptional machinery present at the proximal promoter. Others have reported that TCDD induces AHR/ARNT recruitment to the upstream AHRE-containing enhancer regions of CYP1A1 and CYP1B1 but not to the proximal promoter regions of both genes [28]. This discrepancy is most likely due to cell culture conditions and differences in assay methodologies between laboratories.
Resveratrol is rapidly metabolized following treatment of human hepatoma HepG2 cells, but a single dose of resveratrol inhibits TCDD-induced transcription for at least 48 h in the same cell line [17]. Our analysis of piceatannol, one of several phase I metabolites of resveratrol [18,19] supports the hypothesis that prolonged inhibition of resveratrol might be mediated by its metabolites. In humans and rodents, resveratrol is metabolized to glucuronidated and sulfated conjugates, with resveratrol glucuronide predominantly circulating in human plasma [29]. Similar to resveratrol, kaempferol is predominantly found as a glucuronide conjugate, kaempferol 3-glucuronide, in plasma and urine [30]. The phase II conjugation of resveratrol and kaempferol significantly reduces the bioavailability of both phytochemicals in vivo, which might weaken the in vitro anti-cancer, anti-inflammatory, and anti-atherogenic activities attributed to them. However, resveratrol administered by subcutaneous injection to female Sprague–Dawley rats completely prevented B[a]P-dependent induction of CYP1A1 protein expression in several tissues [4]. Moreover, phase II metabolites of the flavones quercetin, quercetin-3-O-glucoronide and quercetin-3′-O-sulfate retained their anti-oxidant and inhibition of lipoxygenase activity, respectively. These findings demonstrate the effectiveness of resveratrol at inhibiting AHR-mediated activity in vivo and show that phase II metabolic products of certain phytochemicals retain biological activity.
Our analysis of the estrogenic activity of resveratrol, kaempferol and piceatannol is similar to previous studies [20,31] but with some notable differences. In agreement with these studies, we observed super-induction of ERE-regulated reporter activity with resveratrol but not with kaempferol at the doses examined. Both compounds induced a lower level of GREB1 mRNA expression but induced a similar level of recruitment of ERα to GREB1 enhancer 3 upstream regulatory region. Interestingly, NCoA3 occupancy at GREB1 enhancer 3 induced by resveratrol or kaempferol was lower than that induced by E2. This suggests that resveratrol- and kaempferol-ERα might induce structurally distinct conformations from that of E2-ERα altering the effectiveness of the ligand-bound receptor to interact or recruit co-activators similar to that reported for genistein-ERα conformation [32]. Piceatannol displayed weak estrogenic activities in T-47D cells, which contrasts a report describing this compound as a strong estrogen receptor agonist [33]. Estrogenic as well as anti-estrogenic activities have been reported for both resveratrol and kaempferol [34,35]. Resveratrol has been shown to inhibit cell proliferation as well as induce apoptosis and cell cycle arrest in several breast cancer cell lines [36]. Resveratrol decreases ROS generation and inhibits breast tumour cell invasiveness and delays the development of spontaneous mammary tumours in HER-2/neu transgenic mice [37]. However, resveratrol has been reported to accelerate N-methyl-N-nitrosourea-induced mammary carcinogenesis in pre-pubertal rats [38] and induce receptor-mediated proliferation in osteoblasts and breast cancer cells [20]. Collectively, these studies suggest that the effects of resveratrol are influenced by cellular conditions, assay parameters and model system used.
Inhibitory ER-AHR crosstalk may contribute to the AHR antagonist activities by certain phytochemicals. Despite the estrogenic action of resveratrol and kaempferol, siRNA-mediated knockdown of ERα did not affect the ability of either compound to inhibit AHR-dependent transcription in T-47D cells. Both compounds also inhibited TCDD-induced CYP1A1 and CYP1B1 mRNA expression levels in ERα-negative MDA-MB-231 and BT-549 human breast cancer cells. Since T-47D cells express low levels of ERβ and both BT-549 and MDA-MB-231 express ERβ [39],we cannot exclude the possible role of ERβ in mediating the inhibitory action of resveratrol or kaempferol on the AHR signalling pathway. However, resveratrol effectively inhibits AHR-mediated transcription in HepG2 human hepatoma cells which do not express ERα or ERβ [40]. Pharmacological dissection of the role of ERs in resveratrol- or kaempferol-mediated inhibition of AHR action must be carefully considered since 4-hydroxytamoxifen and ICI 182,780 have recently be reported to activate AHR [41].
Another mechanism by which resveratrol may inhibit AHR-dependent transcription is through regulation and/or activation of NAD-dependent deacetylase sirtuin-1 (SIRT1) [42]. SIRT1 is a class III histone deacetylase that modulates the activity of histones as well as a number of transcription factors, including p53 and forkhead protein O3a (FOXO3a) factors [43]. Recent studies have questioned whether resveratrol is a bona fide activator of SIRT1 [44]. We did not observe any recruitment of SIRT1 to AHR-regulated genes in the presence of TCDD alone or in combination with resveratrol (data not shown).
In summary, our findings show that the ability of resveratrol and kaempferol to inhibit AHR-dependent transcription is independent of ERα expression. We also provide evidence that metabolites of phytochemicals may also contribute to the ability of resveratrol to inhibit AHR-mediated action and may also be important in mediating other biological actions and anti-neoplastic properties of these compounds.
Acknowledgments
We would like to thank Shaimaa Ahmed, Raymond Lo and Melanie Powis for their helpful discussions and for their comments. This work was supported by the Canadian Institute of Health Research and the Canadian Breast Cancer Research Alliance [MOP-82715 to J.M.]. J.M. is also a recipient of a Canadian Institute of Health Research New Investigator Award.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 2.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 3.Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- 4.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–790. [PubMed] [Google Scholar]
- 5.Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 6.Puga A, Xia Y, Elferink C. Role of the aryl hydrocarbon receptor in cell cycle regulation. Chem Biol Interact. 2002;141:117–130. doi: 10.1016/s0009-2797(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 7.Matthews J, Gustafsson JA. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl Recept Signal. 2006;4:e016. doi: 10.1621/nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson S, Gustafsson JA. Estrogen receptor transcription and transactivation: Basic aspects of estrogen action. Breast Cancer Res. 2000;2:360–366. doi: 10.1186/bcr81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 10.Liu S, Abdelrahim M, Khan S, Ariazi E, Jordan VC, Safe S. Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in MCF-7 breast cancer cells. Biol Chem. 2006;387:1209–1213. doi: 10.1515/BC.2006.149. [DOI] [PubMed] [Google Scholar]
- 11.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. J Am Diet Assoc. 2002;102:1414–1420. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 13.Kris-Etherton PM, Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol. 2002;13:41–49. doi: 10.1097/00041433-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Mikstacka R, Baer-Dubowska W, Wieczorek M, Sobiak S. Thiomethylstilbenes as inhibitors of CYP1A1, CYP1A2 and CYP1B1 activities. Mol Nutr Food Res. 2008;52(Suppl 1):S77–83. doi: 10.1002/mnfr.200700202. [DOI] [PubMed] [Google Scholar]
- 15.Ung D, Nagar S. Trans-resveratrol-mediated inhibition of beta-oestradiol conjugation in MCF-7 cells stably expressing human sulfotransferases SULT1A1 or SULT1E1, and human liver microsomes. Xenobiotica. 2009;39:72–79. doi: 10.1080/00498250802604082. [DOI] [PubMed] [Google Scholar]
- 16.Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999;340(Pt 3):715–722. [PMC free article] [PubMed] [Google Scholar]
- 17.Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009;110:61–67. doi: 10.1093/toxsci/kfp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancon A, Hanet N, Jannin B, Delmas D, Heydel JM, Lizard G, Chagnon MC, Artur Y, Latruffe N. Resveratrol in human hepatoma HepG2 cells: metabolism and inducibility of detoxifying enzymes. Drug Metab Dispos. 2007;35:699–703. doi: 10.1124/dmd.106.013664. [DOI] [PubMed] [Google Scholar]
- 19.Piver B, Fer M, Vitrac X, Merillon JM, Dreano Y, Berthou F, Lucas D. Involvement of cytochrome P450 1A2 in the biotransformation of trans-resveratrol in human liver microsomes. Biochem Pharmacol. 2004;68:773–782. doi: 10.1016/j.bcp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or Beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 2005;230:558–568. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- 22.Matthews J, Wihlen B, Thomsen J, Gustafsson JA. Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor alpha to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol Cell Biol. 2005;25:5317–5328. doi: 10.1128/MCB.25.13.5317-5328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang YM, Wo YY, Stewart J, Hawkins AL, Griffin CA, Sutter TR, Greenlee WF. Isolation and characterization of the human cytochrome P450 CYP1B1 gene. J Biol Chem. 1996;271:28324–28330. doi: 10.1074/jbc.271.45.28324. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed S, Valen E, Sandelin A, Matthews J. Dioxin increases the interaction between aryl hydrocarbon receptor and estrogen receptor alpha at human promoters. Toxicol Sci. 2009;111:254–266. doi: 10.1093/toxsci/kfp144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38:1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen JS, Wang X, Hines RN, Safe S. Restoration of aryl hydrocarbon (Ah) responsiveness in MDA-MB-231 human breast cancer cells by transient expression of the estrogen receptor. Carcinogenesis. 1994;15:933–937. doi: 10.1093/carcin/15.5.933. [DOI] [PubMed] [Google Scholar]
- 27.Puppala D, Gairola CG, Swanson HI. Identification of kaempferol as an inhibitor of cigarette smoke-induced activation of the aryl hydrocarbon receptor and cell transformation. Carcinogenesis. 2007;28:639–647. doi: 10.1093/carcin/bgl169. [DOI] [PubMed] [Google Scholar]
- 28.Taylor RT, Wang F, Hsu EL, Hankinson O. Roles of coactivator proteins in dioxin induction of CYP1A1 and CYP1B1 in human breast cancer cells. Toxicol Sci. 2009;107:1–8. doi: 10.1093/toxsci/kfn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 30.DuPont MS, Day AJ, Bennett RN, Mellon FA, Kroon PA. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 2004;58:947–954. doi: 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- 31.Levenson AS, Gehm BD, Pearce ST, Horiguchi J, Simons LA, Ward JE, 3rd, Jameson JL, Jordan VC. Resveratrol acts as an estrogen receptor (ER) agonist in breast cancer cells stably transfected with ER alpha. Int J Cancer. 2003;104:587–596. doi: 10.1002/ijc.10992. [DOI] [PubMed] [Google Scholar]
- 32.Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engstrom O, Ljunggren J, Gustafsson JA, Carlquist M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. Embo J. 1999;18:4608–4618. doi: 10.1093/emboj/18.17.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggiolini M, Recchia AG, Bonofiglio D, Catalano S, Vivacqua A, Carpino A, Rago V, Rossi R, Ando S. The red wine phenolics piceatannol and myricetin act as agonists for estrogen receptor alpha in human breast cancer cells. J Mol Endocrinol. 2005;35:269–281. doi: 10.1677/jme.1.01783. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2005;94:431–443. doi: 10.1016/j.jsbmb.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Oh SM, Kim YP, Chung KH. Biphasic effects of kaempferol on the estrogenicity in human breast cancer cells. Arch Pharm Res. 2006;29:354–362. doi: 10.1007/BF02968584. [DOI] [PubMed] [Google Scholar]
- 36.Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 37.Gunther S, Ruhe C, Derikito MG, Bose G, Sauer H, Wartenberg M. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer Lett. 2007;250:25–35. doi: 10.1016/j.canlet.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Sato M, Pei RJ, Yuri T, Danbara N, Nakane Y, Tsubura A. Prepubertal resveratrol exposure accelerates N-methyl-Nnitrosourea-induced mammary carcinoma in female Sprague-Dawley rats. Cancer Lett. 2003;202:137–145. doi: 10.1016/j.canlet.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Weitsman GE, Skliris G, Ung K, Peng B, Younes M, Watson PH, Murphy LC. Assessment of multiple different estrogen receptor-beta antibodies for their ability to immunoprecipitate under chromatin immunoprecipitation conditions. Breast Cancer Res Treat. 2006;100:23–31. doi: 10.1007/s10549-006-9229-5. [DOI] [PubMed] [Google Scholar]
- 40.Holownia A, Braszko JJ. Tamoxifen cytotoxicity in hepatoblastoma cells stably transfected with human CYP3A4. Biochem Pharmacol. 2004;67:1057–1064. doi: 10.1016/j.bcp.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 41.Dusell CD, Nelson ER, Wittmann BM, Fretz JA, Kazmin D, Thomas RS, Pike JW, McDonnell DP. Regulation of aryl hydrocarbon receptor function by selective estrogen receptor modulators. Mol Endocrinol. 24:33–46. doi: 10.1210/me.2009-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 43.Vaziri H, Dessain SK, Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 44.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]