In the mid-1990s, case reports of myocardial infarction (MI) in young patients infected with human immunodeficiency virus (HIV) sparked interest in the relationship between HIV infection and cardiovascular disease (CVD).1,2 Although the initial focus was primarily on the relationship between dyslipidemia associated with antiretroviral therapy (ART) and cardiovascular risk, a broader appreciation of the complex interplay between traditional risk factors for CVD and HIV infection has emerged more recently. Several groups of investigators have designed studies to examine various aspects of the relationship between HIV infection, traditional cardiovascular risk factors, ART, and short- and longer-term cardiovascular risk3–11 (see also Working Group 1). Studies have included both clinical end points (MI, hospitalization for MI or angina, and revascularization) and surrogate markers of atherosclerosis (endothelial function or carotid intima-media thickness). Successive studies have generally improved in quality, with inclusion of data on traditional risk factors, longer follow-up, and more diverse patient populations.
HIV and ART can contribute to an altered risk of CVD in 3 principal ways: (1) HIV may serve as a marker to identify a subgroup of the general population with an altered prevalence of traditional cardiovascular risk factors, unrelated to HIV or ART (eg, HIV-infected patients may have higher smoking rates); (2) HIV or ART may affect the risk of developing a traditional cardiovascular risk factor (eg, HIV or ART may worsen dyslipidemia); and (3) HIV or ART may affect the pathogenetic process that leads to CVD in ways other than via an effect on traditional risk factors (eg, through effects on inflammation or endothelial function). Importantly, there is substantial evidence to suggest that all 3 mechanisms are in operation and affect the risk of CVD in patients infected with HIV. All 3 factors should be considered in epidemiological studies assessing the relationship between CVD and HIV disease.
The objectives of this section are to assess the state of the science with respect to (1) the epidemiological evidence linking coronary heart disease (CHD) and HIV; (2) the specific risk factors for CHD in HIV populations, including ART use; and (3) the effects of ART interruption on CVD risk associated with ART use. Finally, gaps in our knowledge and priorities for future research will be highlighted.
Methodological Considerations
Data on CVD in HIV-infected adult populations are derived from 4 main types of studies: retrospective cohort studies, administrative and clinical databases, prospective HIV cohort studies, and randomized clinical trials of ART. Often, data sources developed for other purposes (administrative databases from healthcare organizations or randomized clinical trials) have been used to add to our understanding of the association between HIV and CVD. These studies vary in the types of end points used, methods of end-point collection and validation, degree to which data on traditional risk factors (eg, presence and magnitude of smoking or hypertension, family history, and diabetes mellitus) were captured, and the amount of information available about the type and duration of ART exposure. When HIV-uninfected control groups have been included, they are often not matched on important, traditional risk factors that may be more prevalent in HIV groups, such as smoking. It is important that data from studies including HIV and non–HIV-infected control subjects contain complete information on traditional risk factors, including smoking, to assess the relative contribution of these risk factors to CVD rates and to determine whether HIV per se is a marker for a subgroup of patients with increased traditional CVD risk markers. Despite methodological limitations inherent in the use of preexisting or administrative databases, some consistent themes have emerged from the existing research in this area, namely, that traditional CVD risk factors and relative CVD disease rates are increased in HIV-infected patients, although absolute rates remain low in the HIV population.
Overall Risk of CVD in HIV Patients
A number of studies have shown that there may be increased risk of CVD in HIV-infected versus uninfected populations. Table 1 includes the event rates for CVD end points in selected published studies3–6,8,11 of MI and clinical CVD events. Variations have been observed, which possibly reflect differences in end-point definitions and ascertainment or differences in underlying cardiovascular risk in the various populations studied. Using a large clinical/administrative database in their health maintenance organization setting, researchers at Kaiser Permanente provided one of the first reports comparing rates of hospitalization for CVD in HIV-infected adults with those of uninfected control subjects.11 With annual updating, these authors have consistently demonstrated that HIV patients have a higher risk of hospitalizations for CVD and specifically for acute MI relative to HIV-uninfected controls. Using claims data among California Medicaid recipients, Currier et al3 demonstrated an increased incidence of CVD in younger HIV-infected men and women compared with HIV-uninfected recipients. Two more recent studies also reported an excess relative risk of CVD among HIV-infected adults compared with HIV-uninfected control subjects.6,10 Triant et al6 compared MI rates (based on hospital claims data) among HIV-infected adults receiving longitudinal care at 2 large hospitals in Boston, Mass, and demonstrated higher rates of acute MIs in HIV-infected individuals than in HIV-uninfected adults. These authors also noted a higher prevalence of conventional risk factors for CVD, such as hypertension, diabetes mellitus, and dyslipidemia, in the HIV-positive group, factors that separately and especially in combination could explain, at least in part, the excessive risk of CVD in the HIV-infected group. An important limitation of this study and others in which claims data are used is the lack of systematically collected data on smoking. In addition, very few studies have attempted to quantify smoking when data are available. Using data from the Danish HIV cohort study, Obel et al10 also reported that in the HAART era, the risk of CVD was higher in HIV-infected patients than in control subjects. To date, there does not appear to have been a major increase in absolute CVD rates over time, perhaps as a result of greater attention to conventional risk factor reduction for HIV-positive individuals.5,10
Table 1.
Rates of MI Across Cohort and Database Studies
| First Author/Cohort | No. of Patients/No. of Events: HIV Group | Event Rate per 1000-Patient HIV+ Group | Event Rate per 1000 HIV− Subjects |
|---|---|---|---|
| Friis-Møller4,5 | |||
| DAD I | 23 468/126 | 3.5 | NA |
| DAD II | 23 437/345 | 3.6 | NA |
| Bozzette8 | |||
| VA | 36 766/1207 | 8.1 | NA |
| Klein11 | |||
| Kaiser 2002 | 4159/47 | 4.3 | 2.9 |
| Kaiser 2007 | 5000/162 | 3.7 | 2.2 |
| Triant6 | |||
| MGH | 3851/189 | 11.13 | 6.98 |
| Currier3 | |||
| MediCal | 28 512/294 | 4.12 | 3.32 |
NA indicates not assessed; VA, Veterans Affairs; MGH, Massachusetts General Hospital; and MediCal, Medicaid recipients in California.
Assessment of Traditional Risk Factors
It is critical to understand whether increased CVD rates are causally related to HIV-related factors or merely reflect differences in the prevalence of underlying traditional risk factors. Importantly, the studies that have controlled for these factors have consistently demonstrated a significant effect of traditional risk factors on CVD events in HIV-infected patients. Age, smoking, hypertension, and diabetes mellitus are all strong predictors of CVD risk in HIV-infected patients.5,6 Additionally, the background prevalence rates of these factors in many HIV cohorts are high and may, for some, predate acquisition of HIV infection. In particular, rates of smoking in HIV populations are consistently high and exceed those for age-matched controls in several studies.12–15 The limited data available on dietary intake suggest that HIV-infected patients may have diets high in saturated fat compared with community control groups.16 Finally, the prevalence of dyslipidemia, whether genetically determined or influenced by ART (elevated triglycerides, total cholesterol, and low-density lipoprotein cholesterol) or HIV infection (low high-density lipoprotein [HDL] cholesterol), is consistently higher in HIV groups. Unfortunately, few studies have pre-HIV lipid values or pretreatment values available to assist in the determination of the role of treatment- or disease-related lipid changes and CVD risk in HIV patients.17
Table 2 summarizes the association between traditional cardiovascular risk factors and MI in HIV-infected adults compared with studies conducted in the general population.18–24 Of note, the relative contribution of each of the cardiovascular risk factors depicted is similar in HIV-infected and uninfected populations, which suggests that these factors contribute to cardiovascular risk in a comparable way irrespective of HIV status. Consistent with this, analyses that compare the observed incidence of CHD in HIV-infected populations with that predicted from risk equations developed in the general population have reported reasonably similar outcomes25 (see Working Group 5 for discussion of prediction algorithms for CVD risk in HIV). Hence, traditional cardiovascular risk factors contribute in important ways to the risk of CVD in HIV.
Table 2.
Do Traditional Cardiovascular Risk Factors Predict the Risk of CHD/CVD in HIV-Infected Persons Similarly to HIV-Uninfected Persons?
| Cardiovascular Risk Factor | Unit | % Increase in Risk per Unit for Each Study | ||
|---|---|---|---|---|
|
| ||||
| HIV-Positive5,9 | HIV-Negative (No. of Studies)* | |||
|
| ||||
| Iloeje et al9 | Friis-Møller et al5 | |||
| Age | Per 1 year older | 9% | 6% | 6% to 9% (7) |
| Sex | Male vs female | NS | 110% | 110% to 160% (2) |
| Diabetes mellitus | Yes vs no | 260% | 90% | 140% to 252% (3) |
| Smoking | Yes vs no | 140% | 290% | 70% to 290% (3) |
| Hypertension | Yes vs no | 30% | 80% | 80% to 90% (3) |
| Total cholesterol | Per 1-mmol/L increase† | · · · | 26% | 25% to 33% (3) |
| HDL cholesterol | Per 1-mmol/L increase† | · · · | −28% | −52% (1) |
Given the limitations of data from observational studies, in which treatment decisions might be somehow linked to unmeasured risk factors for the outcome of interest, a randomized clinical trial might be an optimal method to determine whether HIV infection, ART, or a particular class of ART drugs is associated with an increase in CVD in HIV patients. However, ART comprises several different drugs, each with its own metabolic profile (albeit with some commonalities) that can be combined in multiple ways. As new drugs are developed, the preferred drugs change, and the risks and benefits of existing drugs become better understood. A sufficiently powered randomized trial would require a very large patient sample size and would take several years to complete. To date, there has not been sufficient clinical equipoise and research support available to undertake such a trial. In the absence of a well-powered randomized trial, the prospective cohort study remains the next best methodology for addressing these important questions.
Taken together, the data from available cohort studies suggest that HIV-infected adults appear to have an increased relative risk of CVD compared with non-HIV patients. Owing to the incomplete data on traditional risk factors, it is not possible to determine whether HIV infection per se, exposure to ART, or other HIV-specific risk factors are the cause of this risk or whether HIV-positive status simply serves as a marker for differences in the prevalence of traditional risk factors such as smoking.
Role of ART as a Risk Factor for CVD
Early reports linking treatment of HIV infection to dyslipidemia led to several investigations4,5,7,9,26–30 into the associations between the introduction of ART and CVD rates (Table 3). Researchers in the Veterans Affairs healthcare system8,31 reported that overall deaths due to HIV dramatically declined in the early HAART era without any increase in admissions for cardiovascular or cerebrovascular events. Although limited by the short duration of exposure to combination ART and by possibly incomplete capture of events that occurred outside the Veterans Affairs system, this study suggested that over the short term, the benefits of ART clearly outweighed the risk for CVD.
Table 3.
Does ART (or Its Components) Increase Risk of CV Disease?
| Study/First Author | No. of Persons/No. of Events | Type of Event | Main Findings Related to ART | Adjustment for CV RF?* | NRTI Effect?† | NNRTI Effect?† | PI Effect?† |
|---|---|---|---|---|---|---|---|
| Randomized, controlled trial | |||||||
| Coplan, 200326 | 10 986/19 | MI | PI vs no PI: 69% (NS) | NA | NA | NA | Yes |
| Phillips, 200827 | 2752 (VS)/31 | CVD | PI exp: 13% increase per y (P =0.06) | Yes | NA | No | Yes |
| Prospective observational studies | |||||||
| Holmberg, 200228 | 5672/21 | CVD | PI vs no PI: increase 6.5-fold (NS) | Yes | NA | NA | Yes |
| Iloeje, 20059 | 7542/127 | CVD | PI vs no PI: increase 71% (P <0.05) | Yes | NA | NA | Yes |
| DAD I, 20075 | 23 437/345 | MI | PI exp: 16% increase per year (P <0.001) | Yes | NA | No | Yes |
| DAD II, 20075 | 10 002/40 | MI | ART: 24% increase per year (P <0.05) | Yes | NA | NA | NA |
| Retrospective observational studies | |||||||
| Mary-Krause, 20037 | 34 976/66 | MI | >18 mts exp to PI: increased risk | Partial | NA | NA | Yes |
| Rickerts, 200029 | 4993/29 | MI | ART exp: increased risk | Partial | NA | NA | NA |
| Administrative databases | |||||||
| Klein, 200730 | 5000/162 | CAD adm (/1000 PY) | Increase 7.1 (PI) vs 4.9 (no PI) (P=0.02) | Partial | NA | NA | Yes |
| Bozzette, 20038 | 36 766/1207 | CAD adm | No change | Partial | NA | No | No |
| Currier, 20033 | 28 513/1360 | CAD adm | Increase, but only in young persons | Partial | NA | NA | NA |
CV RF indicates cardiovascular risk factors; NRTI, nucleoside reverse-transcriptase inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; NS, nonsignificant; NA, not assessed; VS, viral suppression arm of trial; PI exp, protease inhibitor exposure; mts exp to PI, months exposed to protease inhibitors; and CAD adm, hospital admission for coronary artery disease.
Was the ART identified after proper adjustment for traditional cardiovascular risk factors?
In studies in which ART or components thereof were identified as cardiovascular risk factors, was this effect associated with exposure to a specific ART drug class?
Subsequently, several published studies have examined the contribution of ART to the excess risk of CHD events observed among HIV-infected patients. A positive association between exposure to ART and risk of CHD has been observed in one3 of 3 administrative database studies,8,11 9 of 14 cross-sectional carotid IMT studies,32–41 1 meta-analysis of randomized clinical trials,24 and 4 prospective observational databases.9,42
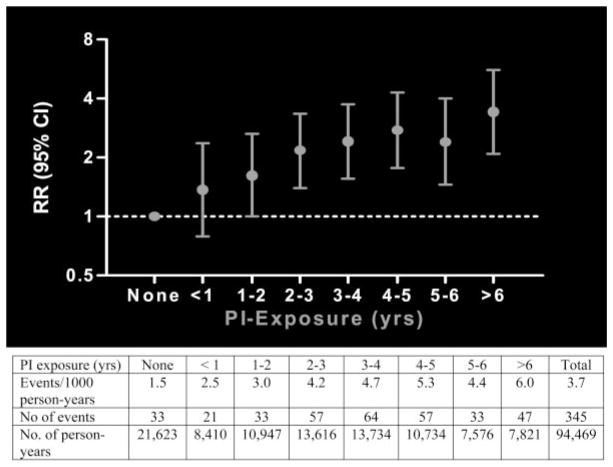
The largest prospective study of cardiovascular risk with ART is the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study.4 Of 23 437 participants, 345 (1.5%) developed a first MI, an incidence of 3.7 per 1000 person-years. Of these, 29% were fatal, representing 10% of all deaths in the study. Incidence of MI increased directly with longer exposure to ART (relative risk 1.16, 95% CI 1.09 to 1.23, per year of exposure, P<0.0001) for up to 6 to 7 years of exposure. Information on longer-term associations is unavailable. Importantly, this relative association between exposure to ART and increased risk of MI was comparable irrespective of age or gender. In further analyses evaluating the impact of individual antiretroviral drug classes, the relative risk of protease inhibitor therapy was also 1.16 (95% CI 1.10 to 1.23, P<0.001) (Figure), whereas the annual relative risk for nonnucleoside reverse-transcriptase inhibitor–based therapy was not significant (relative risk 1.05, 95% CI 0.98 to 1.13). As of early 2007, too few MI events had occurred to determine the relative risk for individual antiretroviral drugs, although such an analysis is planned.
Figure 1.
Relative rate of MI according to protease inhibitor exposure. The shown dose response equates to an adjusted* relative rate (RR) per year of exposure to protease inhibitor (PI) therapy of 1.16 (95% CI 1.10 to 1.23). Reproduced from Friis-Møller et al5 with permission of the publisher. Copyright © 2007 Massachusetts Medical Society. All rights reserved.
In contrast to the associations with ART, the DAD study did not find any relationship between MIs and markers of HIV disease such as a history of acquired immune deficiency syndrome (AIDS), lower CD4+ lymphocyte counts, or higher levels of plasma HIV RNA, either currently or previously. Importantly, traditional cardiovascular risk factors remained significantly associated with incident MIs in this study. Furthermore, the relative risks for increasing age, male sex, current smoking, elevated total cholesterol, low levels of HDL cholesterol, and diabetes mellitus were very similar to those observed in studies of HIV-uninfected adults18–24 (Table 2). Although the risk of MI remained significant in relation to duration of protease inhibitor–based ART, this risk was approximately halved in analyses that controlled for increased total cholesterol levels and lower HDL cholesterol levels, which suggests that ART-induced lipid abnormalities contributed to the increased risk observed.5 Thus, a substantial proportion of the risk attributed to protease inhibitors remains unexplained. Although the DAD study4 demonstrates a relative increase in risk with increased duration of ART, in part due to the presence of 1 or more traditional risk factors, the absolute risk of CVD will remain low for most patients. Because the absolute CVD rates remain low, the relative increase in these rates may not have clinical significance; however, this situation may change in the future as HIV patients live longer with successful ART.
Collectively, these studies suggest an association, in relative terms, between exposure to ART, specifically therapy with protease inhibitors, and risk of MI, attributable in part to proatherogenic effects of protease inhibitors on lipids. However, the design of the studies does not allow for a formal causal relationship to be established; this can only be established in randomized, controlled trials. As noted above, the magnitude of the impact of ART on cardiovascular risk, in absolute terms, will likely be mediated by the underlying cardiovascular risk, which is determined by both modifiable and unmodifiable factors.
Impact of Discontinuing ART
The Strategic Management of Antiretroviral Therapy (SMART) study was initiated in response to concerns about the evident toxicities of ART, as well as its incomplete potency.41 SMART randomly allocated 5472 HIV-infected participants with CD4+ lymphocyte counts >350 cells/mm3 at >330 sites in 33 countries to a strategy of continuous ART aimed at continuous virological suppression or to a strategy of intermittent ART that was ceased when CD4 counts were >350 cells/mm3 and initiated when CD4 counts fell to <250 cells/mm3 (this threshold was chosen because the risk of AIDS begins to increase substantially when the CD4 count is <200 cells/mm3).
It was hypothesized that intermittent ART might be associated with a modestly increased risk of HIV disease progression but that this would be offset by a lower rate of major toxicities such as CVD, which, until that time, were believed to be largely associated with ART. The study was stopped earlier than expected when an interim analysis found that intermittent ART was associated with more deaths, more progression to AIDS, and a greater rate of other major adverse events, including CVD.
Of note, additional studies specifically focusing on a possible increased risk of CVD and intermittent therapy were hampered by the low number of events.25 A possible mechanism to explain the association of increased CVD risk with intermittent therapy is an increase in the ratio of total cholesterol to HDL cholesterol that results from the interruption of ART, particularly because of an apparent decrease in HDL cholesterol. Additionally, interruption of ART may lead to an inflammatory reaction within the arterial wall.
Controversial Issues, Gaps in Knowledge, and Future Research Priorities
The results of studies summarized in this review highlight issues that remain unresolved by current data and that should be the focus of future investigations. It is apparent that among HIV-infected patients, as in those without HIV, there are multiple factors that both increase and decrease risk simultaneously that may contribute to overall CVD rates. A summary of these divergent factors is listed in Table 4. Questions raised include those addressed below.
Table 4.
Factors That Potentially Influence Cardiovascular Risk in HIV Patients
| Increasing Cardiovascular Risk | Decreasing Cardiovascular Risk |
|---|---|
| Dyslipidemia, insulin resistance, body habitus changes associated with HIV itself and certain components of ART | Control of viral replication with ART improves endothelial function |
| High rates of other cardiovascular risk factors, in particular smoking | Current antiretroviral regimens have more favorable effects on metabolic parameters and morphological changes than earlier regimens |
| Prolongation of survival: Older patients are intrinsically at greater cardiovascular risk | ART reduces inflammatory markers and immune activation HIV providers more aggressive about modification of ART or initiation of lipid-lowering therapies |
Determination of Overall MI Rates
What is the clinical significance of an increase in relative CVD risk, even a 2-fold increase in risk, as suggested by some studies, among HIV-infected patients if the absolute risk is low in this population?
What is the optimal control group with which to compare rates of CVD in young HIV-infected patients in longitudinal studies? How should these studies be designed?
Assessment of Traditional and Nontraditional Risk Factors
How do drug-induced or HIV-related lipid abnormalities compare with those that arise naturally in the pathogenesis of atherosclerosis?
What is the impact of unmeasured confounding factors, such as intensity of smoking, cocaine use, concomitant infections, low socioeconomic status, and depression, on the association between HIV infection and CHD risk?
Are there subgroups of patients who are at greatest risk, and can they be identified by use of genetic markers?
Role of ART and HIV-Specific Risk Factors for CVD
Is HIV viral replication itself a risk factor? Can this be measured through use of established and novel markers of inflammation and immune activation?
Do the differential lipid effects of different antiretroviral drugs translate into differences in cardiovascular outcomes? Are these effects clinically relevant?
Is the protease inhibitor effect seen in the DAD study a class effect, or is it related to the more toxic agents from this class of drugs used in the early treatment era? Did the use of earlier nucleoside reverse-transcriptase inhibitors contribute to this increased risk? How will this affect the development of CVD in developing countries, where these earlier regimens (eg, thymidine nucleosides) are, by necessity, still in use?
By contrast, how clinically relevant are the increases in HDL cholesterol seen with nonnucleoside reverse-transcriptase inhibitor–based therapies?
Some nucleoside reverse-transcriptase inhibitors (the thymidine analogues) may result in depletion of subcutaneous fat and may induce insulin resistance and overt diabetes mellitus. Do these adverse effects lead to a net adverse effect on risk of CVD?
Given that the first agents in the CCR5 antagonist and integrase inhibitor classes have a relatively neutral effect on lipids, how will these agents influence CHD risk?
During the past 25 years, the prognosis for people living with HIV infection who have access to ART has improved dramatically. The treatment of HIV infection now should focus on long-term management over decades. Given the improved survival of patients with HIV that results from the use of ART, diseases of aging, including CHD, have become more important. It is therefore evident that CVD should remain an area of focus for clinical and basic research in this population.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
The opinions expressed in this manuscript are those of the authors and should not be construed as necessarily representing an official position of the US Department of Health and Human Services, the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, or the US government. These opinions are not necessarily those of the editor or the American Heart Association.
The Executive Summary is available in the print issue of the journal (Circulation. 2008;118:198–210). The remaining writing group reports are available online at http://circ.ahajournals.org (Circulation. 2008;118:e20–e28; e36–e40; e41–e47; e48–e53; and e54–e60).
These proceedings were approved by the American Heart Association Science Advisory and Coordinating Committee on February 29, 2008. A copy of these proceedings is available at http://www.americanheart.org/presenter.jhtml?identifier=3003999 by selecting either the “topic list” link or the “chronological list” link (No. 71-0449). To purchase additional reprints, call 843-216-2533 or e-mail kelle.ramsay@wolterskluwer.com.
Expert peer review of AHA Scientific Statements is conducted at the AHA National Center. For more on AHA statements and guidelines development, visit http://www.americanheart.org/presenter.jhtml?identifier=3023366.
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at http://www.americanheart.org/presenter.jhtml?identifier=4431. A link to the “Permission Request Form” appears on the right side of the page.
Note Added in Proof
Recently, it was reported that current (but not previous) exposure to 2 antiretroviral drugs (abacavir and didanosine) was associated with increased risk of myocardial infarction and coronary heart disease. These findings were surprising and unexpected, because these drugs were not known to be primarily causes of metabolic dysfunction, which is the likely driver for why the protease inhibitors are associated with excess risk of myocardial infarction.5 Further studies are warranted to confirm this finding.43
Disclosures
Potential conflicts of interest for members of the writing groups for all sections of these conference proceedings are provided in a disclosure table included with the Executive Summary, which is available online at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.107.189622.
This article has been copublished in the Journal of Acquired Immune Deficiency Syndromes.
References
- 1.Henry K, Melroe H, Huebsch J, Hermundson J, Levine C, Swensen L, Daley J. Severe premature coronary artery disease with protease inhibitors. Lancet. 1998;351:1328. doi: 10.1016/S0140-6736(05)79053-X. [DOI] [PubMed] [Google Scholar]
- 2.Vittecoq D, Escaut L, Monsuez JJ. Vascular complications associated with use of HIV protease inhibitors. Lancet. 1998;351:1959. doi: 10.1016/s0140-6736(05)78644-x. [DOI] [PubMed] [Google Scholar]
- 3.Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Maa JF, Hodder S. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, Thiébaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Law MG, Kirk O, Phillips AN, Lundgren JD Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction [published correction appears in N Engl J Med. 2004;350:955] N Engl J Med. 2003;349:1993–2003. [Google Scholar]
- 5.Friis-Møller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, Thiébaut R, De Wit S, Kirk O, Fontas E, Law MG, Phillips A, Lundgren JD DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 6.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mary-Krause M, Cotte L, Simon A, Partisani M, Costagliola D Clinical Epidemiology Group From the French Hospital Database. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 8.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 9.Iloeje UH, Yuan Y, L’Italien G, Mauskopf J, Holmberg SD, Moorman AC, Wood KC, Moore RD. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med. 2005;6:37–44. doi: 10.1111/j.1468-1293.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 10.Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sørensen HT, Gerstoft J. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–1631. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 11.Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Friis-Møller N, Weber R, Reiss P, Thiébaut R, Kirk O, d’Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M, El-Sadr W, De Wit S, Sabin CA, Phillips AN, Lundgren JD DAD Study Group. Cardiovascular disease risk factors in HIV patients: association with antiretroviral therapy: results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 13.Savès M, Chêne G, Ducimetière P, Leport C, Le Moal G, Amouyel P, Arveiler D, Ruidavets JB, Reynes J, Bingham A, Raffi F French WHO MONICA Project and the APROCO (ANRS EP11) Study Group. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 14.Neumann T, Woiwod T, Neumann A, Ross B, Von Birgelen C, Volbracht L, Brockmeyer NH, Gerken G, Erbel R. Cardiovascular risk factors and probability for cardiovascular events in HIV-infected patients, part II: gender differences. Eur J Med Res. 2004;9:55–60. [PubMed] [Google Scholar]
- 15.Kaplan RC, Kingsley LA, Sharrett AR, Li X, Lazar J, Tien PC, Mack WJ, Cohen MH, Jacobson L, Gange SJ. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–1081. doi: 10.1086/521935. [DOI] [PubMed] [Google Scholar]
- 16.Joy T, Keogh HM, Hadigan C, Lee H, Dolan SE, Fitch K, Liebau J, Lo J, Johnsen S, Hubbard J, Anderson EJ, Grinspoon S. Dietary fat intake and relationship to serum lipid levels in HIV-infected patients with metabolic abnormalities in the HAART era. AIDS. 2007;21:1591–1600. doi: 10.1097/QAD.0b013e32823644ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, Palella F, Visscher B, Evans R, Kingsley LA. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 18.Mensah GA, Dietz WH, Harris VB, Henson R, Labarthe DR, Vinicor F, Wechsler H Centers for Disease Control and Prevention. Prevention and control of coronary heart disease and stroke: nomenclature for prevention approaches in public health: a statement for public health practice from the Centers for Disease Control and Prevention. Am J Prev Med. 2005;29(suppl 1):152–157. doi: 10.1016/j.amepre.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen TF, McGee D, Davidsen M, Jørgensen T. A cross-validation of risk-scores for coronary heart disease mortality based on data from the Glostrup Population Studies and Framingham Heart Study. Int J Epidemiol. 2002;31:817–822. doi: 10.1093/ije/31.4.817. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren A, Hagman M, Wedel H, Wilhelmsen L. Serum cholesterol and long-term prognosis in middle-aged men with myocardial infarction and angina pectoris: a 16-year follow-up of the Primary Prevention Study in Göteborg, Sweden. Eur Heart J. 1997;18:754–761. doi: 10.1093/oxfordjournals.eurheartj.a015340. [DOI] [PubMed] [Google Scholar]
- 22.Cooper JA, Miller GJ, Humphries SE. A comparison of the PROCAM and Framingham point-scoring systems for estimation of individual risk of coronary heart disease in the Second Northwick Park Heart Study. Atherosclerosis. 2005;181:93–100. doi: 10.1016/j.atherosclerosis.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Liu X, Li X, Li Y, Zhao L, Chen Z, Li Y, Rao X, Zhou B, Detrano R, Liu K USA-PRC Collaborative Study of Cardiovascular and Cardiopulmonary Epidemiology Research Group; China Multicenter Collaborative Study of Cardiovascular Epidemiology Research Group. Estimation of 10-year risk of fatal and nonfatal ischemic cardiovascular diseases in Chinese adults. Circulation. 2006;114:2217–2225. doi: 10.1161/CIRCULATIONAHA.105.607499. [DOI] [PubMed] [Google Scholar]
- 24.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 25.Law MG, Friis-Møller N, El-Sadr WM, Weber R, Reiss P, D’Arminio Monforte A, Thiébaut R, Morfeldt L, De Wit S, Pradier C, Calvo G, Kirk O, Sabin CA, Phillips AN, Lundgren JD D:A:D Study Group. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D:A:D Study. HIV Med. 2006;7:218–230. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 26.Coplan PM, Nikas A, Japour A, Cormier K, Maradit-Kremers H, Lewis R, Xu Y, DiNubile MJ. Incidence of myocardial infarction in randomized clinical trials of protease inhibitor-based antiretroviral therapy: an analysis of four different protease inhibitors. AIDS Res Hum Retroviruses. 2003;19:449–455. doi: 10.1089/088922203766774487. [DOI] [PubMed] [Google Scholar]
- 27.Phillips A, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, Williams I, Drummond F, Duprez D, Belloso WH, Goebel FD, Grund B, Hatzakis A, Vera J, Lundgren JD. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antiviral Therapy. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 28.Holmberg SD, Moorman AC, Williamson JM, Tong TC, Ward DJ, Wood KC, Greenberg AE, Janssen RS HIV Outpatient Study (HOPS) Investigators. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 29.Rickerts V, Brodt H, Staszewski S, Stille W. Incidence of myocardial infarctions in HIV-infected patients between 1983 and 1998: the Frankfurt HIV-cohort study. Eur J Med Res. 2000;5:329–333. [PubMed] [Google Scholar]
- 30.Klein D, Hurley L, Silverberg M, Horberg M, Quesenberry M, Sidney S. Surveillance data for myocardial infarction hospitalizations among HIV+ and HIV− Northern Californians: 1994–2006. Presented at: 14th Conference on Retroviruses and Opportunistic Infections; February 27, 2007; Los Angeles, Calif. Abstract No. 807. [Google Scholar]
- 31.Bozzette SA, Ake C, Carpenter A, Bommakanty U, Leung V, Tam H, Smith R, Schepps A, Louis T. Cardio- and cerebrovascular outcomes with changing process of anti-HIV therapy in 36,766 US veterans. Presented at: 9th Conference on Retroviruses and Opportunistic Infections; February 28, 2002; Seattle, Wash. Paper No. LB9. [Google Scholar]
- 32.Maggi P, Serio G, Epifani G, Fiorentino G, Saracino A, Fico C, Perilli F, Lillo A, Ferraro S, Gargiulo M, Chirianni A, Angarano G, Regina G, Pastore G. Premature lesions of the carotid vessels in HIV-1-infected patients treated with protease inhibitors. AIDS. 2000;14:F123–F128. doi: 10.1097/00002030-200011100-00001. [DOI] [PubMed] [Google Scholar]
- 33.Depairon M, Chessex S, Sudre P, Rodondi N, Doser N, Chave JP, Riesen W, Nicod P, Darioli R, Telenti A, Mooser V Swiss HIV Cohort Study. Premature atherosclerosis in HIV-infected individuals: focus on protease inhibitor therapy. AIDS. 2001;15:329–334. doi: 10.1097/00002030-200102160-00005. [DOI] [PubMed] [Google Scholar]
- 34.Seminari E, Pan A, Voltini G, Carnevale G, Maserati R, Minoli L, Meneghetti G, Tinelli C, Testa S. Assessment of atherosclerosis using carotid ultrasonography in a cohort of HIV-positive patients treated with protease inhibitors. Atherosclerosis. 2002;162:433–438. doi: 10.1016/s0021-9150(01)00736-5. [DOI] [PubMed] [Google Scholar]
- 35.Jericó C, Knobel H, Calvo N, Sorli ML, Guelar A, Gimeno-Bayón JL, Saballs P, López-Colomés JL, Pedro-Botet J. Subclinical carotid atherosclerosis in HIV-infected patients: role of combination antiretroviral therapy. Stroke. 2006;37:812–817. doi: 10.1161/01.STR.0000204037.26797.7f. [DOI] [PubMed] [Google Scholar]
- 36.Mercié P, Thiébaut R, Aurillac-Lavignolle V, Pellegrin JL, Yvorra-Vives MC, Cipriano C, Neau D, Morlat P, Ragnaud JM, Dupon M, Bonnet F, Lawson-Ayayi S, Malvy D, Roudaut R, Dabis F Groupe d’Epidemiologie Clinique du Sida en Aquitaine (GECSA) Carotid intima-media thickness is slightly increased over time in HIV-1-infected patients. HIV Med. 2005;6:380–387. doi: 10.1111/j.1468-1293.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 37.Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 38.de Saint Martin L, Vandhuick O, Guillo P, Bellein V, Bressollette L, Roudaut N, Amaral A, Pasquier E. Premature atherosclerosis in HIV positive patients and cumulated time of exposure to antiretroviral therapy (SHIVA study) Atherosclerosis. 2006;185:361–367. doi: 10.1016/j.atherosclerosis.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 39.van Wijk JP, de Koning EJ, Cabezas MC, Joven J, op’t Roodt J, Rabelink TJ, Hoepelman AM. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47:1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 40.Lorenz MW, Stephan C, Harmjanz A, Staszewski S, Buehler A, Bickel M, von Kegler S, Ruhkamp D, Steinmetz H, Sitzer M. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 41.Johnsen S, Dolan SE, Fitch KV, Kanter JR, Hemphill LC, Connelly JM, Lees RS, Lee H, Grinspoon S. Carotid intimal medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4916–4924. doi: 10.1210/jc.2006-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmberg SD, Moorman AC, Greenberg AE. Trends in rates of myocardial infarction among patients with HIV. N Engl J Med. 2004;350:730–732. doi: 10.1056/NEJM200402123500719. [DOI] [PubMed] [Google Scholar]
- 43.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fätkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 44.D:A:D Study Group. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–1425. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]