High-dose methotrexate (HDMTX), defined as a dose higher than 500 mg/m2, is used to treat a range of adult and childhood cancers. Although HDMTX is safely administered to most patients, it can cause significant toxicity, including acute kidney injury. This article provides comprehensive recommendations for prevention of toxicity from HDMTX, along with detailed treatment guidance to mitigate acute kidney injury and subsequent toxicity.
Keywords: Methotrexate, High-dose methotrexate, Acute kidney injury, Leucovorin, Pharmacokinetics, Glucarpidase
Abstract
High-dose methotrexate (HDMTX), defined as a dose higher than 500 mg/m2, is used to treat a range of adult and childhood cancers. Although HDMTX is safely administered to most patients, it can cause significant toxicity, including acute kidney injury (AKI) in 2%–12% of patients. Nephrotoxicity results from crystallization of methotrexate in the renal tubular lumen, leading to tubular toxicity. AKI and other toxicities of high-dose methotrexate can lead to significant morbidity, treatment delays, and diminished renal function. Risk factors for methotrexate-associated toxicity include a history of renal dysfunction, volume depletion, acidic urine, and drug interactions. Renal toxicity leads to impaired methotrexate clearance and prolonged exposure to toxic concentrations, which further worsen renal function and exacerbate nonrenal adverse events, including myelosuppression, mucositis, dermatologic toxicity, and hepatotoxicity. Serum creatinine, urine output, and serum methotrexate concentration are monitored to assess renal clearance, with concurrent hydration, urinary alkalinization, and leucovorin rescue to prevent and mitigate AKI and subsequent toxicity. When delayed methotrexate excretion or AKI occurs despite preventive strategies, increased hydration, high-dose leucovorin, and glucarpidase are usually sufficient to allow renal recovery without the need for dialysis. Prompt recognition and effective treatment of AKI and associated toxicities mitigate further toxicity, facilitate renal recovery, and permit patients to receive other chemotherapy or resume HDMTX therapy when additional courses are indicated.
Implications for Practice:
High-dose methotrexate (HDMTX), defined as a dose higher than 500 mg/m2, is used for a range of cancers. Although HDMTX is safely administered to most patients, it can cause significant toxicity, including acute kidney injury (AKI), attributable to crystallization of methotrexate in the renal tubular lumen, leading to tubular toxicity. When AKI occurs despite preventive strategies, increased hydration, high-dose leucovorin, and glucarpidase allow renal recovery without the need for dialysis. This article, based on a review of the current associated literature, provides comprehensive recommendations for prevention of toxicity and, when necessary, detailed treatment guidance to mitigate AKI and subsequent toxicity.
Introduction
Methotrexate is an antimetabolite that interferes with the metabolism of folic acid. After entry into the cell, methotrexate is polyglutamated, binds dihydrofolate reductase (DHFR) with an affinity 1,000-fold greater than that of folate, and competitively inhibits conversion of dihydrofolate to tetrahydrofolate (Fig. 1). Tetrahydrofolate is essential for biosynthesis of thymidine and purines, which are needed for synthesis of DNA. Blockade of tetrahydrofolate synthesis by methotrexate leads to inability of cells to divide and to produce proteins. Methotrexate is an essential component of therapy for acute lymphoblastic leukemia (ALL) and is active against many types of cancer; this justifies its presence on the World Health Organization's list of essential medicines.
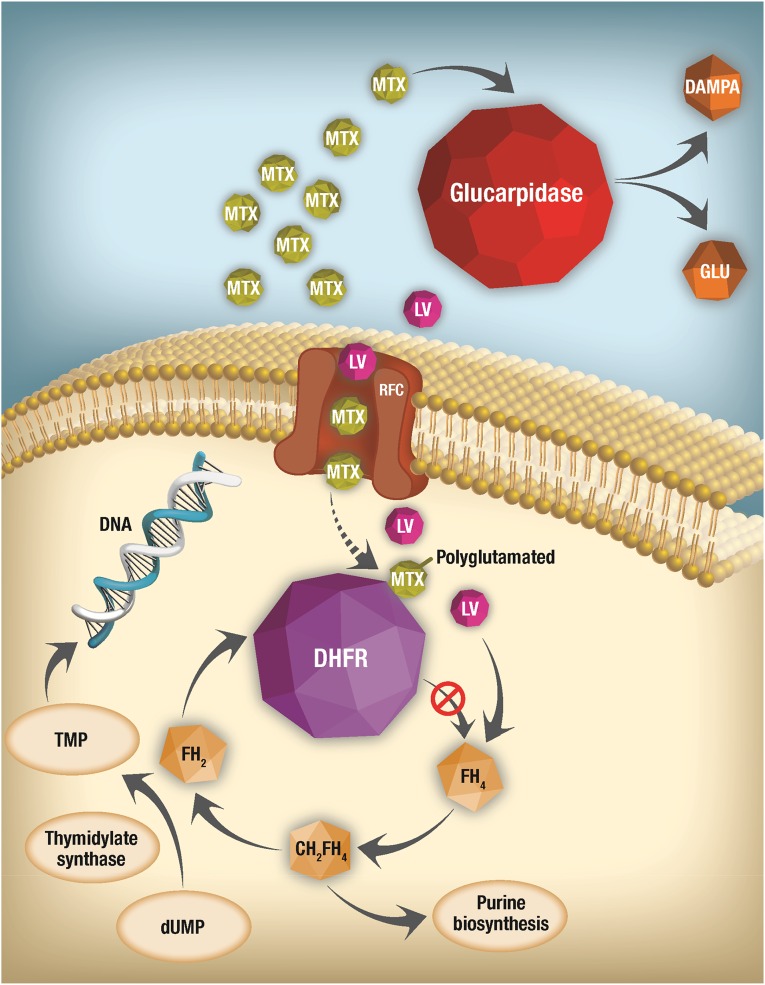
Figure 1.
Mechanism and site of action of MTX and of rescue strategies for delayed MTX elimination. After MTX enters cells through the RFC, it is polyglutamated, then competitively and reversibly inhibits the activity of DHFR, thus preventing formation of FH4 from FH2. The lack of FH4 inhibits DNA, RNA, and protein synthesis. LV enters cells through the RFC and allows formation of FH4 despite the presence of MTX, which effectively rescues cells. However, when MTX elimination is impaired and it is present at very high concentrations, very high doses of LV are necessary to allow entry of a sufficient amount to rescue cells from MTX toxicity. Glucarpidase eliminates extracellular MTX by converting it to nontoxic DAMPA and therefore should always be given with LV to provide intracellular rescue even as the glucarpidase prevents further accumulation of intracellular MTX by removing it from the extracellular compartment.
Abbreviations: DAMPA, 4-deoxy-4-amino-N-10-methylpteroic acid; DHFR, dihydrofolate reductase; dUMP, deoxyuridine monophosphate; FH2, dihydrofolate; FH4, tetrahydrofolate; FH5, tetrahydrofolate; LV, leucovorin; MTX, methotrexate; RFC, reduced folate carrier; TMP, thymidine monophosphate.
Methotrexate is administered at doses that range from 12 mg intrathecally and 20 mg/m2 orally, intramuscularly, or intravenously as weekly maintenance chemotherapy for ALL to doses as high as 33,000 mg/m2 intravenously for some other indications [1]. Doses of 500 mg/m2 or higher given intravenously are defined as high-dose methotrexate (HDMTX) and are used to treat a variety of adult and pediatric cancers, including ALL, osteosarcoma, and lymphomas [2–4]. HDMTX therapy can cause significant toxicity, which not only leads to morbidity and occasional mortality but may also interrupt cancer treatment, potentially leading to inferior anticancer outcomes. To prevent unacceptable toxicity, it must be given with rigorously standardized supportive care [1], which differs across tumor types and treatment protocols (Table 1). When patients experience delayed methotrexate excretion, without timely recognition and treatment, the prolonged exposure to toxic methotrexate concentrations can lead to significant morbidity and mortality [1]. Aggressive monitoring and prompt intervention usually promote methotrexate excretion, prevent toxicity, and allow patients to receive subsequent HDMTX treatment [1, 5]. Here, we describe common toxicities, review supportive care strategies, explore approaches to manage toxicity, and suggest subsequent HDMTX therapy after treatment-related toxicity occurs.
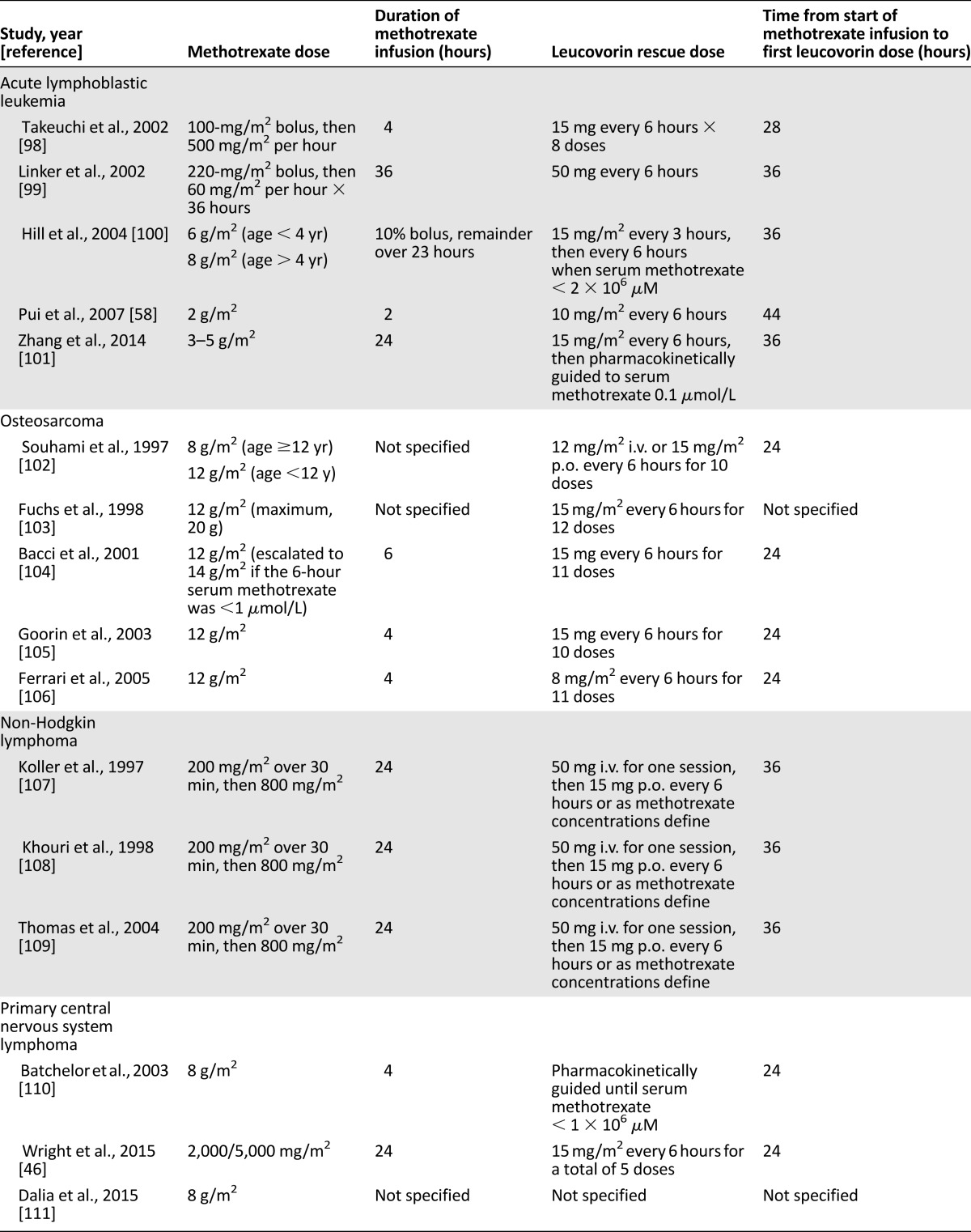
Table 1.
Selected protocols that include high-dose methotrexate for acute lymphoblastic leukemia and osteosarcoma
Potential Toxicities of High-Dose Methotrexate
Acute Kidney Injury
Despite appropriate supportive care measures during administration of HDMTX, acute kidney injury (AKI) develops in 2%–12% of patients [6]. The incidence depends on host factors, supportive measures used, and the dose and schedule of HDMTX. For example, 9.1% of HDMTX cycles in patients with lymphoma are complicated by AKI, compared with only 1.5% of cycles in patients with sarcomas [7, 8]. Nephrotoxicity caused by HDMTX arises through crystal nephropathy, which occurs when methotrexate and its metabolites precipitate within the renal tubules. Because methotrexate is acidic, drug crystals are not present in urine with an alkaline pH, as alkalinization greatly increases methotrexate solubility and excretion. Crystal-induced nephropathy initially manifests as an asymptomatic elevation in serum creatinine and then progresses to tubular necrosis and more severe renal injury.
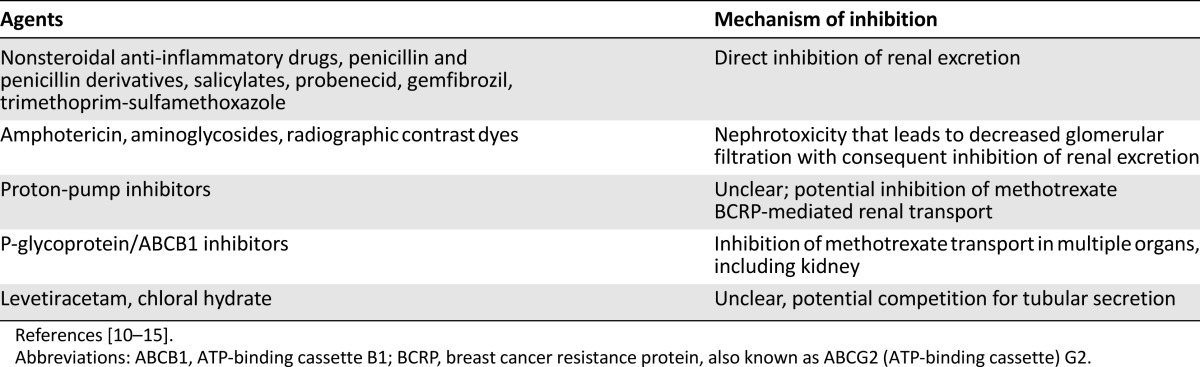
Because volume depletion and acidic urine are major risk factors for AKI, hyperhydration and urine alkalinization are mandatory during HDMTX treatment (discussed further in the section on supportive care measures) [7]. Drug-drug interactions can also contribute to delayed methotrexate excretion and subsequent nephrotoxicity [7]. Agents that pose the highest risk of adverse interaction are those that interfere with methotrexate clearance by competing for renal tubular secretion (Table 2) [1]. Careful reviews of all medications and close monitoring of all patients are warranted [9–15]. Whether prophylactic trimethoprim-sulfamethoxazole should be discontinued during HDMTX infusion is controversial. Although some protocols suggest deferring it until adequate HDMTX clearance is documented, no strong data support this approach.
Table 2.
Drugs that impair methotrexate clearance
Other Toxicities
Acute kidney injury impairs the renal clearance of methotrexate, resulting in the accumulation of toxic concentrations and an increased risk for additional adverse events [7]. Prolonged renal dysfunction with increased systemic methotrexate exposure can cause myelosuppression, mucositis, hepatotoxicity, and, in severe cases, multiorgan failure [16]. Emesis occurs in 10%–30% of patients receiving HDMTX even when appropriate antiemetics are used; in this subgroup, antiemetics should be escalated to completely control vomiting and additional hydration provided to replace lost fluid [17]. The American Society of Clinical Oncology clinical practice guideline for antiemetic therapy classifies HDMTX as having low emetic risk and recommends dexamethasone to reduce the risk for nausea and vomiting. Because 5-HT3 antagonists are used almost universally, dexamethasone is often omitted [17]. Transient liver toxicity may include reversible chemical hepatitis in up to 60% and hyperbilirubinemia in 25% of courses, respectively [18]. In up to 15% of HDMTX courses, patients report transient disturbances of the central nervous system (CNS), and a subset of these experience significant symptoms, such as cortical blindness, hemiparesis, and seizure [19]. Chemical conjunctivitis occurs rarely and can be managed with local treatment; indeed, methotrexate can be safely administered intraocularly to control autoimmune diseases that affect the eye [20]. Pulmonary toxicity is observed in 0.5% of patients per year who receive weekly low-dose methotrexate, but rarely with HDMTX [18, 21–23]. Pulmonary function testing or other screening is not warranted in patients with cancer, in whom methotrexate-induced pneumonitis is rare, unless pre-existing lung compromise or other risk factors warrant surveillance.
Animal models, particularly rats, have been used to study toxicities of other therapeutics [24] and diseases, such as nonalcoholic steatohepatitis [25]. Rodent models have been used for pharmacokinetic modeling of methotrexate [26], exploring leucovorin rescue [27], and preclinical studies of investigational interventions to reduce toxicity (including pentoxifylline [28], amifostine [29], melatonin [30], and activators of peroxisome proliferator activator receptor α and γ [31]). Critically, the mechanism of MTX crystal formation in the renal tubule was elucidated in monkeys [32], and the concept of enzymatic cleavage of MTX using glucarpidase was first described in a mouse model by Chabner et al. in 1972 [33].
Risk Factors for Toxicity During Treatment With High-Dose Methotrexate
Several patient-related factors can increase the risk for AKI [7]. Volume depletion is perhaps the most important and can result from fluid losses due to vomiting or diarrhea, adrenal insufficiency, or renal salt wasting [7]. Reductions in intravascular volume lead to renal hypoperfusion with subsequent decreased urine output [7]. Precipitation of methotrexate crystals occurs in acidic urine (pH < 5.5) when the concentration of methotrexate in the renal tubules exceeds 2 × 10−3 molar. Intrarenal crystal formation can lead to tubule obstruction, direct toxic damage to the renal tubular epithelium (due to prolonged contact with methotrexate), and hypoperfusion from afferent arteriolar vasoconstriction, each of which independently can worsen AKI [34–36]. Polyuria leading to severe shifts in fluid balance has also been reported with methotrexate infusion. Although the mechanism remains unclear, patients with methotrexate-associated polyuria require especially close monitoring of fluid status and frequent adjustments in intravenous fluids to maintain fluid balance and prevent renal hypoperfusion [37].
Patients with a history of toxicity with prior courses of HDMTX have higher risk for subsequent renal toxicity [1]. However, even when toxicity is severe, subsequent HDMTX courses can generally be administered safely after the patient recovers [5]. As many as 60% of adult cancer patients have some degree of renal dysfunction, which increases their risk for AKI [38]. Lower creatinine clearance (CrCl) before administration of HDMTX predicts renal toxicity, and both CrCl and serum creatinine concentration before HDMTX administration can be useful in predicting plasma methotrexate concentrations after infusion [8, 39]. Specific CrCl cutoff values for dose reduction or omission of subsequent HDMTX have not been established, with upper cutoffs for dose reduction starting at 50–60 mL/min and recommendations to omit further HDMTX when CrCl falls below 10–30 mL/min [40, 41]. Additional host factors that contribute to AKI risk include pre-existing nephropathy because of previous drug toxicity (e.g., from cisplatin) or associated disease, metabolic derangements due to the presence of tumor, advanced age, and pharmacogenetic factors (such as hyperhomocysteinemia with concurrent relative or absolute folate deficiency) (Table 2) [9, 42]. In this regard, methotrexate clearance is also associated with polymorphisms of SLCO1B1, which encode a hepatic solute carrier organic anion transporter that mediates disposition of many medications, including methotrexate [43–45].
Delayed methotrexate excretion has been associated with extravascular fluid collections, including ascites, pleural effusions, or intracranial fluid; whether HDMTX should be deferred to a later date in such situations depends on the balance of risks and the benefits of deferral, but even more rigorous monitoring is warranted if one proceeds [46]. Pre-existing nephropathy is associated with substantial toxicity even with low doses of methotrexate [47], suggesting a need for even greater diligence during HDMTX administration in the presence of renal dysfunction [7, 48]. Finally, delays between recognition of toxicity and initiation of treatment can directly contribute to renal and systemic toxicities [6]. In the setting of AKI, the rise in serum creatinine values lags behind progressive intrinsic renal damage, such that precise measurement of function at a specific moment is difficult [49]. Instead, a decrease in urine output, positive fluid balance, or weight change may help identify patients with early AKI after HDMTX administration, even before creatinine increases. Nevertheless, in some cases irreversible damage to renal tubule epithelial cells may have already occurred before the onset of oliguria or detection of clinically significant increases in serum creatinine concentration.
Delayed methotrexate excretion has been associated with extravascular fluid collections, including ascites, pleural effusions, or intracranial fluid; whether HDMTX should be deferred to a later date in such situations depends on the balance of risks and the benefits of deferral, but even more rigorous monitoring is warranted if one proceeds.
Supportive Care Measures
In most patients with normal renal function, HDMTX can be given safely with the use of several supportive care strategies. These should include adjusting medications with potential interactions, vigorous hydration, and urinary alkalinization (target pH ≥ 7) before starting methotrexate infusions. The goal is to enhance the solubility and dilution of methotrexate in the urine and apply leucovorin rescue guided by serial serum methotrexate levels to protect against potentially lethal toxicity.
Suspending Medications That Interfere With Methotrexate Clearance
All prescribed, over-the-counter, and nontraditional medications must be reconciled and documented before HDMTX administration begins. Because of their long half-life, some medications (e.g., naproxen sodium) can interfere with methotrexate elimination for many hours, with potentially disastrous delays in methotrexate elimination. Patients who take such medications daily should be provided with an alternative starting the day before HDMTX and continuing until methotrexate clearance. Studies in mice documented inhibition of renal tubule methotrexate transporters by indomethacin and ketoprofen, with reduced methotrexate elimination and prolonged elevated levels and toxicity [24].
Hydration
More than 90% of methotrexate is eliminated by the kidneys [1]. The use of fluids to promote high urinary flow rates and alkalinize the urine protects the kidney from injury during treatment with HDMTX [7]. Many pediatric protocols recommend at least 2 hours of hyperhydration of a minimum of 200 mL/m2 per hour or 100–150 mL/m2 per hour beginning 12 hours before the start of methotrexate infusion and continuing for 24–48 hours or longer if the patient has a history of methotrexate toxicity or develops delayed methotrexate elimination [1]. In adults, rates of 150–200 mL of bicarbonate-containing fluids per hour to a total of 2 L before HDMTX infusion are often used. Strict monitoring of fluid intake and output is recommended during and after administration of methotrexate.
Urine Alkalinization
Methotrexate and its metabolites, including 7-OH-methotrexate and 4-deoxy-4-amino-N-10-methylpteroic acid (DAMPA), are poorly soluble at an acidic pH [1, 50]. An increase in urine pH from 6.0 to 7.0 increases the solubility of methotrexate and its metabolites by five- to eightfold, and alkalinization is imperative to reduce intratubular crystal formation (precipitation) [1, 50]. Thus, administration of fluids with 40 mEq/L sodium bicarbonate is recommended during and after HDMTX administration [1, 7]. A urine pH of 7 or greater should be required before administration of methotrexate to reduce crystal formation. It is also important to check urine pH values with each void during the infusion to ensure no extended periods of time with acidic urine, which could increase the risk for precipitation, nephrotoxicity, and delayed methotrexate elimination. The ability of the laboratory to process samples rapidly and notify clinicians when the pH decreases to 7 or less facilitates safer HDMTX administration. If a urine pH of 6.5 is identified, sodium bicarbonate at a dose of 12.5 mEq/m2 is administered, and for urine pH <6.5, a dose of 25 mEq/m2 is given; urine pH is measured hourly throughout HDMTX infusion because sometimes boluses of sodium bicarbonate must be repeated to achieve alkaline urine [51]. In patients with serum alkalosis and inadequate urine alkalinization, the carbonic anhydrase inhibitor acetazolamide (250–500 mg p.o. four times daily) may be added to directly alkalinize the urine by increasing renal excretion of sodium, water, and bicarbonate, without increasing serum pH [52].
Leucovorin
For more than 30 years, leucovorin rescue has been a cornerstone of HDMTX treatment (Fig. 2) [18]. Leucovorin is particularly effective in the prevention of myelosuppression, gastrointestinal toxicity, and neurotoxicity during treatment with HDMTX. Chemotherapy protocols that include HDMTX also include recommendations for the timing, dose, and duration of leucovorin administration to protect normal cells from injury (Table 1) [1, 18]. Because leucovorin effectively neutralizes the effects of methotrexate, it must not be started too early because it would then reduce not only toxicity but also anticancer efficacy. In this regard, if a patient is taking leucovorin at the time HDMTX treatment is scheduled to begin, the leucovorin should be discontinued and the HDMTX deferred until the following day.
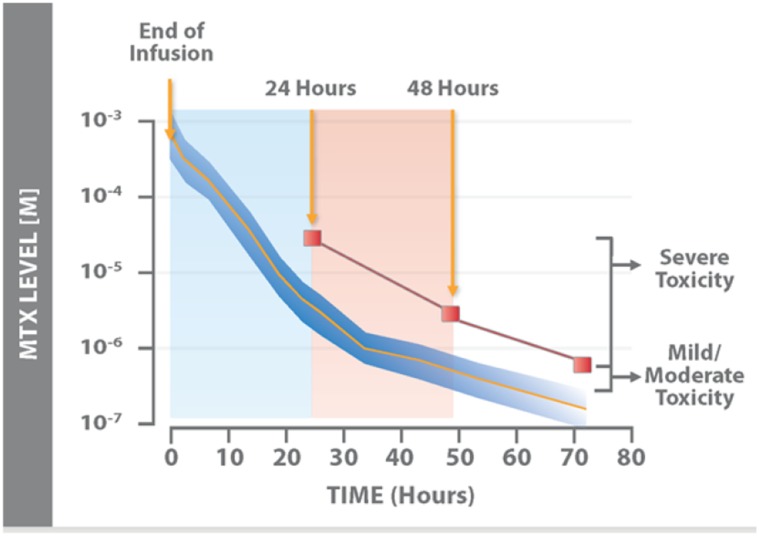
Figure 2.
Nomogram for the expected time-dependent decrease in serum MTX levels after completion of MTX infusion. Nomogram for expected serum MTX levels as a function of time from the end of a 6-hour infusion of methotrexate at a dose of 7.5 g/m2. The dark blue area represents ±2 SD from the mean (orange line). Values above the red line indicate impending or severe toxicity. Adapted from [112] with permission.
Abbreviation: MTX, methotrexate.
Other Supportive Care Measures
Other supportive care measures can be tailored according to individual patient risk factors. For instance, HDMTX doses can be reduced in patients with pre-existing renal dysfunction or severe toxicity after a prior course of HDMTX, and serum methotrexate levels can be measured early (e.g., at hour 6 of a 24-hour infusion) to make sure that there is no excessive accumulation [7]. During treatment with HDMTX, patients must also minimize exposure to other potential nephrotoxins, including those listed in Table 2 [7, 10].
Monitoring During Treatment With High-Dose Methotrexate
The pharmacokinetics of methotrexate dictate the degree of supportive care and monitoring needed after treatment. After a HDMTX infusion with a fixed dose and duration, plasma concentrations can vary widely between patients and within a patient on different cycles. Plasma protein binding, effusions, renal function, and, to a lesser degree, hepatic function can all contribute to peak concentrations after infusion. Serial methotrexate concentrations are obtained, with the primary focus on values that easily fit within the leucovorin nomogram timeframe (i.e., 42 hours and later) (Fig. 2). However, pharmacokinetic modeling data from Evans et al. [53] show that values above 10 μM at 24 hours after the start of the infusion confers a high risk for toxicity.
Methotrexate is primarily eliminated by the kidney, so renal function must be assessed before, during, and after each course of HDMTX. Currently used measures of kidney function include serum creatinine, urine output, urine pH, and blood urea nitrogen [54]. A rise in serum creatinine concentration and other parameters above normal values indicates potential renal dysfunction and delayed methotrexate elimination [2]. Incorporation of clinical decision support in the electronic health record can alert clinicians of acute changes in serum creatinine or prescription of medicines that may delay elimination of methotrexate. Automated early warnings may facilitate prompt interventions, such as increasing the rate of intravenous fluids, substitution of an alternative drug that does not interfere with methotrexate clearance, and, in extreme cases, even stopping the methotrexate infusion early to prevent toxicity. Treatment protocols that include HDMTX sometimes outline strategies for dose reduction in patients with reduced creatinine clearance [1]. The utility of alternate biomarkers for earlier detection of renal injury is an area of active study [55].
Plasma methotrexate concentrations should be monitored closely to detect any delay in methotrexate clearance during each cycle of therapy [56]. Depending on the regimen, plasma methotrexate assays may be appropriate at 24, 48, and 72 hours after the start of methotrexate infusion [57]. Other protocols may require serum methotrexate measurements at 36 hours (i.e., 12 hours after the end of a 24-hour infusion) or at 42 hours from the start [58]. Importantly, leucovorin doses are adjusted according to plasma methotrexate concentrations, and hydration and alkalization can be fine-tuned to optimize safety [1]. Serum methotrexate concentrations should be monitored with ongoing adjustments in hydration, alkalinization, and leucovorin rescue until the target of less than 0.05–0.1 μM is reached [1]. Plasma methotrexate monitoring is a reliable indicator specifically of nephrotoxicity but may be a limited predictor of other toxicities [59]. However, in centers where methotrexate levels cannot be monitored, assiduous monitoring of urine pH and output, serum creatinine, and twice-daily examination of mucosal membranes for evidence of inflammation can allow safe administration of HDMTX for most patients; indeed, this practice in Recife, Brazil, where methotrexate levels were not available, allowed for safe administration of hundreds of courses of HDMTX [60].
Management of Specific Toxicities Associated With High-Dose Methotrexate
Nephrotoxicity
Aggressive supportive care measures are needed when AKI occurs after HDMTX. Continuing to administer alkalinized i.v. fluids with addition of acetazolamide when needed to keep urine pH > 7 maximizes methotrexate elimination and reduces further crystal formation in nephrons. Increasing infusion rates to the maximum tolerated amount (≥3 L/m2 per day) is recommended to maximize urine output. Attention to fluid balance, frequent symptom assessment, pulmonary examination, pulse oximetry, and chest radiography or echocardiography of patients at risk for heart failure allow aggressive hydration with minimal risks. Although pleural effusions may occur with very aggressive hydration, the risk-benefit relationship favors continued hydration in most cases, as delayed methotrexate elimination in most patients is primarily driven by renal dysfunction.
Extracorporeal techniques to remove excessive methotrexate have been used and are logical on the basis of the distribution of methotrexate in serum and its limited protein binding (58%) [61]. However, results have been mixed; because of ethical considerations, studies have lacked suitable control patients who did not receive dialysis and have been confounded by differing concomitant interventions (e.g., leucovorin doses, glucarpidase use) [1, 62]. Furthermore, retrospective analyses of differing approaches without control groups, including plasmapheresis, charcoal hemoperfusion, high-flux hemodialysis, conventional hemodialysis, and peritoneal dialysis make it difficult to identify one optimal extracorporeal strategy. High-flux hemodialysis is likely to be the most effective based on technique and flow rates and reduced methotrexate concentrations during a 6-hour period in one series, whereas peritoneal dialysis is unlikely to be effective [63]. Unfortunately, even when hemodialysis is effective, many patients experience a rebound in serum methotrexate concentrations of 10%–220% of the postprocedure values [64]. Complications of dialysis must also be considered, especially in critically ill patients who are at higher risk for electrolyte abnormalities, bleeding at catheter sites, and cardiac arrest. Rapid institution of extracorporeal methods is critical if they are used, but the variable results and rebound rise in methotrexate concentrations necessitate continuous monitoring and repeat dialysis as needed. In all cases, high doses of leucovorin should be administered until methotrexate has been completely eliminated; in very ill patients, continuing it for another day or two thereafter is warranted. Leucovorin is removed by dialysis, and so it should be redosed afterward [65].
Hepatotoxicity
Hepatotoxicity after HDMTX is much less common than with the lower, long-term oral methotrexate dosing that is used in patients with rheumatoid arthritis, who are at risk for liver fibrosis and require regular monitoring of liver enzyme levels [66]. Indeed, almost all patients have elevations of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values after HDMTX, but these laboratory findings have no clinical significance and require no adjustment of subsequent courses of HDMTX because most cases are transient and reversible and do not lead to chronic liver disease [67]. Measurement of AST, ALT, and bilirubin before each course of HDMTX is advisable to assure there is no evidence of hepatic inflammation or dysfunction that could be worsened by methotrexate [68]. Risk factors for hepatotoxicity in long-term methotrexate dosing include alcohol and hepatitis B and C virus infection [69–71]. Although these have not been documented to complicate HDMTX, avoiding alcohol and controlling hepatitis infection before HDMTX is warranted to minimize risk. Conversely, existing steatohepatitis may increase methotrexate toxicity [25].
Neurotoxicity
CNS toxicity may occur after HDMTX, and concurrent intrathecal treatment, cranial irradiation, infiltration of malignant cells, or concomitant CNS toxins increase the risk, as well as confound the etiology. Depending on the population studied, up to 11% of patients may have CNS events, including confusion, seizures, somnolence, and headaches with or without radiographic evidence of leukoencephalopathy [72]. For example, 3% of pediatric ALL patients had acute encephalopathy, and pharmacokinetic parameters did not predict the onset [72]. Symptoms typically occur within 24 hours, often resolve spontaneously, and rarely have long-term sequelae [19, 73]. Elevated ratios of methotrexate to leucovorin at 42 hours may predict those at risk for CNS toxicity, and polymorphisms in genes associated with neurodevelopment (e.g., TRIO, PRKG1, ANK1, COL4A2, NTN1, and ASTN2) may also increase risk [19].
A potential mechanism of neurotoxicity is the accumulation of adenosine after MTX-induced reductions in purine synthesis [74]. The finding of increased adenosine in the CNS in patients with toxicity led some investigators to evaluate 1-hour 2.5-mg/kg infusions of aminophylline in pediatric ALL patients on the basis of their ability to displace adenosine from central receptors [75]. In the 6 patients treated, 4 had complete resolution of symptoms that had not improved after other measures (e.g., corticosteroids), and 2 had persistent nausea but no other symptoms. However, no definitive studies demonstrate the efficacy of aminophylline in treating or preventing methotrexate-induced neurotoxicity. Patients who develop CNS toxicity should have all potential neurotoxins discontinued and magnetic resonance imaging examination should be performed, particularly if symptoms do not improve within 24 hours of onset.
Mucositis
Oral mucositis can become a dose-limiting toxicity, require the use of opioids, increase infectious risk, and lead to chemotherapy delays. The biological processes associated with mucositis ultimately result in mucosal barrier injury, based on a systematic cascade of cellular and tissue interactions involving the endothelium, extracellular matrix, metalloproteinase, submucosal reactions, and connective tissue [76–78]. Mucositis after HDMTX is caused by cellular damage to rapidly dividing epithelial cells along the entire gastrointestinal tract; inadequate or delayed leucovorin rescue can lead to impaired epithelial cell growth and regeneration.
Grade IV mucositis is an oncologic emergency and is associated with infections, the need for parenteral nutrition, increased use of health care resources, delayed chemotherapy, and even death [77]. A variety of methods have been used to prevent or treat oral mucositis, including ice chips [79, 80], glutamine and N-acetylcysteine, benzydamine hydrochloride, benzydamine hydrochloride, and prostaglandin E1 and E2, but none has proven benefits in patients receiving prolonged infusions of HDMTX [78, 81]. Palifermin, a recombinant human keratinocyte growth factor that stimulates growth of epithelial cells, reduces the incidence of mucositis after HDMTX [82, 83]. Interventions to prevent and treat mucositis have recently been reviewed, but none is standard practice in patients receiving HDMTX [84, 85].
Small animal models, mainly rats, have been used to investigate the mechanisms of gastrointestinal methotrexate toxicity [86, 87], the effect on the resident microbiome [88], and a variety of approved and experimental interventions.
Myelosuppression
A dose-limiting toxicity of HDMTX in the absence of leucovorin is severe, prolonged myelosuppression; however, the frequency and severity of cytopenias with pharmacokinetically guided leucovorin is low. When elimination is delayed because of third spacing and fluid accumulations or as a result of renal injury, neutropenia and thrombocytopenia may be severe [89]. When myelosuppression occurs, standard therapies for febrile neutropenia and transfusions are provided as clinically indicated. The only known strategies to prevent myelosuppression are to prevent delayed methotrexate elimination by avoiding interacting medications around the time of infusion and draining effusions before treatment (or delaying HDMTX until effusions resolve) and to ensure optimal leucovorin dosing.
Glucarpidase
Enzymatic cleavage of MTX using glucarpidase (a recombinant bacterial carboxypeptidase G2) was first described in 1972 [33]. Glucarpidase was approved by the U.S. Food and Drug Administration in 2012 for patients with delayed methotrexate elimination or AKI and plasma methotrexate concentrations >1 μmol/L [50]. Glucarpidase cleaves methotrexate into DAMPA and glutamate, two nontoxic metabolites, and thus provides an enzymatic method to rapidly remove methotrexate in patients with renal dysfunction (Fig. 1). A single dose of glucarpidase (50 U/kg i.v. over 5 minutes) reduces plasma methotrexate concentrations by 97% or more within 15 minutes [50]. However, despite the decrease in the magnitude and duration of systemic exposure to methotrexate after glucarpidase, it has no effect on intracellular methotrexate concentrations [6, 16]. Therefore, just as with dialysis, the coadministration of high-dose leucovorin is required to protect cells from toxic methotrexate concentrations until renal function recovers sufficiently to clear any residual methotrexate as it is released from cells (Fig. 1). In fact, after glucarpidase administration, leucovorin should be continued until methotrexate concentrations have been maintained at close to undetectable levels for several more days. Leucovorin should not be administered within 2 hours before or after a dose of glucarpidase because, like methotrexate, leucovorin is a substrate for glucarpidase. Hydration and urine alkalinization should also be continued in patients requiring glucarpidase [50].
Within 48 hours of glucarpidase administration, only a chromatographic (high-performance liquid chromatography) method can reliably measure methotrexate concentrations because the DAMPA produced by enzymatic breakdown of methotrexate cross-reacts with methotrexate in the standard immunoassay and artificially elevates the level [50]. The long half-life of DAMPA (approximately 9 hours) precludes use of immunoassays for several days after glucarpidase administration.
Correct timing of leucovorin dosing relative to glucarpidase is essential and is based on pharmacologic principles (Fig. 3) [1]. Glucarpidase is confined to the plasma; therefore, interstitial and intracellular leucovorin concentrations are not directly affected by glucarpidase. Glucarpidase is given as a single i.v. infusion over 5 minutes and has a half-life of 5.6 hours. Leucovorin should be given 2–3 hours after glucarpidase, then every 3–6 hours at doses guided by the methotrexate concentration (pharmacokinetically guided dosing; Fig. 3) [1]. Reduction of the plasma methotrexate concentration reduces the competition between methotrexate and leucovorin for intracellular access. Therefore, the combination of a lower plasma methotrexate level and the transient presence of glucarpidase probably enhances the intracellular transport of leucovorin [90]. Leucovorin is continued for 48 hours after glucarpidase at the dose appropriate for the preglucarpidase methotrexate concentration [1]. It is important to note that in the presence of very high methotrexate concentrations, no plasma leucovorin concentration may be sufficiently high to reverse intracellular toxicity because of competition with the shared active transport mechanism. Urgent hemodialysis plus glucarpidase and very-high-dose leucovorin are warranted to reduce mortality [91].
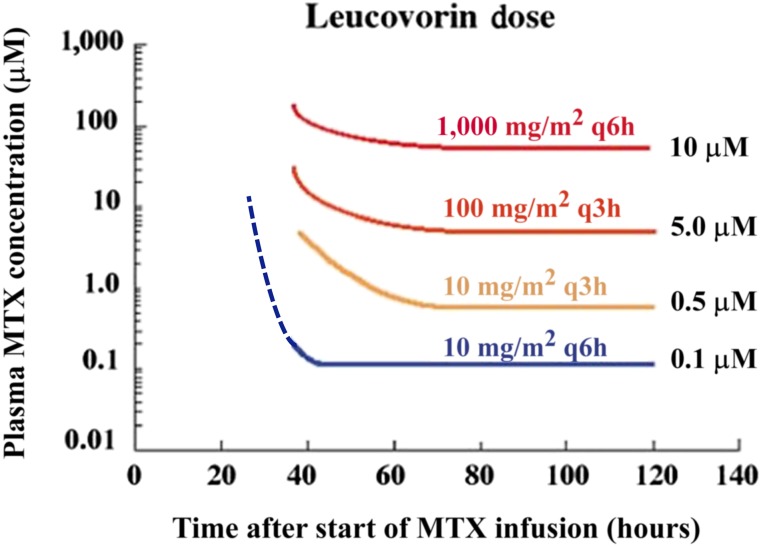
Figure 3.
Pharmacokinetically guided leucovorin rescue based on plasma MTX levels after high-dose MTX. Leucovorin dosing must be increased dramatically when plasma MTX levels are elevated above 5 µM at 42 hours after the start of the MTX infusion because leucovorin must compete with MTX to enter cells via the reduced folate carrier and the goal of leucovorin rescue is to achieve a high intracellular concentration of leucovorin. In color are the recommended doses of leucovorin based on the plasma MTX concentration at each time point after the start of the MTX infusion. For example, if at hour 60 the MTX concentration is 100 µM, it falls above the red line and the recommended leucovorin dose would be 1,000 mg/m2 every 6 hours. If at 100 hours the methotrexate concentration decreases to 3 µM (above the yellow line, below the orange line), then the recommended leucovorin dose would decrease to 10 mg/m2 every 3 hours. The dotted lines indicate extrapolated values based on modeling and clinical trial experience following the original publication [113].
Abbreviation: MTX, methotrexate.
Reduction of the plasma methotrexate concentration reduces the competition between methotrexate and leucovorin for intracellular access. Therefore, the combination of a lower plasma methotrexate level and the transient presence of glucarpidase probably enhances the intracellular transport of leucovorin.
Many curable cancers require multiple courses of HDMTX therapy. If doses are skipped or delayed, treatment outcomes may be adversely affected [1]. For patients with delayed methotrexate clearance, the early use of glucarpidase rescue can facilitate a return to acceptable renal function that allows safe administration of subsequent HDMTX courses. Christensen et al. [5] reviewed the clinical courses of 1,141 pediatric oncology patients who received a total of 4,909 courses of HDMTX (≥1 g/m2) at St. Jude Children’s Research Hospital from 1998 through 2010 and identified 20 (1.8% of patients, 0.4% of HDMTX courses) who developed AKI and delayed methotrexate excretion and required glucarpidase. All patients had a return to baseline creatinine values, none died of methotrexate toxicity, and 13 of 20 received a total of 39 subsequent courses of HDMTX, which was well-tolerated in all cases [5]. In a pooled analysis of efficacy data from four multicenter, single-arm compassionate-use clinical trials, Widemann et al. [43] showed that glucarpidase resulted in a 99% or greater sustained reduction of serum methotrexate concentrations in renally impaired patients. Of concern is the declining glomerular function later in life among childhood cancer survivors who received methotrexate and other nephrotoxic chemotherapeutics; thus, prevention of AKI is preferable to managing it [92].
Efficacy of Glucarpidase
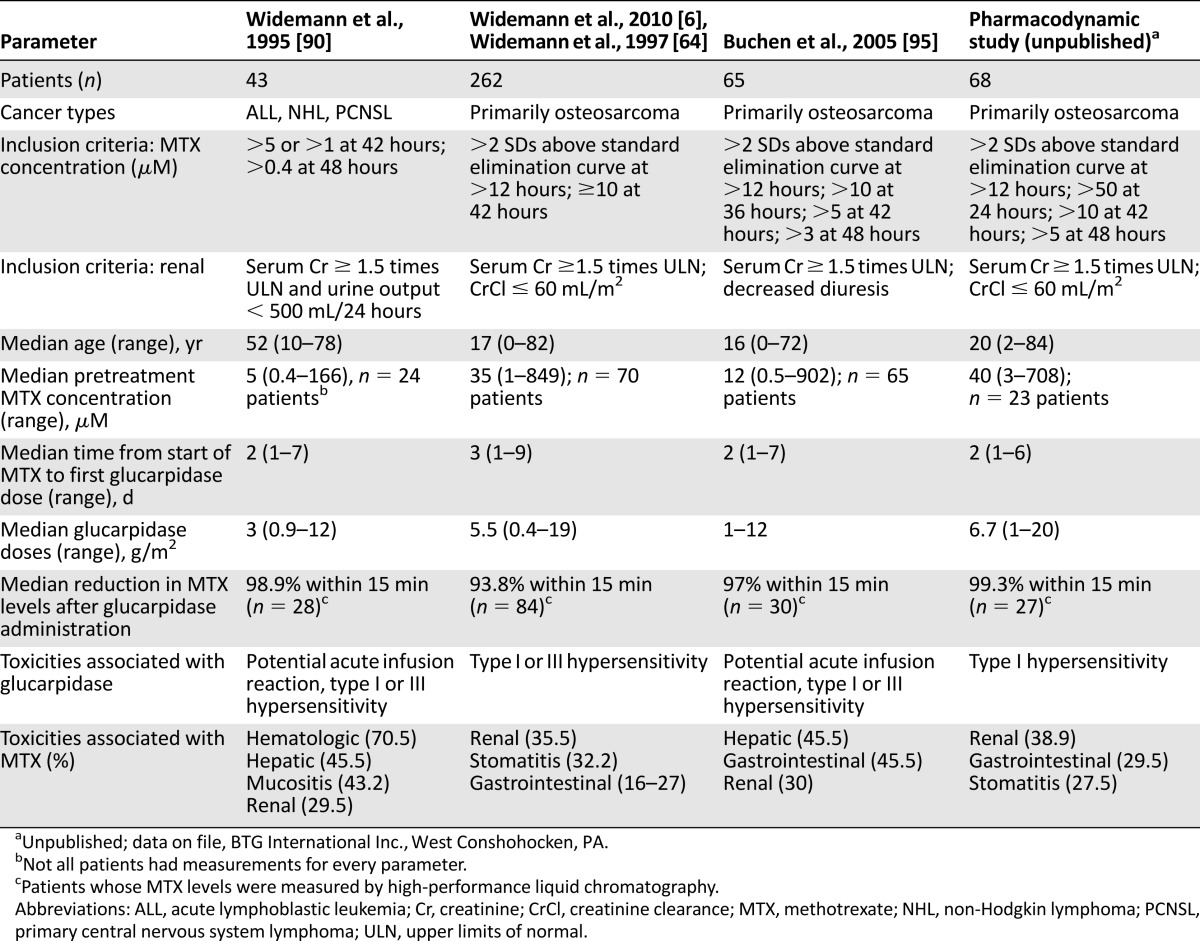
A series of compassionate-use studies that used glucarpidase in conjunction with standard management approaches for patients with signs of renal toxicity were published by Widemann and colleagues about their 15-year experience with glucarpidase for methotrexate toxicity in 492 cancer patients between November 1993 and June 2009 (Table 3) [6, 50, 64, 93–95].
Table 3.
Selected studies that used glucarpidase
Widemann and colleagues [6] reported additional experience with glucarpidase, leucovorin, and thymidine in 100 patients treated with 1–3 doses of glucarpidase and standard leucovorin rescue. An initial cohort of 35 patients received thymidine by continuous infusion. Thereafter, thymidine was restricted to patients with prolonged methotrexate exposure (>96 hours) or with substantial methotrexate toxicity. Plasma methotrexate concentrations decreased by 99% within 15 minutes after the first glucarpidase dose [6]. This analysis underscores the importance of early leucovorin dose adjustment and timely glucarpidase administration. Of 12 deaths, 6 were considered directly related to methotrexate because patients experienced grade 4 myelosuppression (n = 5), grade 3 or 4 mucositis (n = 4), sepsis (n = 5), and toxic epidermal necrolysis (n = 2). All 6 patients had received thymidine. Predictors of grade 4 and 5 toxicity included the presence of grade 4 toxicity before glucarpidase administration, inadequate initial increase in leucovorin dosing, and administration of glucarpidase more than 96 hours after the start of the methotrexate infusion. The other patient deaths were attributed primarily to rapid cancer progression. The major risk factors for severe toxicity are preexisting toxicity, inappropriate leucovorin increase, and delayed glucarpidase administration [6].
Conclusion
HDMTX can be safely administered to patients with normal renal function using hyperhydration, urine alkalization, and pharmacokinetically guided leucovorin rescue. Successful management of methotrexate toxicity requires timely recognition of delayed methotrexate elimination and renal dysfunction. In particular, rising serum creatinine concentration or decreased urine output after HDMTX indicates a medical emergency. Increased hydration, high-dose leucovorin, and glucarpidase (when necessary) effectively reduce serum methotrexate concentrations and protect cells from methotrexate, but these measures must be administered as early as possible to prevent further toxicity, facilitate renal recovery, and allow patients to resume HDMTX therapy after normalization of renal function [96, 97].
Acknowledgments
We thank Thomas King, M.P.H. (BTG International Inc.) for preparation of key figures and editorial assistance. Funding was provided in part by the National Institutes of Health Cancer Center Support Core Grant (CA-21765) and the American Lebanese Syrian Associated Charities. Dr. Pui is an American Cancer Society professor.
Author Contributions
Conception/Design: Scott C. Howard, John McCormick, Randall K. Buddington, R. Donald Harvey
Provision of study material or patients: Scott C. Howard, Ching-Hon Pui, R. Donald Harvey
Collection and/or assembly of data: Scott C. Howard, Ching-Hon Pui, Randall K. Buddington, R. Donald Harvey
Data analysis and interpretation: Scott C. Howard, John McCormick, Ching-Hon Pui, Randall K. Buddington, R. Donald Harvey
Manuscript writing: Scott C. Howard, John McCormick, Ching-Hon Pui, R. Donald Harvey
Final approval of manuscript: Scott C. Howard, John McCormick, Ching-Hon Pui, Randall K. Buddington, R. Donald Harvey
Disclosures
Scott C. Howard: Sanofi, Sigma Tau (C/A), Jazz Pharmaceuticals (RF), Sanofi, Sigma Tau, Jazz Pharmaceuticals (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. The Oncologist. 2006;11:694–703. doi: 10.1634/theoncologist.11-6-694. [DOI] [PubMed] [Google Scholar]
- 2.Stoller RG, Hande KR, Jacobs SA, et al. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297:630–634. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell MS, Wawro NW, DeConti RC, et al. Effectiveness of high-dose infusions of methotrexate followed by leucovorin in carcinoma of the head and neck. Cancer Res. 1968;28:1088–1094. [PubMed] [Google Scholar]
- 4.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 5.Christensen AM, Pauley JL, Molinelli AR, et al. Resumption of high-dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients. Cancer. 2012;118:4321–4330. doi: 10.1002/cncr.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widemann BC, Balis FM, Kim A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28:3979–3986. doi: 10.1200/JCO.2009.25.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perazella MA, Moeckel GW. Nephrotoxicity from chemotherapeutic agents: Clinical manifestations, pathobiology, and prevention/therapy. Semin Nephrol. 2010;30:570–581. doi: 10.1016/j.semnephrol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 8.May J, Carson KR, Butler S, et al. High incidence of methotrexate associated renal toxicity in patients with lymphoma: A retrospective analysis. Leuk Lymphoma. 2014;55:1345–1349. doi: 10.3109/10428194.2013.840780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Miguel D, García-Suárez J, Martín Y, et al. Severe acute renal failure following high-dose methotrexate therapy in adults with haematological malignancies: A significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23:3762–3766. doi: 10.1093/ndt/gfn503. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, Doki K, Homma M, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009;67:44–49. doi: 10.1111/j.1365-2125.2008.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dao K, Ivanyuk A, Buclin T, et al. Pharmacokinetic interaction between methotrexate and chloral hydrate. Pediatr Blood Cancer. 2013;60:518–520. doi: 10.1002/pbc.24393. [DOI] [PubMed] [Google Scholar]
- 12.Bain E, Birhiray RE, Reeves DJ. Drug-drug interaction between methotrexate and levetiracetam resulting in delayed methotrexate elimination. Ann Pharmacother. 2014;48:292–296. doi: 10.1177/1060028013511951. [DOI] [PubMed] [Google Scholar]
- 13.Parentelli AS, Phulpin-Weibel A, Mansuy L, et al. Drug-drug interaction between methotrexate and levetiracetam in a child treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2013;60:340–341. doi: 10.1002/pbc.24371. [DOI] [PubMed] [Google Scholar]
- 14.McBride A, Antonia SJ, Haura EB, et al. Suspected methotrexate toxicity from omeprazole: a case review of carboxypeptidase G2 use in a methotrexate-experienced patient with methotrexate toxicity and a review of the literature. J Pharm Pract. 2012;25:477–485. doi: 10.1177/0897190012442717. [DOI] [PubMed] [Google Scholar]
- 15.Bezabeh S, Mackey AC, Kluetz P, et al. Accumulating evidence for a drug-drug interaction between methotrexate and proton pump inhibitors. The Oncologist. 2012;17:550–554. doi: 10.1634/theoncologist.2011-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyers PA, Flombaum C. High-dose methotrexate-induced renal dysfunction: is glucarpidase necessary for rescue? J Clin Oncol. 2011;29:e180–e180; author reply e181. doi: 10.1200/JCO.2010.32.8245. [DOI] [PubMed] [Google Scholar]
- 17.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackland SP, Schilsky RL. High-dose methotrexate: A critical reappraisal. J Clin Oncol. 1987;5:2017–2031. doi: 10.1200/JCO.1987.5.12.2017. [DOI] [PubMed] [Google Scholar]
- 19.Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949–959. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor SR, Banker A, Schlaen A, et al. Intraocular methotrexate can induce extended remission in some patients in noninfectious uveitis. Retina. 2013;33:2149–2154. doi: 10.1097/IAE.0b013e31828ac07d. [DOI] [PubMed] [Google Scholar]
- 21.D’Elia T. Methotrexate-induced pneumonitis: Heterogeneity of bronchoalveolar lavage and differences between cancer and rheumatoid arthritis. Inflamm Allergy Drug Targets. 2014;13:25–33. doi: 10.2174/1871528112666131230013059. [DOI] [PubMed] [Google Scholar]
- 22.Jakubovic BD, Donovan A, Webster PM, et al. Methotrexate-induced pulmonary toxicity. Can Respir J. 2013;20:153–155. doi: 10.1155/2013/527912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathi N, Chikura B, Kaushik VV, et al. How common is methotrexate pneumonitis? A large prospective study investigates. Clin Rheumatol. 2012;31:79–83. doi: 10.1007/s10067-011-1758-6. [DOI] [PubMed] [Google Scholar]
- 24.Elmorsi YM, El-Haggar SM, Ibrahim OM, et al. Effect of ketoprofen and indomethacin on methotrexate pharmacokinetics in mice plasma and tumor tissues. Eur J Drug Metab Pharmacokinet. 2013;38:27–32. doi: 10.1007/s13318-012-0113-x. [DOI] [PubMed] [Google Scholar]
- 25.Hardwick RN, Clarke JD, Lake AD, et al. Increased susceptibility to methotrexate-induced toxicity in nonalcoholic steatohepatitis. Toxicol Sci. 2014;142:45–55. doi: 10.1093/toxsci/kfu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekstrøm PO, Anderson A, Warren DJ, et al. Pharmacokinetics of different doses of methotrexate at steady state by in situ microdialysis in a rat model. Cancer Chemother Pharmacol. 1995;36:283–289. doi: 10.1007/BF00689044. [DOI] [PubMed] [Google Scholar]
- 27.Fuskevåg O-M, Kristiansen C, Lindal S, et al. Leucovorin and maximum tolerated dose toxicity of methotrexate in rats. Pediatr Hematol Oncol. 2000;17:651–658. doi: 10.1080/08880010050211358. [DOI] [PubMed] [Google Scholar]
- 28.Asvadi I, Hajipour B, Asvadi A, et al. Protective effect of pentoxyfilline in renal toxicity after methotrexate administration. Eur Rev Med Pharmacol Sci. 2011;15:1003–1009. [PubMed] [Google Scholar]
- 29.Chen C, Tian L, Zhang M, et al. Protective effect of amifostine on high-dose methotrexate-induced small intestinal mucositis in mice. Dig Dis Sci. 2013;58:3134–3143. doi: 10.1007/s10620-013-2826-3. [DOI] [PubMed] [Google Scholar]
- 30.Abraham P, Kolli VK, Rabi S. Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem Funct. 2010;28:426–433. doi: 10.1002/cbf.1676. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim MA, El-Sheikh AA, Khalaf HM, et al. Protective effect of peroxisome proliferator activator receptor (PPAR)-α and -γ ligands against methotrexate-induced nephrotoxicity. Immunopharmacol Immunotoxicol. 2014;36:130–137. doi: 10.3109/08923973.2014.884135. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs SA, Stoller RG, Chabner BA, et al. 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. J Clin Invest. 1976;57:534–538. doi: 10.1172/JCI108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chabner BA, Johns DG, Bertino JR. Enzymatic cleavage of methotrexate provides a method for prevention of drug toxicity. Nature. 1972;239:395–397. doi: 10.1038/239395b0. [DOI] [PubMed] [Google Scholar]
- 34.Garnick M, Mayer R, Abelson H. Acute renal failure associated with cancer treatment. In: Brenner BM, Lazarus JM, eds. Acute Renal Failure. New York: Churchill Livingstone, 1988:621–657 [Google Scholar]
- 35.Pitman S, Parker L, Tattersall M, et al. Clinical trial of high-dose methotrexate (nsc-740) with citrovorum factor (nsc-3590)-toxicologic and therapeutic observations. Cancer Chemother Rep. 1975;6:43–49. [Google Scholar]
- 36.Pitman SW, Frei E., 3rd Weekly methotrexate-calcium leucovorin rescue: effect of alkalinization on nephrotoxicity; pharmacokinetics in the CNS; and use in CNS non-Hodgkin’s lymphoma. Cancer Treat Rep. 1977;61:695–701. [PubMed] [Google Scholar]
- 37.Lau KK, Weiss AR, Jones DP. Polyuria associated with high-dose methotrexate in two patients with acute lymphoblastic leukaemia. J Oncol Pharm Pract. 2005;11:31–33. doi: 10.1191/1078155205jp148oa. [DOI] [PubMed] [Google Scholar]
- 38.Sahni V, Choudhury D, Ahmed Z. Chemotherapy-associated renal dysfunction. Nat Rev Nephrol. 2009;5:450–462. doi: 10.1038/nrneph.2009.97. [DOI] [PubMed] [Google Scholar]
- 39.Xu W, Zhang L-y, Chen X-y, et al. Serum creatinine and creatinine clearance for predicting plasma methotrexate concentrations after high-dose methotrexate chemotherapy for the treatment for childhood lymphoblastic malignancies. Cancer Chemother Pharmacol. 2014;73:79–86. doi: 10.1007/s00280-013-2319-2. [DOI] [PubMed] [Google Scholar]
- 40.Kintzel PE, Dorr RT. Anticancer drug renal toxicity and elimination: Dosing guidelines for altered renal function. Cancer Treat Rev. 1995;21:33–64. doi: 10.1016/0305-7372(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 41.Aronoff GR, Berns J, Brier M, et al. Drug prescribing in renal failure:Dosing guidelines for adults. 1999448. [DOI] [PubMed] [Google Scholar]
- 42.Chiusolo P, Giammarco S, Bellesi S, et al. The role of MTHFR and RFC1 polymorphisms on toxicity and outcome of adult patients with hematological malignancies treated with high-dose methotrexate followed by leucovorin rescue. Cancer Chemother Pharmacol. 2012;69:691–696. doi: 10.1007/s00280-011-1751-4. [DOI] [PubMed] [Google Scholar]
- 43.Treviño LR, Shimasaki N, Yang W, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. J Clin Oncol. 2009;27:5972–5978. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramsey LB, Bruun GH, Yang W, et al. Rare versus common variants in pharmacogenetics: SLCO1B1 variation and methotrexate disposition. Genome Res. 2012;22:1–8. doi: 10.1101/gr.129668.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsey LB, Panetta JC, Smith C, et al. Genome-wide study of methotrexate clearance replicates SLCO1B1. Blood. 2013;121:898–904. doi: 10.1182/blood-2012-08-452839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright KD, Panetta JC, Onar-Thomas A, et al. Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections. Cancer Chemother Pharmacol. 2015;75:27–35. doi: 10.1007/s00280-014-2614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gregory RE, Pui CH, Crom WR. Raised plasma methotrexate concentrations following intrathecal administration in children with renal dysfunction. Leukemia. 1991;5:999–1003. [PubMed] [Google Scholar]
- 48.Kelly H, Harvey D, Moll S. A cautionary tale: fatal outcome of methotrexate therapy given for management of ectopic pregnancy. Obstet Gynecol. 2006;107:439–441. doi: 10.1097/01.AOG.0000172374.72125.3e. [DOI] [PubMed] [Google Scholar]
- 49.Chen S. Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J Am Soc Nephrol. 2013;24:877–888. doi: 10.1681/ASN.2012070653. [DOI] [PubMed] [Google Scholar]
- 50.Widemann BC, Schwartz S, Jayaprakash N, et al. Efficacy of glucarpidase (carboxypeptidase g2) in patients with acute kidney injury after high‐dose methotrexate therapy. Pharmacotherapy. 2014;34:427–439. doi: 10.1002/phar.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12:1667–1672. doi: 10.1200/JCO.1994.12.8.1667. [DOI] [PubMed] [Google Scholar]
- 52.Shamash J, Earl H, Souhami R. Acetazolamide for alkalinisation of urine in patients receiving high-dose methotrexate. Cancer Chemother Pharmacol. 1991;28:150–151. doi: 10.1007/BF00689708. [DOI] [PubMed] [Google Scholar]
- 53.Evans WE, Pratt CB, Taylor RH, et al. Pharmacokinetic monitoring of high-dose methotrexate. Early recognition of high-risk patients. Cancer Chemother Pharmacol. 1979;3:161–166. doi: 10.1007/BF00262416. [DOI] [PubMed] [Google Scholar]
- 54.Al-Turkmani MR, Law T, Narla A, et al. Difficulty measuring methotrexate in a patient with high-dose methotrexate-induced nephrotoxicity. Clin Chem. 2010;56:1792–1794. doi: 10.1373/clinchem.2010.144824. [DOI] [PubMed] [Google Scholar]
- 55.Ylinen E, Jahnukainen K, Saarinen-Pihkala UM, et al. Assessment of renal function during high-dose methotrexate treatment in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2014;61:2199–2202. doi: 10.1002/pbc.25137. [DOI] [PubMed] [Google Scholar]
- 56.Aumente D, Buelga DS, Lukas JC, et al. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45:1227–1238. doi: 10.2165/00003088-200645120-00007. [DOI] [PubMed] [Google Scholar]
- 57.Plard C, Bressolle F, Fakhoury M, et al. A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;60:609–620. doi: 10.1007/s00280-006-0394-3. [DOI] [PubMed] [Google Scholar]
- 58.Pui C-H, Sandlund JT, Pei D, et al. Improved outcome for children with acute lymphoblastic leukemia: Results of Total Therapy Study XIIIB at St Jude Children’s Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 59.Tsurusawa M, Gosho M, Mori T, et al. Statistical analysis of relation between plasma methotrexate concentration and toxicity in high-dose methotrexate therapy of childhood nonHodgkin lymphoma. Pediatr Blood Cancer. 2014 doi: 10.1002/pbc.25305. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Howard SC, Pedrosa M, Lins M, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource-poor area. JAMA. 2004;291:2471–2475. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- 61.Maia MB, Saivin S, Chatelut E, et al. In vitro and in vivo protein binding of methotrexate assessed by microdialysis. Int J Clin Pharmacol Ther. 1996;34:335–341. [PubMed] [Google Scholar]
- 62.Saland JM, Leavey PJ, Bash RO, et al. Effective removal of methotrexate by high-flux hemodialysis. Pediatr Nephrol. 2002;17:825–829. doi: 10.1007/s00467-002-0946-7. [DOI] [PubMed] [Google Scholar]
- 63.Wall SM, Johansen MJ, Molony DA, et al. Effective clearance of methotrexate using high-flux hemodialysis membranes. Am J Kidney Dis. 1996;28:846–854. doi: 10.1016/s0272-6386(96)90384-4. [DOI] [PubMed] [Google Scholar]
- 64.Widemann BC, Balis FM, Murphy RF, et al. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997;15:2125–2134. doi: 10.1200/JCO.1997.15.5.2125. [DOI] [PubMed] [Google Scholar]
- 65.Relling MV, Stapleton FB, Ochs J, et al. Removal of methotrexate, leucovorin, and their metabolites by combined hemodialysis and hemoperfusion. Cancer. 1988;62:884–888. doi: 10.1002/1097-0142(19880901)62:5<884::aid-cncr2820620506>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 66.Lindsay K, Fraser AD, Layton A, et al. Liver fibrosis in patients with psoriasis and psoriatic arthritis on long-term, high cumulative dose methotrexate therapy. Rheumatology (Oxford) 2009;48:569–572. doi: 10.1093/rheumatology/kep023. [DOI] [PubMed] [Google Scholar]
- 67.Weber BL, Tanyer G, Poplack DG, et al. Transient acute hepatotoxicity of high-dose methotrexate therapy during childhood NCI Monogr 1987207–212. [PubMed]
- 68.Locasciulli A, Mura R, Fraschini D, et al. High-dose methotrexate administration and acute liver damage in children treated for acute lymphoblastic leukemia. A prospective study. Haematologica. 1992;77:49–53. [PubMed] [Google Scholar]
- 69.Nyfors A. Liver biopsies from psoriatics related to methotrexate therapy. 3. Findings in post-methotrexate liver biopsies from 160 psoriatics. Acta Pathol Microbiol Scand [A] 1977;85:511–518. doi: 10.1111/j.1699-0463.1977.tb03882.x. [DOI] [PubMed] [Google Scholar]
- 70.Watson WA, Litovitz TL, Rodgers GC, Jr, et al. 2004 annual report of the American Association of Poison Control Centers toxic exposure surveillance system. Am J Emerg Med. 2005;23:589–666. doi: 10.1016/j.ajem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Kremer JM, Alarcón GS, Lightfoot RW, Jr, et al. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. Arthritis Rheum. 1994;37:316–328. doi: 10.1002/art.1780370304. [DOI] [PubMed] [Google Scholar]
- 72.Mahoney DH, Jr, Shuster JJ, Nitschke R, et al. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: An association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol. 1998;16:1712–1722. doi: 10.1200/JCO.1998.16.5.1712. [DOI] [PubMed] [Google Scholar]
- 73.Rubnitz JE, Relling MV, Harrison PL, et al. Transient encephalopathy following high-dose methotrexate treatment in childhood acute lymphoblastic leukemia. Leukemia. 1998;12:1176–1181. doi: 10.1038/sj.leu.2401098. [DOI] [PubMed] [Google Scholar]
- 74.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J Clin Invest. 1993;92:2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernini JC, Fort DW, Griener JC, et al. Aminophylline for methotrexate-induced neurotoxicity. Lancet. 1995;345:544–547. doi: 10.1016/s0140-6736(95)90464-6. [DOI] [PubMed] [Google Scholar]
- 76.Sonis ST. Mucositis as a biological process: A new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39–43. doi: 10.1016/s1368-8375(97)00053-5. [DOI] [PubMed] [Google Scholar]
- 77.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 78.Sonis ST. A biological approach to mucositis. J Support Oncol. 2004;2:21–32; discussion 35–36. [PubMed] [Google Scholar]
- 79.Dumontet C, Sonnet A, Bastion Y, et al. Prevention of high dose L-PAM-induced mucositis by cryotherapy. Bone Marrow Transplant. 1994;14:492–494. [PubMed] [Google Scholar]
- 80.Edelman MJ, Gandara DR, Perez EA, et al. Phase I trial of edatrexate plus carboplatin in advanced solid tumors: Amelioration of dose-limiting mucositis by ice chip cryotherapy. Invest New Drugs. 1998;16:69–75. doi: 10.1023/a:1006026928733. [DOI] [PubMed] [Google Scholar]
- 81.Matejka M, Nell A, Kment G, et al. Local benefit of prostaglandin E2 in radiochemotherapy-induced oral mucositis. Br J Oral Maxillofac Surg. 1990;28:89–91. doi: 10.1016/0266-4356(90)90128-8. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt E, Thoennissen NH, Rudat A, et al. Use of palifermin for the prevention of high-dose methotrexate-induced oral mucositis. Ann Oncol. 2008;19:1644–1649. doi: 10.1093/annonc/mdn179. [DOI] [PubMed] [Google Scholar]
- 83.Maiguma T, Kaji H, Makino K, et al. Protective effects of amifostine and cyclooxygenase-1 inhibitor against normal human epidermal keratinocyte toxicity induced by methotrexate and 5-fluorouracil. Basic Clin Pharmacol Toxicol. 2009;105:1–9. doi: 10.1111/j.1742-7843.2009.00400.x. [DOI] [PubMed] [Google Scholar]
- 84.Worthington HV, Clarkson JE, Bryan G, et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2011;4:CD000978. doi: 10.1002/14651858.CD000978.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarkson JE, Worthington HV, Furness S, et al. Interventions for treating oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2010;8:CD001973. doi: 10.1002/14651858.CD001973.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sukhotnik I, Pollak Y, Coran AG, et al. Glutamine attenuates the inhibitory effect of methotrexate on TLR signaling during intestinal chemotherapy-induced mucositis in a rat. Nutr Metab (Lond) 2014;11:17. doi: 10.1186/1743-7075-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamada K, Kakigawa N, Sekine S, et al. Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother Pharmacol. 2013;72:757–765. doi: 10.1007/s00280-013-2238-2. [DOI] [PubMed] [Google Scholar]
- 88.Fijlstra M, Tissing WJ, Stellaard F, et al. Reduced absorption of long-chain fatty acids during methotrexate-induced gastrointestinal mucositis in the rat. Clin Nutr. 2013;32:452–459. doi: 10.1016/j.clnu.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Goh TS, Wong KY, Lampkin B, et al. Evaluation of 24-hour infusion of high-dose methotrexate--pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 1979;3:177–180. doi: 10.1007/BF00262419. [DOI] [PubMed] [Google Scholar]
- 90.Widemann BC, Hetherington ML, Murphy RF, et al. Carboxypeptidase-G2 rescue in a patient with high dose methotrexate-induced nephrotoxicity. Cancer. 1995;76:521–526. doi: 10.1002/1097-0142(19950801)76:3<521::aid-cncr2820760325>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 91.Pinedo HM, Zaharko DS, Bull JM, et al. The reversal of methotrexate cytotoxicity to mouse bone marrow cells by leucovorin and nucleosides. Cancer Res. 1976;36:4418–4424. [PubMed] [Google Scholar]
- 92.Mulder RL, Knijnenburg SL, Geskus RB, et al. Glomerular function time trends in long-term survivors of childhood cancer: A longitudinal study. Cancer Epidemiol Biomarkers Prev. 2013;22:1736–1746. doi: 10.1158/1055-9965.EPI-13-0036. [DOI] [PubMed] [Google Scholar]
- 93.Schwartz S, Borner K, Müller K, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. The Oncologist. 2007;12:1299–1308. doi: 10.1634/theoncologist.12-11-1299. [DOI] [PubMed] [Google Scholar]
- 94.Widemann BC, Jayaprakash N, Howard SC, et al. Clinical trial and compassionate use experience with glucarpidase for methotrexate toxicity. J Clin Oncol. 2012;30(suppl):6530. [Google Scholar]
- 95.Buchen S, Ngampolo D, Melton RG, et al. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Br J Cancer. 2005;92:480–487. doi: 10.1038/sj.bjc.6602337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin J, Howard SC, Pillai A, et al. The weaned pig as a model for Doxorubicin-induced mucositis. Chemotherapy. 2014;60:24–36. doi: 10.1159/000365725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Li G. Mechanisms of methotrexate resistance in osteosarcoma cell lines and strategies for overcoming this resistance. Oncol Lett. 2015;9:940–944. doi: 10.3892/ol.2014.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeuchi J, Kyo T, Naito K, et al. Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: The JALSG-ALL93 study. Leukemia. 2002;16:1259–1266. doi: 10.1038/sj.leu.2402526. [DOI] [PubMed] [Google Scholar]
- 99.Linker C, Damon L, Ries C, et al. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:2464–2471. doi: 10.1200/JCO.2002.07.116. [DOI] [PubMed] [Google Scholar]
- 100.Hill FG, Richards S, Gibson B, et al. Successful treatment without cranial radiotherapy of children receiving intensified chemotherapy for acute lymphoblastic leukaemia: Results of the risk-stratified randomized central nervous system treatment trial MRC UKALL XI (ISRC TN 16757172) Br J Haematol. 2004;124:33–46. doi: 10.1046/j.1365-2141.2003.04738.x. [DOI] [PubMed] [Google Scholar]
- 101.Zhang HN, He XL, Wang C, et al. Impact of SLCO1B1 521T>C variant on leucovorin rescue and risk of relapse in childhood acute lymphoblastic leukemia treated with high-dose methotrexate. Pediatr Blood Cancer. 2014;61:2203–2207. doi: 10.1002/pbc.25191. [DOI] [PubMed] [Google Scholar]
- 102.Souhami RL, Craft AW, Van der Eijken JW, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: A study of the European Osteosarcoma Intergroup. Lancet. 1997;350:911–917. doi: 10.1016/S0140-6736(97)02307-6. [DOI] [PubMed] [Google Scholar]
- 103.Fuchs N, Bielack SS, Epler D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group’s protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–899. doi: 10.1023/a:1008391103132. [DOI] [PubMed] [Google Scholar]
- 104.Bacci G, Briccoli A, Ferrari S, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: Long-term results of the Rizzoli’s 4th protocol. Eur J Cancer. 2001;37:2030–2039. doi: 10.1016/s0959-8049(01)00229-5. [DOI] [PubMed] [Google Scholar]
- 105.Goorin AM, Schwartzentruber DJ, Devidas M, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21:1574–1580. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 106.Ferrari S, Smeland S, Mercuri M, et al. Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: A joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol. 2005;23:8845–8852. doi: 10.1200/JCO.2004.00.5785. [DOI] [PubMed] [Google Scholar]
- 107.Koller CA, Kantarjian HM, Thomas D, et al. The hyper-CVAD regimen improves outcome in relapsed acute lymphoblastic leukemia. Leukemia. 1997;11:2039–2044. doi: 10.1038/sj.leu.2400861. [DOI] [PubMed] [Google Scholar]
- 108.Khouri IF, Romaguera J, Kantarjian H, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: An active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16:3803–3809. doi: 10.1200/JCO.1998.16.12.3803. [DOI] [PubMed] [Google Scholar]
- 109.Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood. 2004;104:1624–1630. doi: 10.1182/blood-2003-12-4428. [DOI] [PubMed] [Google Scholar]
- 110.Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96-07. J Clin Oncol. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 111.Dalia S, Price S, Forsyth P, et al. What is the optimal dose of high-dose methotrexate in the initial treatment of primary central nervous system lymphoma? Leuk Lymphoma. 2015;56:500–502. doi: 10.3109/10428194.2014.927458. [DOI] [PubMed] [Google Scholar]
- 112.Abelson HT, Fosburg MT, Beardsley GP, et al. Methotrexate-induced renal impairment: Clinical studies and rescue from systemic toxicity with high-dose leucovorin and thymidine. J Clin Oncol. 1983;1:208–216. doi: 10.1200/JCO.1983.1.3.208. [DOI] [PubMed] [Google Scholar]
- 113.Bleyer WA. Methotrexate: Clinical pharmacology, current status and therapeutic guidelines. Cancer Treat Rev. 1977;4:87–101. doi: 10.1016/s0305-7372(77)80007-8. [DOI] [PubMed] [Google Scholar]