Markers of bone metabolism are prognostic in breast cancer (BC) patients with bone metastases (BMs). The survival effect of the N-telopeptide of type I collagen (NTX) response to zoledronic acid (ZA) and the influence of extraskeletal metastatic disease on NTX variation were evaluated. An early response to ZA was strongly associated with long-term NTX control. Patients with BMs plus extraskeletal metastases had erratic NTX variations.
Keywords: Breast cancer, Bone metastases, N-telopeptide of type I collagen, Zoledronic acid
Abstract
Background.
Markers of bone metabolism, such as N-telopeptide of type I collagen (NTX), have been demonstrated to be prognostic in previous trials of breast cancer (BC) patients with bone metastases (BMs). In the present study, we tested the survival effect of the NTX response to zoledronic acid (ZA) at 3 and 12 months in a contemporaneous cohort of BC patients with BMs and evaluated the influence of extraskeletal metastatic disease on NTX variation.
Patients and Methods.
The present study was a prospective cohort study of consecutive BC patients diagnosed and treated at a single center. Patients presenting with de novo radiological evidence of BMs who started monthly intravenous ZA were included. Urinary NTX was measured at baseline and 1, 3, 6, 9, and 12 months after ZA introduction.
Results.
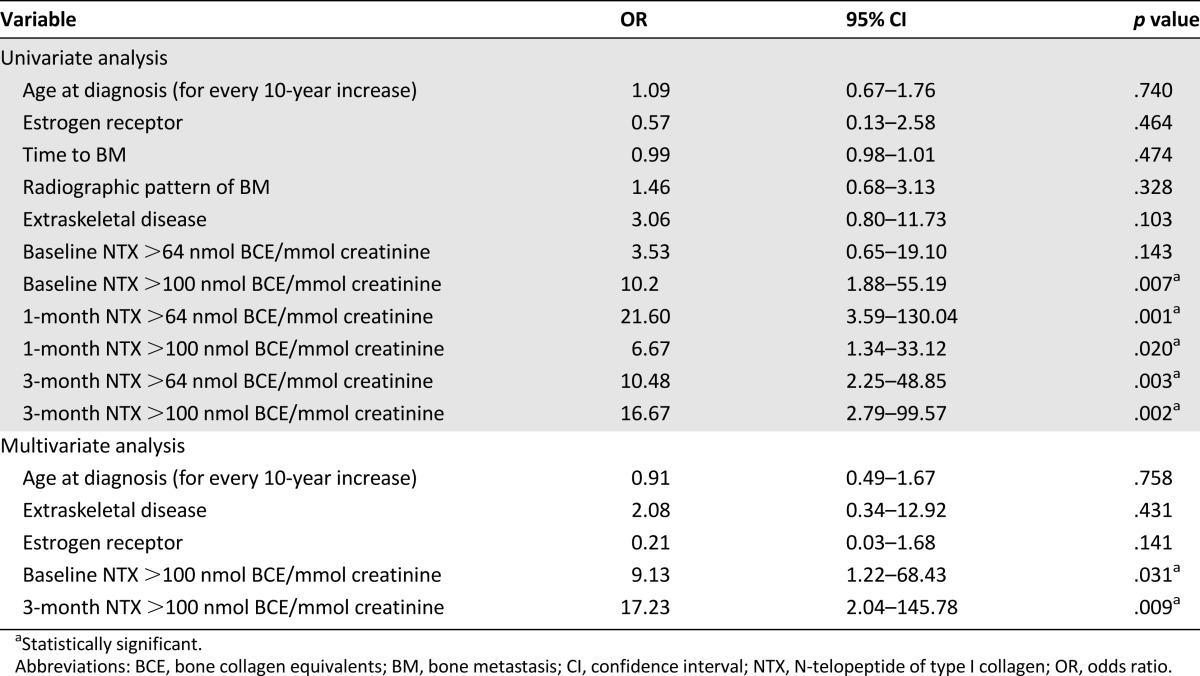
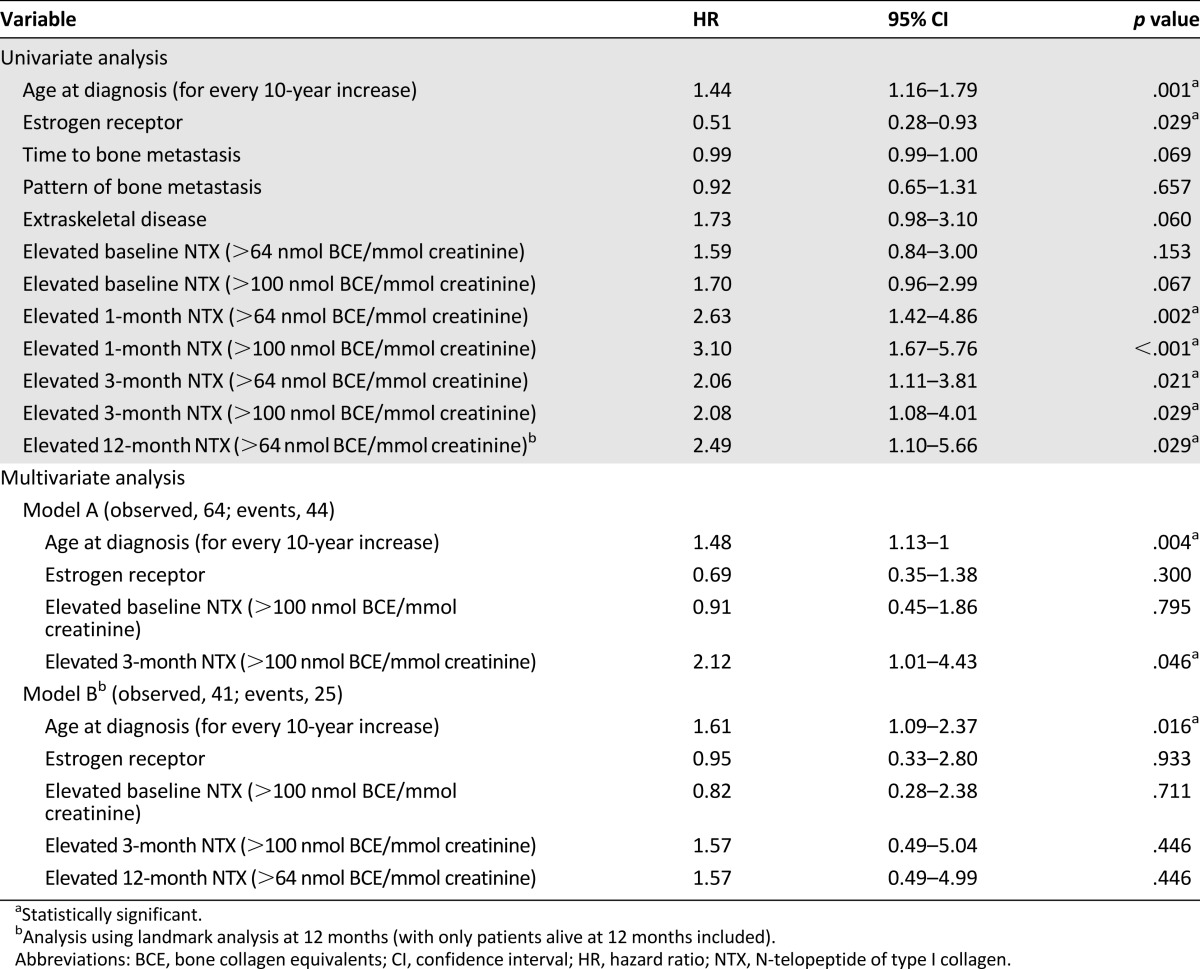
Overall, 71 patients were enrolled, 32 with BMs and 39 with BMs plus extraskeletal metastases. The proportion of patients with elevated NTX at baseline and 3 and 12 months was 49.3%, 26.6%, and 34.2%, respectively. The variables associated with survival included age at diagnosis, tumor estrogen receptor status, and NTX at 3 and 12 months. Multivariate analysis showed that, in addition to age at diagnosis, only the 3-month NTX level was significantly associated with survival. Patients with BMs plus extraskeletal metastases had an erratic NTX variation pattern, unrelated to survival.
Conclusion.
In the present contemporaneous cohort of BC patients with BMs, the NTX response at 3 months was strongly associated with survival. Furthermore, an early response to ZA was strongly associated with long-term NTX control. Finally, patients with BMs plus extraskeletal metastases had an erratic NTX variation.
Implications for Practice:
The present study showed that when accommodating recent therapy innovations and longer patient survival, the N-telopeptide (NTX) variation at 3 months is strongly associated with survival. In this setting, in addition to a few other clinicopathological features, NTX is a powerful prognostic marker. Moreover, early NTX correction associates with persistently normal NTX. This might identify a subgroup of patients with a good prognosis who are eligible for premature zoledronic acid (ZA) de-escalation. Finally, patients with bone plus extraskeletal metastases showed an erratic variation of NTX, raising concerns that a single ZA regimen might not fit all patients. Future trials should test its effect according to the presence of extraskeletal involvement.
Introduction
Bone is the most common site of metastatic disease in breast cancer (BC), affecting up to 80% of patients with metastatic disease [1]. Bone metastases (BMs) significantly affect patients’ quality of life and survival [2]. In addition to local symptoms, the morbidity of BMs mostly derives from the development of skeletal-related events (SREs), a composite of several clinical events, such as pathologic bone fractures, the need for radiotherapy or orthopedic surgery for symptomatic control, hypercalcemia of malignancy, and spinal cord compression [3]. Pathologic fractures are further associated with decreased survival [4].
When cancer cells invade bone, the balanced and coupled activity of the dyad osteoclast (bone-degrading cells) to osteoblast (bone-forming cells) is disrupted [5]. Cancer cells growing in bone activate bone metabolism, leading to the release of growth factors entrapped in the bone matrix, which ultimately enhance tumor growth. Tumor growth further activates bone metabolism, a step that closes the positive reinforcing loop of cancer-mediated activation of bone metabolism referred to as the vicious cycle of BMs [5, 6]. In BC, the activation of osteoclasts by cancer cells often leads to the development of lytic lesions. Because osteoclast activation is a central piece in the vicious cycle of bone metastases, current treatments include antiosteoclastic agents, such as bisphosphonates or denosumab [7]. These bone-targeted agents (BTAs) are given in association with anticancer therapy.
The bone metabolism releases a set of biochemical products amenable to quantification in the serum and urine. Although bone resorption releases byproducts of collagen breakdown, such as N-telopeptide of type I collagen (NTX) and C-telopeptide of type I collagen, bone formation releases bone-specific alkaline phosphatase, a biomarker of osteoblast activity. The use of these markers provides a noninvasive method to broadly assess ongoing bone turnover and hence indirectly inform about the action of tumor cells in the bone [8]. NTX, one of the most studied markers of bone metabolism, is very specific for the monitoring of bone metastases [9, 10]. It outperforms conventional tumor markers in predicting the progression of metastatic disease in the bone [10]. Moreover, NTX decreases with the use of BTAs [11, 12]. Both baseline and serial NTX levels after BTA therapy are strongly prognostic [5]. In a large study, higher baseline and on-study NTX levels doubled the risk of a SRE or bone disease progression and increased, by a factor of four to six times, the risk of death [13]. In contrast, early NTX normalization (at 3 months) after treatment with zoledronic acid (ZA) was associated with an approximately twofold lower risk of experiencing an SRE or death compared with patients with persistently elevated NTX levels despite treatment with ZA [14].
During the past 15 years, several therapeutic innovations in the BC arena were brought into clinical practice, changing the standard management of metastatic BC and extending survival. The prognostic value of NTX in this setting is not known. Moreover, the correlation of early and late variations of NTX and their clinical utility are incompletely understood. Furthermore, it is unclear whether the presence of extraskeletal disease affects NTX behavior. In the present study, we assessed the prognostic role of early (at 3 months) and later (at 12 months) NTX levels after the introduction of ZA in a cohort of contemporaneous BC patients. We also investigated the effect of extraskeletal metastases on the NTX levels.
Patients and Methods
Study Population and Design
In the present prospective cohort study, we included consecutive BC patients diagnosed and treated in the medical oncology department from Santa Maria Hospital by a single provider. These patients had presented with de novo radiological evidence of bone metastases (not excluding patients with concomitant extraskeletal metastasis) and started monthly intravenous ZA from 2002 to 2009. We evaluated the presence of metastases at baseline and then prospectively every 3 to 4 months for 1 year. Urine samples for NTX evaluation were also collected at baseline and 1, 3, 6, 9, and 12 months after ZA introduction. Overall cancer treatment was provided as per the institutional guidelines and was in compliance with the international oncology society guidelines at the time of diagnosis. We then retrospectively collected a set of clinical information, namely concomitant therapy, radiographic pattern of bone metastases (lytic, blastic, or mixed lesions), number and timing of SREs, date of disease progression (bone and extrabone progression), and survival. The institutional review boards provided ethical approval of the study, which complied with all national regulations.
NTX Determination
Urinary NTX was quantified by an experienced researcher using Osteomark NTx Urine enzyme-linked immunosorbent assay (Inverness; now Alere, Waltham, MA, http://www.alere.com) according to manufacturer’s instructions. The lower detection limit of the NTX assay is 20 nmol bone collagen equivalents (BCE). The urinary levels of NTX in BCE are expressed as a ratio to urine creatinine excretion. Urinary NTX was defined as low (NTX <64 nmol BCE/mmol creatinine) or high (NTX ≥100 nmol BCE/mmol creatinine). The cutoff values for NTX were chosen to reflect the approximate upper limit of normal (ULN). However, the normal range for urinary NTX varies according to age, gender, and endocrine function. The ULN in young healthy adults is approximately 50 nmol/mmol creatinine. For women after menopause, the ULN level is approximately 100 nmol BCE/mmol creatinine [13]. For the present analysis, we used the NTX cutoff of 100 nmol BCE/mmol creatinine at baseline and at 3 months, given that most women were postmenopausal at the date of the bone metastasis diagnosis. We used the cutoff of 64 nmol BCE/mmol creatinine for the 12-month analysis to reflect the normal values of this biomarker for patients taking ZA for a long period. A total of 329 measurements were obtained. Researchers quantifying NTX were unaware of the patients’ outcomes through the use of specific sample identifiers.
Statistical Analysis
We performed descriptive statistics of baseline clinical and pathologic characteristics in the overall cohort and according to the presence of concomitant extraskeletal metastasis at diagnosis and the baseline NTX status. Survival plots were performed using Kaplan-Meier methods. For time-to-event outcomes, we used univariate and multivariate Cox proportional hazards regression models. Departures from the proportional hazards assumption were assessed based on the Schoenfeld residuals. To overcome the immortal time bias, a survival analysis using the NTX levels at 12 months as the dependent variable was performed with a landmark analysis, in which only patients alive at 12 months were included. For dichotomous outcomes, we used logistic regression analysis. For other univariate comparisons, we used the chi-square test or t test, as appropriate. Given the exploratory nature of the present study, no specific sample size was defined in advance. Missing data at 12 months were compared with the baseline characteristics, and no relevant discrepancies were identified; thus, the missing data were considered as missing completely at random (data not shown). The outcome of overall survival (OS) was defined as the time from the diagnosis of bone metastasis to death from any cause. Patients without a vital status change in July 2013 were right censored. The analyses were performed using Stata, version 12.3 (StataCorp LP, College Station, TX, http://www.stata.com).
Results
Baseline Characteristics
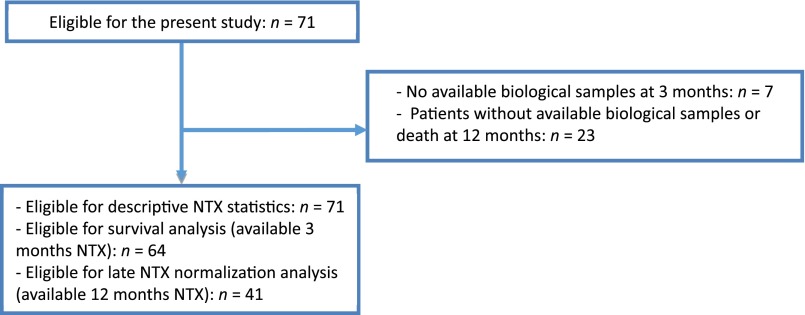
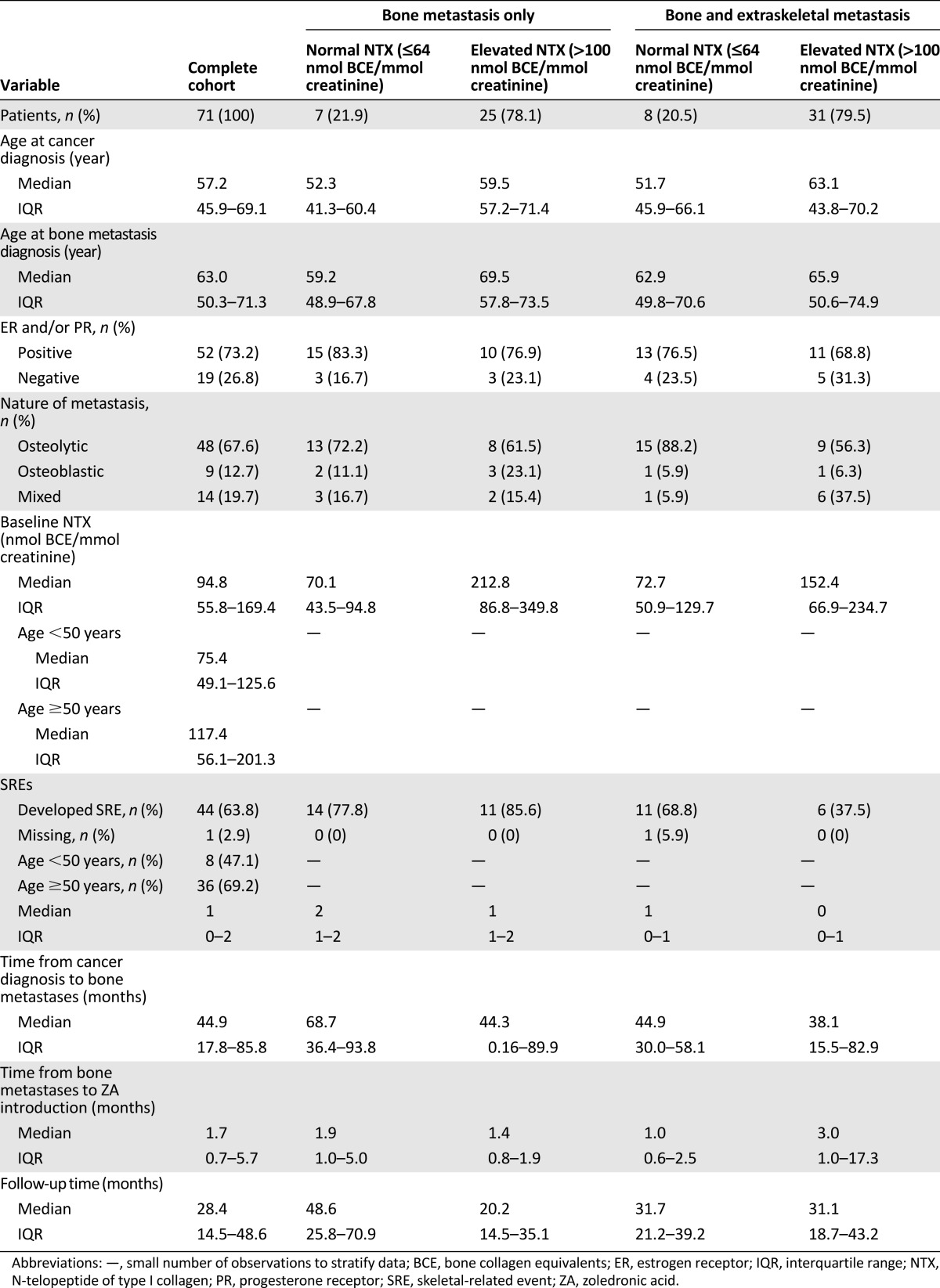
During the study period, we included a total of 71 patients (Fig. 1) with a median age of 57.2 years (interquartile range [IQR], 45.9–71.3). Detailed baseline clinical and pathologic characteristics are summarized in Table 1. Overall, 32 patients presented with bone metastases only, and 39 had bone plus extraskeletal metastases. Most patients presented with lytic bone metastasis (67.6%) and developed at least one SRE (63.8%). The median number of SREs was 1 (IQR, 1–2), with the use of radiotherapy for symptomatic control being the most frequent SRE (63.6% of all SREs; data not shown). The proportion of patients with at least one SRE during the study was 64%. The median time from the diagnosis of BC to the development of bone metastasis was 44.9 months (IQR, 17.8–85.8), and the median time to ZA introduction after the diagnosis of bone metastasis was 1.7 months (IQR, 0.7–5.7).
Figure 1.
Flowchart of study participants.
Abbreviation: NTX, N-telopeptide of type I collagen.
Table 1.
Patient demographics and clinical features, overall and according to site of metastasis and baseline median NTX
NTX Variation
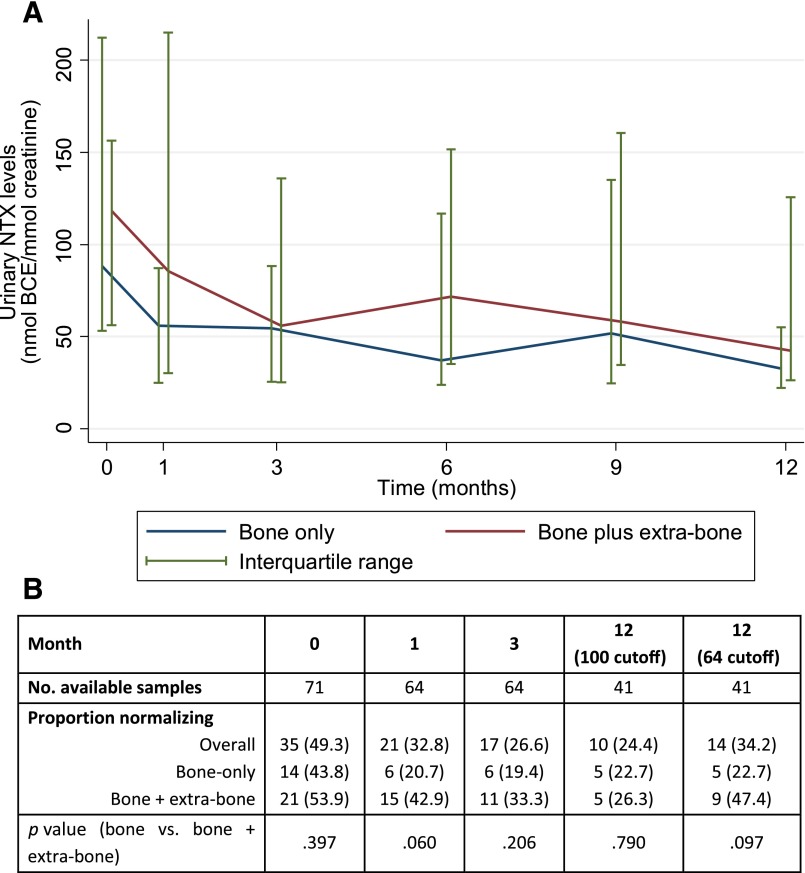
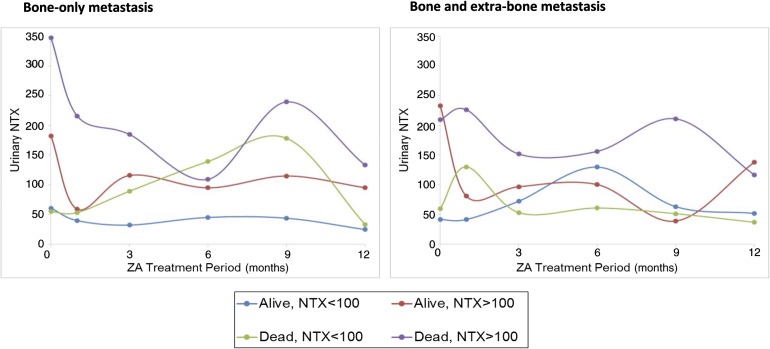
Approximately half the patients had an elevated baseline NTX level (49.3%). Overall, the median baseline NTX value was 94.8 (IQR, 55.8–169.4). The percentage of patients with elevated NTX levels at 3 and 12 months after introduction of bisphosphonates was 26.6% and 34.2%, respectively (Fig. 2; details on the NTX cutoffs are provided in NTX Determination). The percentage of patients with elevated NTX levels at baseline and 1, 3, and 12 months according to extraskeletal involvement did not differ statistically; however, a numerical trend toward a higher proportion of patients with elevated NTX and higher median levels was observed in patients with associated extraskeletal metastases (Fig. 2). Furthermore, the NTX pattern of variation differed according to extraskeletal involvement. Although NTX variation demonstrated a response in line with patient vital status (and thus a response to therapy plus ZA) in patients with bone-only metastasis, patients with simultaneous extraskeletal involvement present with an erratic pattern of NTX variation that was independent of survival (Fig. 3).
Figure 2.
Median NTX (A) and proportion of patients with elevated NTX (B) over time and according to metastatic site (bone only or bone plus extraskeletal metastases). Elevated NTX >100 nmol BCE/mmol creatinine, if not otherwise specified.
Abbreviations: BCE, bone collagen equivalents; NTX, N-telopeptide of type I collagen.
Figure 3.
Median NTX over time according to metastatic site (bone only or bone plus extraskeletal metastases) and survival status at the end of the study follow-up period.
Abbreviations: NTX, N-telopeptide of type I collagen; ZA, zoledronic acid.
The variables associated with persistently elevated NTX levels at month 12 included an elevated baseline and 1- and 3-month NTX level but not age, estrogen receptor status, time to the development of bone metastasis, radiographic pattern of bone metastasis, or the presence of extraskeletal metastasis (Table 2). Patients with extraskeletal disease also demonstrated a trend toward an increased risk of persistently elevated NTX at 12 months. Both an elevated baseline and an elevated 3-month NTX level correlated strongly with survival on univariate and multivariate analyses.
Table 2.
Variables predictive of persistently elevated NTX levels at 12 months (late response) in patients with both 3- and 12-month NTX data (n = 41)
Prognostic Role of Early and Late NTX Levels
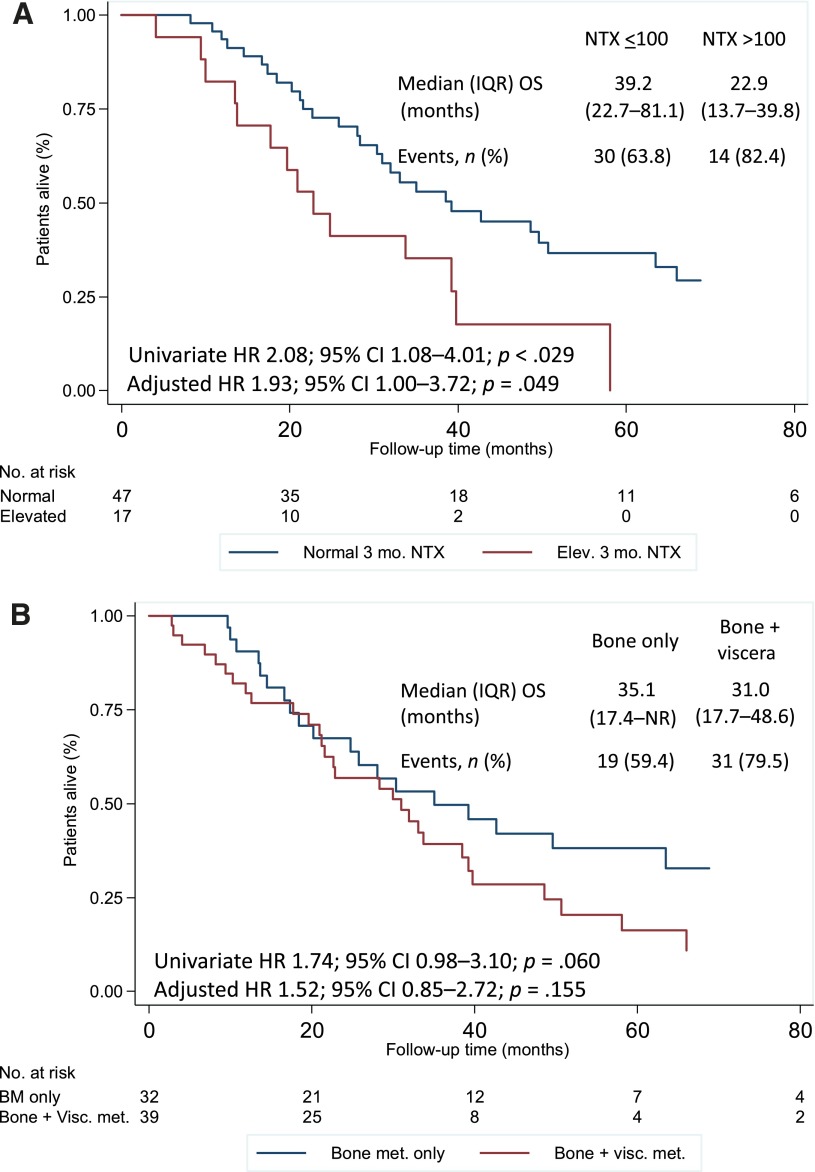
Overall, the median follow-up was 28.4 months (IQR, 14.5–48.6), and 50 patients (70.4%) developed an OS event during the follow-up period. The median OS was 32.0 months (IQR, 17.7–63.5). Variables associated with survival included age at diagnosis, tumor estrogen receptor status, and NTX level at 3 and 12 months, both of which were associated with a twofold increase in the risk of death (Table 3; Fig. 4A). A trend toward worse OS was also noted for patients with disease affecting extraskeletal sites (Fig. 4B) and in those patients with shorter intervals from BC diagnosis to bone involvement. On multivariate analysis, although elevated NTX levels at 3 months maintained significance, elevated NTX levels at 12 months showed only a trend toward an increased risk of death.
Table 3.
Predictors of survival
Figure 4.
Survival according to NTX level at 3 months (A) and extraskeletal involvement (B). Adjusted HR, controlling for age at diagnosis, shown.
Abbreviations: BM, bone metastasis; CI, confidence interval; Elev., elevated; HR, hazard ratio; IQR, interquartile range; met., metastasis; NR, not reached; NTX, N-telopeptide of type I collagen; OS, overall survival; Visc., visceral.
Discussion
In the present study, we report the prognostic role of NTX and its long-term behavior in a contemporaneous cohort of BC patients with bone metastases. We also report for the first time, the effect of extraskeletal disease on this biomarker. In brief, as previously shown in older cohorts of metastatic BC patients, persistently elevated NTX levels at 3 months (NTX >100 nmol BCE/mmol creatinine) after initiation of ZA is a clear marker of worse outcome, in addition to a few other clinicopathological features. Also, despite recent therapeutic innovations and longer survival of metastatic BC patients, early NTX responses correlated with death as well as did later NTX assessments, in our study, at 12 months. We also showed that the NTX levels at 1 and 3 months after the introduction of ZA are strongly associated with long-term NTX levels (at 12 months); thus, good responders to ZA seem to be identifiable early after the introduction of such therapy. Patients with metastatic disease involving extraskeletal sites in association with the bone had a numerical trend for persistently higher NTX levels and an erratic variation of NTX during ZA treatment.
The use of bone remodeling markers is not recommended as a part of routine clinical practice [7, 15–17]. However, a growing body of evidence has moved bone markers from exploratory research to clinical research as tools that can be used to determine the design of clinical trials and support decisions in the context of drug development [17]. The application of bone remodeling markers was further explored in several clinical contexts, including to assess the risk of developing BM, to support the diagnosis of BM, to adjust the schedule of administration of bone-modifying agents, and also to estimate the risk of bone disease progression and the overall prognosis of patients with BM [5]. NTX is one of the most extensively studied markers of bone metabolism. The urinary NTX level at baseline, its early response after treatment with ZA, and its values during treatment were reported to correlate with the risk of developing an SRE, the time to the first SRE, bone disease progression, and death [11, 13, 14, 18]. However, these data were derived from clinical studies conducted before the introduction of major therapeutic innovations in the management of BC, such as aromatase inhibitors, taxanes, and trastuzumab. Despite these innovations, in our contemporaneous cohort of BC patients, the NTX level at 3 months was strongly associated with survival. Moreover, despite the longer survival of these patients (median, 32 months), very early NTX evaluation seems to be associated with survival in a similar way as later evaluations (e.g., at 12 months). Thus, later evaluations do not seem to improve its prognostic value.
Furthermore, the NTX levels 1 and 3 months after starting ZA therapy were consistently associated with long-term biomarker response, showing that early control of bone metabolism after starting ZA is associated with persistent future control. This finding is interesting in light of the results of the ZOOM and OPTIMIZE-2 trials that tested the de-escalation of ZA (from an every 4-week regimen to an every 12-week regimen) in patients treated with ZA for approximately 12 months [19, 20]. Given the strong association of early normalization of NTX with later control (but also longer survival and thus higher cumulative exposure to ZA, with the associated increased risk of adverse events), it could be a reasonable strategy to test the early de-escalation of ZA in those patients with a good biomarker response at 3 months.
We found a greater variation of NTX after the introduction of ZA in those patients with extraskeletal metastatic disease sites, in addition to bone metastasis. These patients showed a trend toward increased bone resorption and a lower rate of NTX normalization (less than 64 nmol BCE/mmol creatinine) compared with patients with bone-only metastases. This observation is relevant when we realize that in all the large phase III trials that led to the approval of bisphosphonates or denosumab to treat BC patients with BMs, the treatment schedule of the antiresorptive agent was the same, regardless of the sites of metastatic disease: bone-only or bone with extraskeletal metastases. We do not know whether this affects the incidence of SREs in patients with bone plus extraskeletal metastases. Furthermore, the observation that extraskeletal metastases influence the status of bone resorption in metastatic BC could be provocative to discover answers to other questions. For example, it is not known whether humoral factors affecting bone resorption are produced by extraskeletal metastases and/or whether cancer cells can circulate from different sites of metastases (outside of bone to bone and vice versa) to restimulate the microenvironment. This appears plausible in light of the evidence that cancer cells can circulate (reseed) from metastases to the primary site [21, 22].
Conclusion
In a contemporaneous cohort of BC patients with bone metastases, persistently elevated NTX levels at 3 months after the introduction of ZA doubled the risk of death. This effect size was similar to that of later evaluations (at 12 months). Furthermore, NTX normalization at 1 and 3 months was strongly associated with long-term control (at 12 months) of bone metabolism in response to the introduction of ZA. Finally, bone resorption, as measured by NTX levels, seems to be influenced by the site of distribution of the metastases. Thus, patients with bone plus extraskeletal metastasis tended to have higher NTX levels and an erratic NTX variation during treatment with ZA. This observation should be further explored to clarify whether patients with bone plus extraskeletal metastases should receive a different treatment regimen of bone-targeted agents.
Acknowledgments
This work was partially supported by Thar Pharmaceutical Inc. This report reflects the authors’ exclusive and best scientific judgment of findings.
This work was partially presented in poster format at the 2013 American Society of Clinical Oncology Annual Meeting.
Footnotes
For Further Reading: Ryota Tanaka, Kan Yonemori, Akihiro Hirakawa et al. Risk Factors for Developing Skeletal-Related Events in Breast Cancer Patients With Bone Metastases Undergoing Treatment With Bone-Modifying Agents. The Oncologist 2016;21:508–513.
Implications for Practice: Retrospectively, risk factors were identified for skeletal-related events (SREs) in breast cancer patients with bone metastasis who were treated with bone-modifying agents (BMAs). For the time to the first SRE and for the SRE frequency, presence of extraskeletal metastases and BMA initiation ≥6 months after the detection of bone metastases were risk factors, respectively. Luminal B type disease, a history of palliative radiation therapy, BMA treatment within 2 years, and elevated serum calcium levels at initial BMA dose were risk factors for both first SRE and SRE frequency. More vigilant observation should be considered for patients with these risk factors.
Author Contributions
Conception/Design: Arlindo R. Ferreira, Irina Alho, Ning Shan, Sandra Casimiro, Luís Costa
Provision of study materials and/or patients: Luís Costa
Collection and/or assembly of data: Arlindo R. Ferreira, Irina Alho, Margarida Matias, Mariana Faria, Luís Costa
Data analysis and interpretation: Arlindo R. Ferreira, Irina Alho, Ning Shan, Sandra Casimiro, Kim Leitzel, Suhail Ali, Allan Lipton, Luís Costa
Manuscript writing: Arlindo R. Ferreira, Irina Alho, Ning Shan, Margarida Matias, Mariana Faria, Sandra Casimiro, Kim Leitzel, Suhail Ali, Allan Lipton, Luís Costa
Final approval of manuscript: Arlindo R. Ferreira, Irina Alho, Ning Shan, Margarida Matias, Mariana Faria, Sandra Casimiro, Kim Leitzel, Suhail Ali, Allan Lipton, Luís Costa
Disclosures
Ning Shan: Thar Pharmaceuticals Inc. (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Galasko C. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert A, editors. Bone Metastases. Boston, MA: G.K. Hall & Co.; 1981. pp. 49–63. [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(suppl):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira A, Alho I, Casimiro S, et al. Bone remodeling markers and bone metastases: From cancer research to clinical implications. Bonekey Rep. 2015;4:668. doi: 10.1038/bonekey.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pluijm G, Löwik C, Papapoulos S. Tumour progression and angiogenesis in bone metastasis from breast cancer: New approaches to an old problem. Cancer Treat Rev. 2000;26:11–27. doi: 10.1053/ctrv.1999.0143. [DOI] [PubMed] [Google Scholar]
- 7.Gralow JR, Biermann JS, Farooki A, et al. NCCN task force report: Bone health in cancer care. J Natl Compre Canc Netw. 2013;11(suppl 3):S1–S50. doi: 10.6004/jnccn.2013.0215. [DOI] [PubMed] [Google Scholar]
- 8.Lipton A, Costa L, Coleman RE. Bone turnover markers: Tools for prognosis and monitoring response to bisphosphonates? Breast Dis. 2011;33:59–69. doi: 10.3233/BD-2010-0327. [DOI] [PubMed] [Google Scholar]
- 9.Lester JE, Brown JE, Hannon RA, et al. The use of a point of care device for monitoring the bone resorption biomarker urinary N-telopeptide in cancer patients with bone metastases. Bone. 2010;46:801–805. doi: 10.1016/j.bone.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Costa L, Demers LM, Gouveia-Oliveira A, et al. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol. 2002;20:850–856. doi: 10.1200/JCO.2002.20.3.850. [DOI] [PubMed] [Google Scholar]
- 11.Brown JE, Cook RJ, Major P, et al. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97:59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 12.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 14.Lipton A, Cook RJ, Major P, et al. Zoledronic acid and survival in breast cancer patients with bone metastases and elevated markers of osteoclast activity. The Oncologist. 2007;12:1035–1043. doi: 10.1634/theoncologist.12-9-1035. [DOI] [PubMed] [Google Scholar]
- 15.Van Poznak CH, Von Roenn JH, Temin S. American Society of Clinical Oncology clinical practice guideline update: Recommendations on the role of bone-modifying agents in metastatic breast cancer. J Oncol Pract. 2011;7:117–121. doi: 10.1200/JOP.2011.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman R, Body JJ, Aapro M, et al. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25(suppl 3):iii124–iii137. doi: 10.1093/annonc/mdu103. [DOI] [PubMed] [Google Scholar]
- 17.Coleman R, Costa L, Saad F, et al. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80:411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Lipton A, Cook R, Saad F, et al. Normalization of bone markers is associated with improved survival in patients with bone metastases from solid tumors and elevated bone resorption receiving zoledronic acid. Cancer. 2008;113:193–201. doi: 10.1002/cncr.23529. [DOI] [PubMed] [Google Scholar]
- 19.Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): A phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14:663–670. doi: 10.1016/S1470-2045(13)70174-8. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel N, Hortobagyi AL, Chew HK, et al. Efficacy and safety of continued zoledronic acid every 4 weeks versus every 12 weeks in women with bone metastases from breast cancer: Results of the OPTIMIZE-2 trial. J Clin Oncol. 2014;32(suppl):LBA9500a. [Google Scholar]
- 21.Kim M-Y, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comen E, Norton L, Massagué J. Clinical implications of cancer self-seeding. Nat Rev Clin Oncol. 2011;8:369–377. doi: 10.1038/nrclinonc.2011.64. [DOI] [PubMed] [Google Scholar]