Metformin might have antineoplastic properties against colon cancer (CC). Patients with stage III CC enrolled in the N0147 study completed a questionnaire regarding diabetes mellitus (DM) and metformin use. Patients with stage III CC undergoing adjuvant chemotherapy who used metformin before the diagnosis of CC experienced survival outcomes similar to those for non-DM patients and DM patients without metformin use.
Keywords: Colon cancer, Recurrence, Survival, Metformin, Diabetes mellitus, Adjuvant chemotherapy
Abstract
Background.
Preclinical and epidemiological data suggest that metformin might have antineoplastic properties against colon cancer (CC). However, the effect of metformin use on patient survival in stage III CC after curative resection is unknown. The survival outcomes were comparable regardless of the duration of metformin use.
Patients and Methods.
Before randomization to FOLFOX (folinic acid, 5-fluorouracil, oxaliplatin) with or without cetuximab, 1,958 patients with stage III CC enrolled in the N0147 study completed a questionnaire with information on diabetes mellitus (DM) and metformin use. Cox models were used to assess the association between metformin use and disease-free survival (DFS), overall survival (OS), and the time to recurrence (TTR), adjusting for clinical and/or pathological factors.
Results.
Of the 1,958 patients, 1,691 (86%) reported no history of DM, 115 reported DM with metformin use (6%), and 152 reported DM without metformin use (8%). The adjuvant treatment arms were pooled, because metformin use showed homogeneous effects on outcomes across the two arms. Among the patients with DM (n = 267), DFS (adjusted hazard ratio [aHR], 0.90; 95% confidence interval [CI], 0.59–1.35; p = .60), OS (aHR, 0.99; 95% CI, 0.65–1.49; p = .95), and TTR (aHR, 0.87; 95% CI, 0.56–1.35; p = .53) were not different for the metformin users compared with the nonusers after adjusting for tumor and patient factors. The survival outcomes were comparable regardless of the duration of metformin use (<1, 1–5, 6–10, ≥11 years) before randomization (ptrend = .64 for DFS, ptrend = .84 for OS, and ptrend = .87 for TTR). No interaction effects were observed between metformin use and KRAS, BRAF mutation status, tumor site, T/N stage, gender, or age.
Conclusions.
Patients with stage III CC undergoing adjuvant chemotherapy who used metformin before the diagnosis of CC experienced DFS, OS, and TTR similar to those for non-DM patients and DM patients without metformin use.
Implications for Practice:
The present study did not find any relationship between metformin use or its duration and disease-free survival, time to recurrence, and overall survival in a large cohort of patients with resected stage III colon cancer receiving adjuvant FOLFOX (folinic acid, fluorouracil, oxaliplatin)-based chemotherapy. This relationship was not modified by KRAS or BRAF mutation or DNA mismatch repair status. Metformin use did not increase or decrease the likelihood of chemotherapy-related grade 3 or higher adverse events.
Introduction
Colorectal cancer (CRC) is an important public health concern, because it is the third most common cancer in the United States in both men and women and is responsible for approximately 10% of all cancer deaths [1]. Several recent clinical trials of novel targeted agents in the adjuvant setting have failed to improve survival in patients with colon cancer (CC) [2–4]. Given the recent lack of advancements in adjuvant therapy, it remains important to explore other potential agents that might enhance the benefit of current standard adjuvant regimens.
Metformin is the most widely used antidiabetic drug in the world, and increasing evidence has shown potential antineoplastic effects. Metformin decreases insulin resistance and indirectly reduces insulin levels, which might explain at least some of its antitumor properties, given the observation that insulin promotes cancer cell growth [5]. Also, metformin has been shown to activate AMP-activated protein kinase (AMPK), a major sensor of the energy status of a cell, causing inhibition of the mammalian target of rapamycin (mTOR) pathway. Metformin also acts through unique AMPK-independent mechanisms, both leading to reduced tumor growth [5–9]. In addition, mutant KRAS has been postulated to be a predictor of cancer cell responsiveness to metformin [10].
Observational studies and meta-analyses have suggested that metformin use might be associated with a reduced risk of CRC [11]. In addition, several epidemiological studies have evaluated the association between metformin use and the survival of patients with CRC but yielded conflicting results [12–16]. A large population-based study in Ireland demonstrated a 31% reduction in all-cause mortality for metformin users versus nonusers, after adjusting for confounders. In that study, high-intensity exclusive metformin use was also associated with a significant reduction in CRC-specific mortality (hazard ratio [HR], 0.44; 95% confidence interval [CI], 0.20–0.95) [16]. Likewise, a meta-analysis reported a 36% reduction in all-cause mortality and a 34% reduction in CRC-specific mortality with metformin use compared with nonuse in CRC patients [17]. However, several other large retrospective studies have offered inconsistent results and have not supported an association between overall survival (OS) in CRC patients and metformin use [12, 13].
Most previous studies were limited by the use of diagnostic codes for defining CRC, instead of histological confirmation, and studied heterogeneous populations that included patients with all stages of CRC. They also lacked details regarding surgery and the concomitant administration of chemotherapeutic drugs. None of these studies explored the effect of KRAS or BRAF mutation status or DNA mismatch repair (MMR) status on the relationship between metformin use and survival. It is thus unknown whether a subgroup of patients with CRC according to the stage or molecular characteristics exists that might benefit from metformin. To address this gap in knowledge, we examined the relationship between metformin use and disease-free survival (DFS), OS, and the time to recurrence (TTR) in a large cohort of patients with resected, stage III colon cancer enrolled in a completed randomized clinical trial of adjuvant chemotherapy. In addition, we investigated the association between metformin use and patient outcomes in relation to tumoral KRAS and BRAF mutation status and MMR status.
Patients and Methods
Study Population
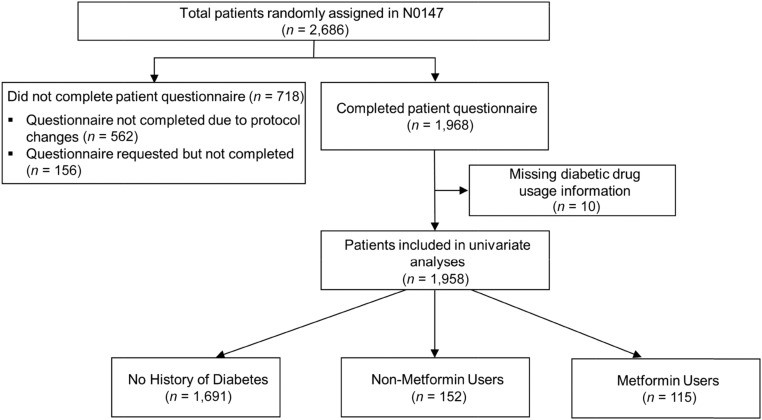
N0147 was a multicenter phase III randomized trial led by the North Central Cancer Treatment Group (NCCTG) in which patients with resected stage III colon cancer were randomly assigned (1:1) to adjuvant treatment with infusional 5-fluorouracil, leucovorin, and oxaliplatin (modified FOLFOX6), with or without cetuximab [2]. (NCCTG is now part of the Alliance for Clinical Trials in Oncology.) Eligible patients had histologically confirmed adenocarcinoma of the colon, at least one pathologically confirmed positive lymph node, complete surgical resection performed at least 56 days before random assignment, and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Patients with evidence of metastatic disease, previous or concurrent malignancies, previous epidermal growth factor receptor inhibitor therapy, age younger than 18 years, or one of several exclusionary comorbid conditions at the time of random assignment were excluded from participation. Between February 10, 2004 and November 25, 2009, 2,686 patients were randomly assigned to one of the treatment arms. From the self-reported patient questionnaires, 1,958 patients provided information on any diagnosis of diabetes mellitus (DM) and metformin use. Centralized molecular analysis for KRAS mutations, BRAF V600E mutations, and MMR status was also conducted as a part of the study. Each participant signed an institutional review board-approved, protocol-specific informed consent form in accordance with federal and institutional guidelines.
Exposure Ascertainment
Until a change in protocol in 2008, the participants in N0147 were asked at study enrollment and before randomization to complete a questionnaire eliciting information on comorbid conditions, family history of cancer, the use of common medicines (including metformin), vitamins, and nutritional supplements, and lifestyle habits (smoking, alcohol use, and physical activity). Specifically, participants were asked to report a history of DM and regular use (defined as at least once a week) of antidiabetic medications, including insulin, metformin (Glucophage), sulfonylureas (DiaBeta, Diabinese, Glucotrol, or Micronase), or thiazolidinediones (Actos, Avandia, or Rezulin). Patients who reported antidiabetic medication use were also asked to report the duration of use (<1 year, ≥1–5 years, ≥6–10 years, or ≥11 years).
Tumor Characterization
Colon tumor tissue blocks from the original surgical resection were obtained for all study participants and sent to Mayo Clinic for centralized KRAS mutation testing and additional molecular analyses. DNA isolated from tumor specimens was used to test for seven mutations in codons 12 and 13 of KRAS exon 2 (TheraScreen; DxS, Manchester, U.K.) and to test for the BRAF V600E mutation, as previously described [18]. DNA MMR status was determined by immunohistochemical assessment of three proteins: hMLH-1, hMSH-2, and hMSH-6 [19]. Patients with tumors exhibiting a loss of protein expression for any of these markers were classified as having defective MMR (dMMR). Patients with no loss of expression were classified as having proficient MMR (pMMR). Assays for tumor characterization were interpreted without knowledge of the treatment, patient, or outcome information.
Survival Outcomes
The primary clinical endpoint for the present analysis was DFS, defined as the time from randomization to the earliest occurrence of either the first documented colon cancer recurrence or death from any cause. The secondary endpoints were OS, defined as the time from randomization to death from any cause, and the TTR. The TTR was defined as the time from randomization to the first documented disease recurrence. Participants who died before any recurrence were censored at their last disease evaluation date. On the basis of the consistency of the available follow-up information, OS and DFS and TTR were censored at 8 and 5 years after randomization, respectively.
Statistical Analysis
Descriptive statistics were tabulated by metformin use and compared between groups using the Kruskal-Wallis and chi-square tests for continuous and categorical factors, respectively. The distributions of survival outcomes were estimated using the Kaplan-Meier method [20] and compared between groups using the log-rank test. Cox regression models [21] were used to evaluate the adjusted associations between metformin use and the time-to-event outcomes, adjusting for relevant baseline factors (age, sex, race, performance status, T/N stage, treatment arm, body mass index [BMI], histologic grade, tumor location, KRAS/BRAF mutation status, and MMR status). The potential differential relationship between metformin use and outcomes per KRAS/BRAF mutation status and MMR status was tested by including interaction terms in the Cox models. Logistic regression analysis was used to evaluate the association between metformin use and toxicity outcomes.
Because no significant interaction effect was found between metformin use and treatment assignment on outcomes (all interaction, p > .39), the analysis was conducted by pooling the two treatments. Based on the total number of events and ratio of patients between the two groups (metformin users/nondiabetic = 1:14.7), the study would be able to detect a minimal effect size with a HR of 1.5 for DFS, with 80% power at a one-sided α of 0.05. The analyses included follow-up data through December 3, 2014 and were performed using Statistical Analysis Systems, version 9.3 (SAS Institute, Cary, NC, http://www.sas.com). Two-sided p values less than .05 were considered statistically significant. All data collection and statistical analyses were performed by the Alliance Statistics and Data Center.
Results
Patient Characteristics According to Metformin Use
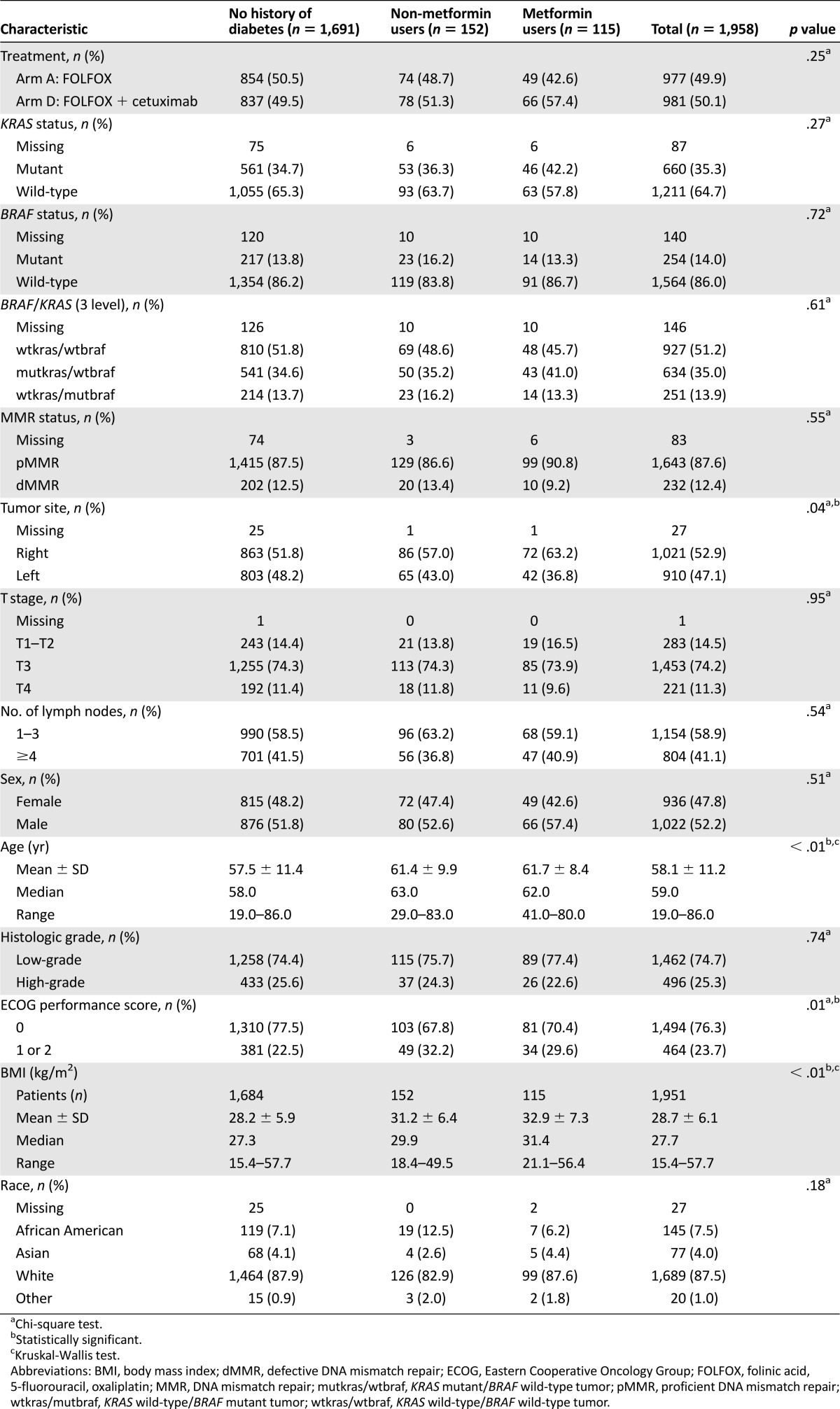
The baseline characteristics for study patients classified by DM and metformin use are presented in Table 1. Of the 1,958 patients, 1,691 (86%) reported no history of DM (nondiabetics), 115 (6%) reported a history of DM and regular metformin use (metformin users), and 152 (8%) reported a history of DM and no metformin use (nonusers) (Fig. 1). The patients who reported metformin use were older (p < .0001), had a higher BMI (p < .001), and were more likely to have right-sided tumors (p = .037) than the nondiabetics and metformin nonusers. Other patient and tumor characteristics were not different among the metformin users and nonusers.
Table 1.
Patient characteristics stratified by metformin drug usage status
Figure 1.
Derivation of metformin analytic cohort in North Central Cancer Treatment Group phase III trial N0147.
Association of Metformin Use With Cancer Recurrence and Death in Overall Population
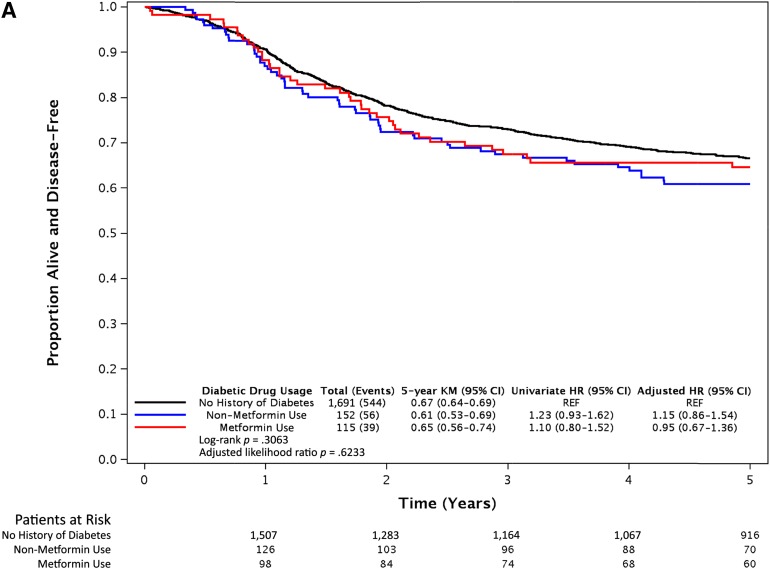
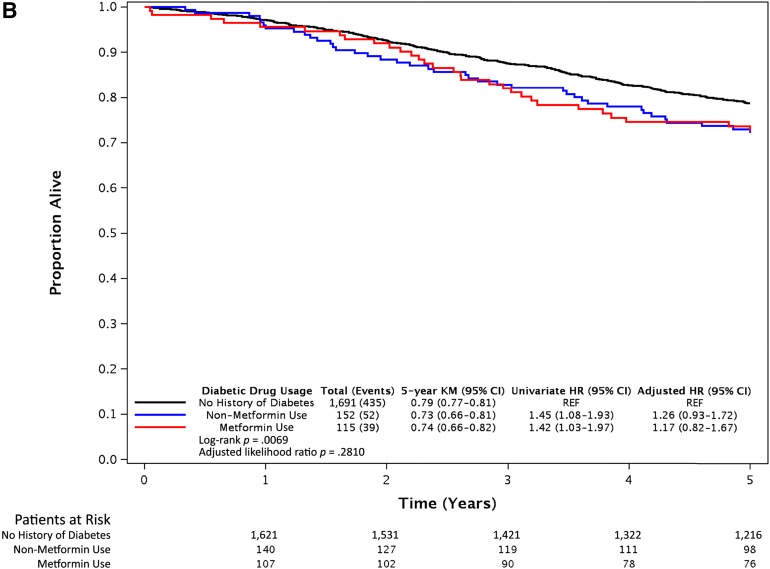
After a median follow-up of 6.5 years, 566 of the 1,958 eligible patients had developed recurrence and 526 had died. Compared with the patients with no history of DM, the patients with DM as a group had no evidence of differences in DFS (adjusted [aHR], 1.06; 95% CI, 0.84–1.35; p = .63), OS (aHR, 1.22; 95% CI, 0.95–1.57; p = .12), and TTR (aHR, 1.04; 95% CI, 0.80–1.34; p = .77). No difference was found in DFS (Fig. 2A), OS (Fig. 2B), or TTR (Fig. 2C) for metformin users or nonusers compared with non-DM patients, after adjusting for tumor and patient factors. Within the cohort of DM patients (n = 267), DFS (aHR, 0.90; 95% CI, 0.59–1.35; p = .60), OS (aHR, 0.99; 95% CI, 0.65–1.49; p = .95), and TTR (aHR, 0.87; 95% CI, 0.56–1.35; p = .53) were not different between the metformin users and nonusers. Although our study detected a minimal effect size of a HR of 1.5 for DFS, with 80% power at one-sided α of 0.05, an inability to detect a true difference owing to a lack of power would have been an issue if we had observed a large HR (e.g., HR, 1.4) without a significant p value. However, the observed univariate HR of 1.10 and aHR of 0.95 for DFS (Fig. 2A) were very close to 1.0; thus, this effect size was not clinically large enough to claim survival differences among the groups.
Figure 2.
Survival outcomes of metformin users, nonusers, and patients without diabetes mellitus from the N0147 trial. Kaplan-Meier curves of disease-free survival (A), overall survival (B), and time to recurrence (C) after a median follow-up period of 6.5 years. Statistical significance was measured using the likelihood ratio p value.
Abbreviations: CI, confidence interval; HR, hazard ratio; KM, Kaplan-Meier; REF, reference value.
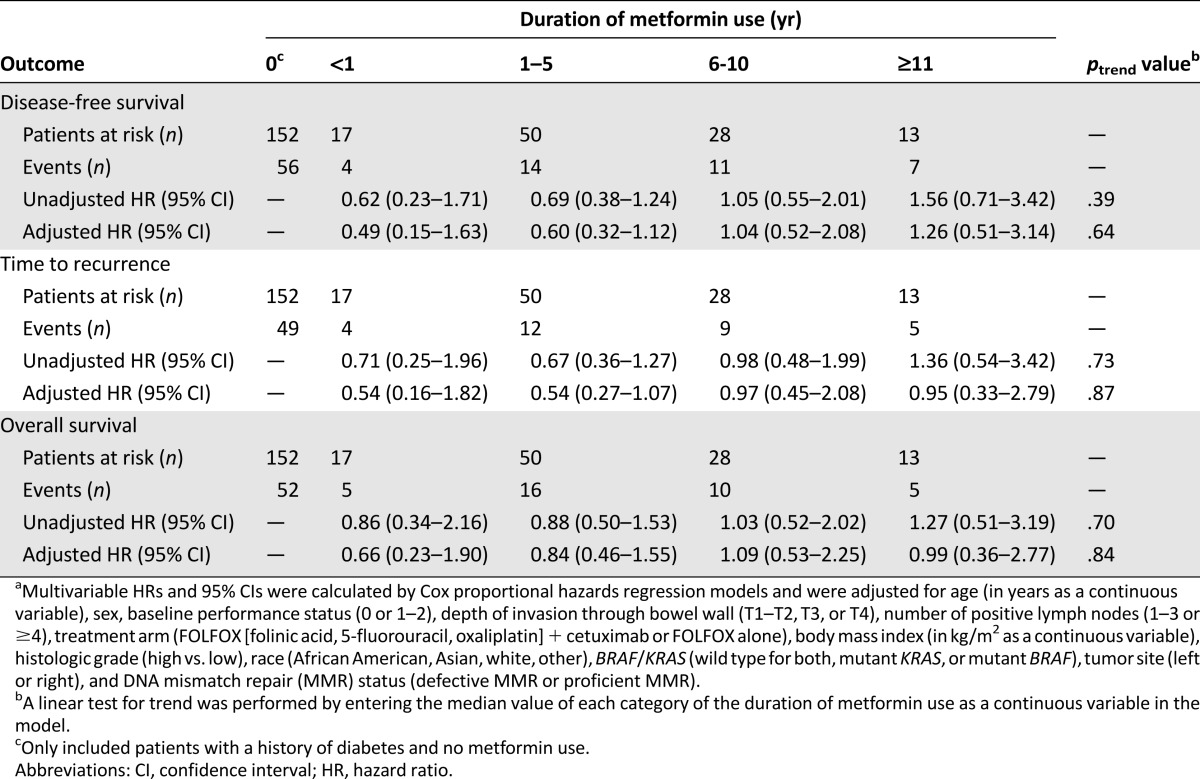
We also investigated the effect of the duration of prerandomization metformin use on patient outcomes (Table 2). Recent metformin use, as reflected by reported use for 1–5 years, did not lead to statistically significantly improved DFS (aHR, 0.60; 95% CI, 0.32–1.12), OS (aHR, 0.84; 95% CI, 0.46–1.55), or TTR (aHR, 0.54; 95% CI, 0.27–1.07) compared with nonusers (Table 2). Moreover, an increasing duration of use was not associated with patient outcome (ptrend = .64 for DFS, ptrend = .87 for TTR, and ptrend = .84 for OS).
Table 2.
Association between duration of metformin use and colon cancer recurrence and mortality stratified by metformin use duration within cohort of diabetes mellitus patients (n = 267)a
Association of Metformin Use and Survival According to KRAS, BRAF Mutation and MMR Status
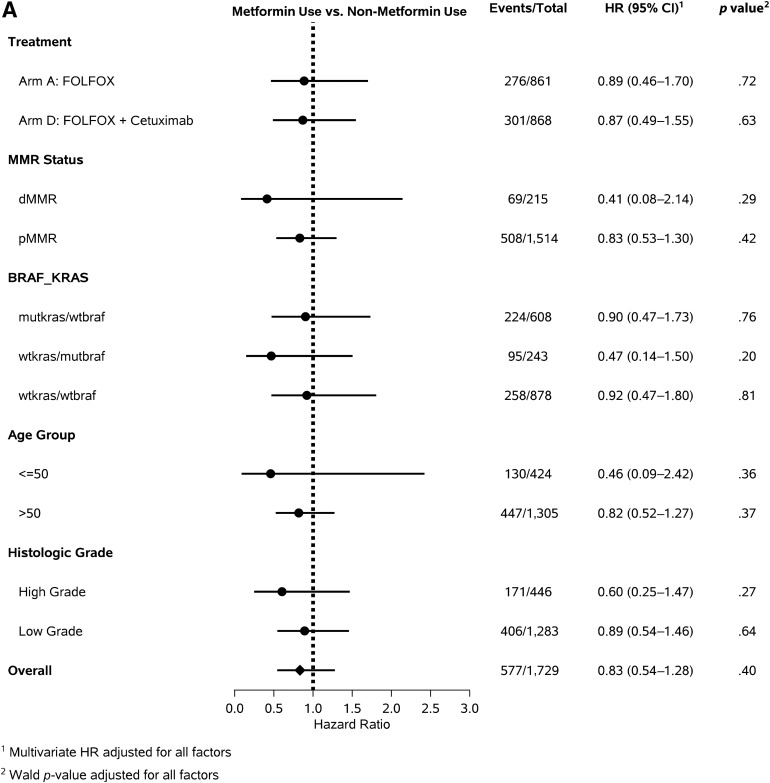
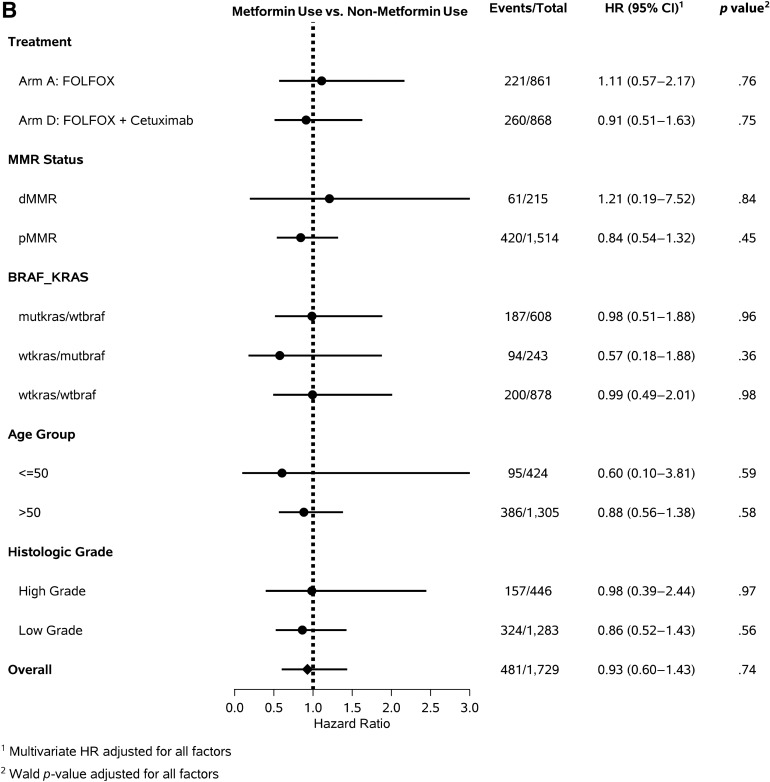
Among the 1,958 patients who reported information on metformin use, 1,812 (92.5%) had data available on KRAS and BRAF mutation status. Among these patients, 634 (35%) had KRAS mutant/BRAF wild-type tumors and 251 (13.9%) had KRAS wild-type/BRAF mutant tumors. Within this overall population, the DFS among metformin users and nonusers was not different, irrespective of the KRAS and BRAF mutation status (KRAS mutant/BRAF wild type, aHR, 0.90; 95% CI, 0.47–1.73; KRAS wild-type/BRAF mutant, aHR, 0.47; 95% CI, 0.14–1.50; KRAS wild-type/BRAF wild-type, aHR, 0.92; 95% CI, 0.47–1.80; pinteraction = .93; Fig. 3A). Also, no difference was seen in OS or TTR among metformin users and nonusers (Fig. 3B, 3C), regardless of KRAS/BRAF mutation status.
Figure 3.
Forest plots showing the risk of cancer recurrence and death among metformin users and nonusers across strata of predictors of cancer outcome. Forest plots for metformin use vs. non-metformin use for disease-free survival (A), overall survival (B), and time to recurrence (C).
Abbreviations: CI, confidence interval; dMMR, defective DNA mismatch repair; FOLFOX, folinic acid, 5-fluorouracil, oxaliplatin; HR, hazard ratio; mutkras/wtbraf, KRAS mutant/BRAF wild-type tumor; pMMR, proficient DNA mismatch repair; wtkras/mutbraf, KRAS wild-type/BRAF mutant tumor; wtkras/wtbraf, KRAS wild-type/BRAF wild-type tumor.
MMR status was available for 1,875 patients (98%), and 232 (12.4%) of these patients had tumors with dMMR expression. No evidence was found of a difference in DFS, OS, or TTR among metformin users and nonusers for both pMMR and dMMR tumors. (Fig. 3).
Interaction Between Metformin Use and Other Predictors of Patient Outcome
We evaluated the influence of metformin use on DFS, TTR, and OS across strata of other predictors of cancer outcome. The relationship between metformin use and DFS, OS, and TTR was similar across the strata of age, treatment arm, histologic grade (Fig. 3), sex, tumor site, ECOG performance status, T stage, and N stage (data not shown).
Relationship Between Metformin Use and Toxicity
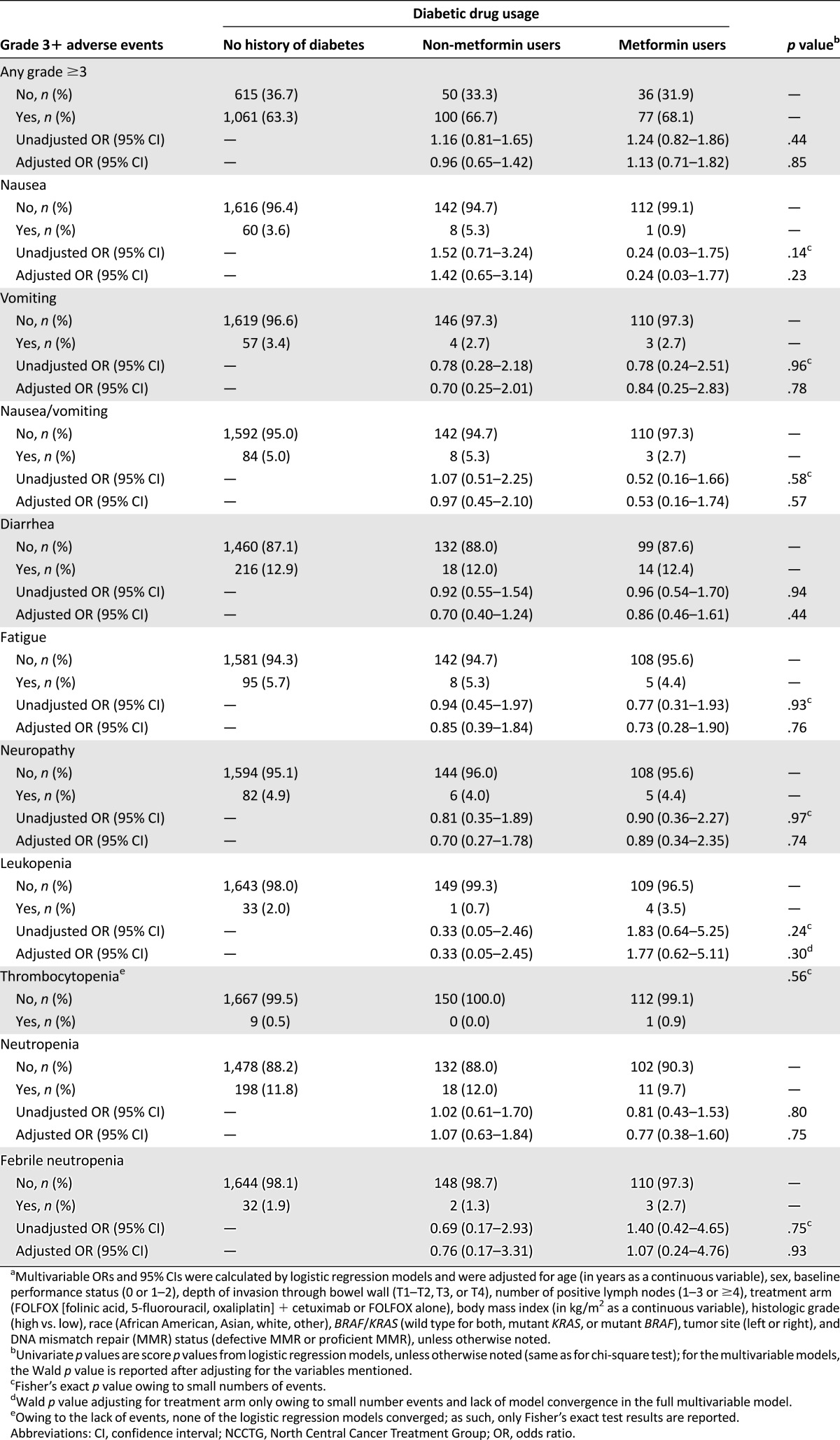
We evaluated the influence of metformin use on the occurrence of selected grade 3 or higher adverse events (Table 3). The likelihood of developing any grade 3 or higher adverse event was not different between metformin users and nonusers after adjusting for potential confounding factors.
Table 3.
Association between diabetic drug usage and incidence of selected grade 3 or higher adverse events in patients from NCCTG trial N0147 (n = 1,939)a
Discussion
We did not find any relationship between a history of metformin use or its duration and DFS, TTR, and OS in this large cohort of patients with resected stage III colon cancer receiving adjuvant FOLFOX-based chemotherapy. Additionally, the relationship between metformin use and patient outcome was not modified by KRAS or BRAF mutation status or MMR status. Metformin use did not increase or decrease the likelihood of chemotherapy-related grade 3 or higher adverse events. To the best of our knowledge, the present study is the first to examine the relationship between metformin use and patient survival in a homogenous cohort of patients with resected stage III colon cancer receiving adjuvant chemotherapy in the context of a prospective clinical trial.
DM is an established, independent risk factor for CRC, with a reported 30%–40% higher risk compared with that for nondiabetic patients [22–24]. Because metformin might interact with diverse signaling pathways critical for colon cancer development and progression, interest has been strong in defining metformin’s role in the chemoprevention and treatment of this malignancy in patients with DM. A comprehensive meta-analysis evaluating 840,787 patients with DM identified 13,871 incident CRC cases and showed a modest, but statistically significant, reduction in the risk of CRC with metformin use (odds ratio, 0.89; 95% CI, 0.81–0.99) [11]. However, few studies have addressed the impact of metformin use on survival among patients with established CRC and have reported conflicting findings. Cossor et al. found no difference in CRC-specific survival for metformin users compared with nonusers among 2,066 postmenopausal women (212 with DM) with CRC (all stages) in the Women’s Health Initiative study [12]. Another cohort study evaluating only stage I-III CRC patients reported that high-intensity metformin use was associated with a significant reduction in CRC-specific mortality (HR, 0.44; 95% CI, 0.20–0.95) [16]. In that cohort study, metformin exposure (yes vs. no) was defined according to whether the individual had a supply of metformin available at any point in the year before the diagnosis of colorectal cancer. Metformin dosing intensity was calculated as the proportion of days covered in the year before the diagnosis of colorectal cancer in which a supply of metformin was available. This was stratified as “low” or “high” at the median. A single institution study evaluating 4,758 CRC patients (424 with DM) found that metformin users had better OS than did diabetic patients treated with other diabetic agents, even after adjustment for covariates (HR, 0.6; 95% CI, 0.5–0.8) [14]. This survival advantage was observed in patients with stage I-III CRC but not in patients with stage IV disease. Specifically, in stage III CRC patients, the estimated median OS was significantly longer at 89.7 months (95% CI, 54.6–124.8) for metformin users (n = 80) compared with 71.5 months (95% CI, 63.8–79.2) for non-metformin users (n = 92; p = .002). Our results might be different from these studies for several reasons. First, the patient cohorts in the other studies were database derived and heterogeneous, including patients with all stages of CRC at various times from diagnosis, receiving various anticancer therapy regimens. Our cohort was homogenous and specific to patients with stage III colon cancer, who were undergoing adjuvant chemotherapy. Second, the comparator arm of “non-metformin” users was defined differently in the various studies, with some even including nondiabetic patients in that group. We have separately analyzed nondiabetic patients and patients with DM who were not taking metformin. Third, the distribution of other antidiabetic treatments (that might have an independent association with CRC outcomes) in the “non-metformin” users might be dissimilar among the studies.
Several mechanisms exist by which metformin exposure might exert antineoplastic effects. Experimental data have indicated that metformin leads to mTOR inhibition, which has been shown to interrupt colon carcinogenesis in mice [25]. Metformin has also been shown to downregulate the Wnt/β-catenin signaling pathway and act synergistically with 5-fluorouracil and oxaliplatin to induce cell death in chemoresistant HT-29 and HCT-116 colon cancer cells and in murine xenograft models [26]. It suppresses tumor progression with inhibition of NFκB/STAT3 inflammatory signaling [27]. Metformin might also inhibit cell growth and promote cell senescence by inhibiting cyclin D1 expression and retinoblastoma protein phosphorylation [28]. Metformin has been shown to decrease the number of breast cancer stem cells and impair their ability to self-renew and proliferate, acting in synergy with chemotherapy and prolonging remission in a mouse xenograft model [29]. In addition to a tumor cell-specific effect, metformin exerts systemic effects such as improving insulin sensitivity and promoting weight loss [30]. Based on these mechanisms, metformin has been postulated to reduce the risk of the development of colon cancer and probably potentiate the effects of chemotherapy in those with established disease. Our study results indicate, however, that metformin might not be active against micrometastatic disease, which is the source of tumor recurrence after adjuvant chemotherapy in resected stage III colon cancer.
Stage III colon cancer patients whose tumors harbor BRAF or KRAS mutations have been shown to have shorter survival compared with patients whose tumors lack these mutations [31]. In preclinical endometrial cancer models, it has been demonstrated that metformin inhibits cell proliferation, induces apoptosis, and decreases tumor growth, with the greatest response observed in cells harboring activating KRAS mutations [32]. These studies have suggested that metformin might improve the cancer prognosis for a subgroup of patients with tumors harboring activating KRAS mutations. However, in our analysis, metformin use was not associated with improved survival in patients with KRAS-mutated colon tumors.
The results from the present analysis should be interpreted in the context of the study limitations. Although the study population was large, the patient numbers were modest within some strata defined by the patient or tumor characteristics, which reduced our ability to detect small differences between metformin users and nonusers and to evaluate associations within subgroups. Second, because we relied on self-reported medication use, misclassification of exposure is possible. In addition, the study questionnaire could not fully capture all aspects of DM and metformin use. The nonrandom nature of metformin usage in the study cohort could also have been a potential source of bias. Nonetheless, exposure was recorded before any knowledge of cancer-related outcomes, thus reducing the likelihood of reporting biases. Third, because information on metformin use was collected only at study enrollment, we were unable to evaluate the associations with postdiagnostic use or changes in metformin use status after the diagnosis. Fourth, because our study was conducted within the context of a randomized clinical trial and patients enrolled in randomized trials represent a more selected population, the generalizability of the study findings to the broader population of patients with colon cancer is not fully known. Finally, DM patients who use metformin might differ from DM patients not using metformin and the general population in the duration and severity of DM, dietary behaviors, BMI, physical activity, and health care usage. Although we controlled for several potentially prognostic variables, residual confounding from unknown variables is possible.
The present analysis also had several important strengths, including the availability of information on the duration of metformin use, molecular markers, and potential confounders. Our analysis was conducted in the context of a prospective clinical trial in which colon cancer was histologically confirmed, the treatment and follow-up protocols were standardized, and the date and nature of recurrence were recorded prospectively. Although the published data evaluating the relationship between metformin use and colon cancer risk are extensive, few studies have evaluated the association between metformin use and survival after a colon cancer diagnosis [12–14, 16]. Furthermore, the present study is the first to evaluate the effect of metformin among patient subgroups stratified by KRAS and BRAF mutation status.
Conclusion
Our study of patients with resected stage III colon cancer found that DFS, TTR, and OS were similar for metformin users and nonusers, independent of KRAS, BRAF mutation and MMR status. Metformin use was not associated with increased toxicity of adjuvant therapy in this patient population. Additional studies are required to fully elucidate the potential role of metformin use in colon cancer recurrence and patient outcome, and questions related to the dose, duration and timing of use remain unanswered. Although our data did not show an association of metformin use with survival in patients with stage III colon cancer, its ability to affect premalignancy or malignancy in larger or pooled patient cohorts remains an important area for further study.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
We acknowledge the accrual of patients by Balkrishna Jahagirdar, Metro-Minnesota National Cancer Institute Community Oncology Research Program, supported by Grant UG1CA189863. This work was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 (to the Alliance for Clinical Trials in Oncology) and U10CA82 (Alliance Statistics and Data Center), P30CA016058, U10CA025224, U10CA076001, U10 CA180820, U10CA180833, U10CA180835, U10CA180847, U10CA180850, and U10CA180888. Support was also received from the Division of Medical Oncology, Mayo Clinic, Rochester, Minnesota (to P.P.S. and S.R.A.). Support for correlative studies was also provided by unrestricted funds from Bristol-Myers Squibb, ImClone, Sanofi-Aventis, and Pfizer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Previously presented at the 2015 American Society of Clinical Oncology Annual Conference, Chicago, IL.
Author Contributions
Conception/Design: Preet Paul Singh, Qian Shi, Axel Grothey, Steven R. Alberts
Provision of study material or patients: Axel Grothey, Suresh G. Nair, Emily Chan, Anthony F. Shields, Richard M. Goldberg, Sharlene Gill, Morton S. Kahlenberg, Frank A. Sinicrope, Steven R. Alberts
Collection and/or assembly of data: Qian Shi, Nathan R. Foster, Frank A. Sinicrope, Daniel J. Sargent
Data analysis and interpretation: Preet Paul Singh, Qian Shi, Nathan R. Foster, Daniel J. Sargent
Manuscript writing: Preet Paul Singh, Qian Shi
Final approval of manuscript: Preet Paul Singh, Qian Shi, Nathan R. Foster, Axel Grothey, Suresh G. Nair, Emily Chan, Anthony F. Shields, Richard M. Goldberg, Sharlene Gill, Morton S. Kahlenberg, Frank A. Sinicrope, Daniel J. Sargent, Steven R. Alberts
Disclosures
Preet Paul Singh: Bayer Healthcare, Merrimack Pharma (C/A); Emily Chan: Taiho, Bayer, EMD Serono, Merrimack (C/A), Aduro, Bristol-Myers Squibb, Merrimack, Dekkun, Karyopharm, Boehringer Ingelheim (RF); Morton S. Kahlenberg: Genentech (H); Frank A. Sinicrope: EMD Serono (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol. 2013;31:359–364. doi: 10.1200/JCO.2012.44.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 5.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, et al. Metformin in cancer therapy: A new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 6.Ben Sahra I, Regazzetti C, Robert G, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 7.Rocha GZ, Dias MM, Ropelle ER, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res. 2011;17:3993–4005. doi: 10.1158/1078-0432.CCR-10-2243. [DOI] [PubMed] [Google Scholar]
- 8.Larsson O, Morita M, Topisirovic I, et al. Distinct perturbation of the translatome by the antidiabetic drug metformin. Proc Natl Acad Sci USA. 2012;109:8977–8982. doi: 10.1073/pnas.1201689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Chhipa RR, Pooya S, et al. Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci USA. 2014;111:E435–E444. doi: 10.1073/pnas.1311121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, Guo FC, Wang W, et al. K‑ras gene mutation as a predictor of cancer cell responsiveness to metformin. Mol Med Rep. 2013;8:763–768. doi: 10.3892/mmr.2013.1596. [DOI] [PubMed] [Google Scholar]
- 11.Singh S, Singh H, Singh PP, et al. Anti-diabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: A systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2013;22:2258–2268. doi: 10.1158/1055-9965.EPI-13-0429. [DOI] [PubMed] [Google Scholar]
- 12.Cossor FI, Adams-Campbell LL, Chlebowski RT, et al. Diabetes, metformin use, and colorectal cancer survival in postmenopausal women. Cancer Epidemiol. 2013;37:742–749. doi: 10.1016/j.canep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie CJ, Poole CD, Jenkins-Jones S, et al. Mortality after incident cancer in people with and without type 2 diabetes: Impact of metformin on survival. Diabetes Care. 2012;35:299–304. doi: 10.2337/dc11-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrett CR, Hassabo HM, Bhadkamkar NA, et al. Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer. 2012;106:1374–1378. doi: 10.1038/bjc.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Kim TI, Jeon SM, et al. The effects of metformin on the survival of colorectal cancer patients with diabetes mellitus. Int J Cancer. 2012;131:752–759. doi: 10.1002/ijc.26421. [DOI] [PubMed] [Google Scholar]
- 16.Spillane S, Bennett K, Sharp L, et al. A cohort study of metformin exposure and survival in patients with stage I-III colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1364–1373. doi: 10.1158/1055-9965.EPI-13-0347. [DOI] [PubMed] [Google Scholar]
- 17.Singh PP, Singh S, Gonsalves WI, Grothey A. Association of metformin with reduced mortality in patients with colorectal cancer: A systematic review and meta-analysis of observational studies. J Clin Oncol. 2014;32:522a [Google Scholar]
- 18.Domingo E, Laiho P, Ollikainen M, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet. 2004;41:664–668. doi: 10.1136/jmg.2004.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindor NM, Burgart LJ, Leontovich O, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–1048. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 22.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 23.Limburg PJ, Anderson KE, Johnson TW, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2005;14:133–137. [PubMed] [Google Scholar]
- 24.Yuhara H, Steinmaus C, Cohen SE, et al. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer. Am J Gastroenterol. 2011;106:1911–1922. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardiman KM, Liu J, Feng Y, et al. Rapamycin inhibition of polyposis and progression to dysplasia in a mouse model. PLoS One. 2014;9:e96023. doi: 10.1371/journal.pone.0096023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nangia-Makker P, Yu Y, Vasudevan A, et al. Metformin: A potential therapeutic agent for recurrent colon cancer. PLoS One. 2014;9:e84369. doi: 10.1371/journal.pone.0084369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan XL, Bhattacharyya KK, Dutta SK, et al. Metformin suppresses pancreatic tumor growth with inhibition of NFκB/STAT3 inflammatory signaling. Pancreas. 2015;44:636–647. doi: 10.1097/MPA.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch HA, Iliopoulos D, Tsichlis PN, et al. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher EJ, LeRoith D. Diabetes, cancer, and metformin: Connections of metabolism and cell proliferation. Ann N Y Acad Sci. 2011;1243:54–68. doi: 10.1111/j.1749-6632.2011.06285.x. [DOI] [PubMed] [Google Scholar]
- 31.Sinicrope FA, Shi Q, Smyrk TC, et al. Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes. Gastroenterology. 2015;148:88–99. doi: 10.1053/j.gastro.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iglesias DA, Yates MS, van der Hoeven D, et al. Another surprise from metformin: Novel mechanism of action via K-Ras influences endometrial cancer response to therapy. Mol Cancer Ther. 2013;12:2847–2856. doi: 10.1158/1535-7163.MCT-13-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]