This study compared the effectiveness of intensity-modulated radiotherapy (IMRT) and three-dimensional conformal radiotherapy (3DCRT) in patients with locally advanced non-small cell lung cancer (LA-NSCLC). Results showed that IMRT may confer superior local-regional progression-free survival and comparable overall survival than 3DCRT in LA-NSCLC, along with the reduction of pulmonary toxicity.
Keywords: Non-small cell lung cancer, Three-dimensional conformal radiation therapy, Intensity-modulated radiation therapy, Survival, Toxicity
Abstract
Background.
Consistent results are lacking as regards the comparative effectiveness of intensity-modulated radiotherapy (IMRT) versus three-dimensional conformal radiotherapy (3DCRT) in patients with locally advanced non-small cell lung cancer (LA-NSCLC).
Patients and Methods.
Patients treated with definitive radiotherapy (RT) between 2002 and 2010 were retrospectively reviewed. Overall survival (OS), local-regional progression-free survival (LRPFS), distant metastasis-free survival (DMFS), and progression-free survival (PFS) were compared among patients irradiated with different techniques. The association between RT technique and survival indexes was assessed in a Cox proportional hazard regression model. Propensity score matching (PSM) was used to balance known confounding factors.
Results.
A total of 652 patients were eligible for analysis, including 206 with 3DCRT and 446 with IMRT. The median OS of the 3DCRT and IMRT groups were 19.4 and 23.3 months, with the 5-year rate of 13% and 19%, respectively (p = .043). Multivariate analysis identified IMRT as an independent favorable factor associated with LRPFS and DMFS. PSM analysis further verified the beneficial effect of IMRT on LRPFS. No difference in OS or PFS was observed between the two techniques. Subgroup analysis revealed that IMRT might be differentially more effective in both OS and LRPFS among patients who were female, nonsmokers, with adenocarcinoma, or without weight loss. There was a significant reduction of lung toxicity and similar esophagus toxicity in the IMRT group when compared with the 3DCRT group.
Conclusion.
IMRT may confer superior LRPFS and comparable OS than can be achieved with 3DCRT in LA-NSCLC, along with the reduction of pulmonary toxicity.
Implications for Practice:
Based on the largest number of patients from a single institution, the present study demonstrated that intensity-modulated radiotherapy (IMRT) could provide superior local-regional progression-free survival and similar overall survival compared with the traditional three-dimensional conformal radiotherapy (3DCRT) for stage III non-small cell lung cancer (NSCLC). IMRT was also found to be associated with the significantly decreased incidence of pulmonary toxicity. These results suggest that IMRT should be considered a surrogate for 3DCRT in locally advanced NSCLC and might be the preferred option for a female nonsmoker with adenocarcinoma and a potentially high risk of pulmonary toxicity from radiotherapy.
Introduction
Combined modality of radiation therapy (RT) and cisplatin-based doublet chemotherapy is the predominant strategy for locally advanced non-small cell lung cancer (LA-NSCLC), and concomitant chemoradiotherapy is considered the optimal option for patients with good performance status [1]. Notwithstanding a lack of randomized controlled trials, several retrospective studies have consistently demonstrated the superiority of three-dimensional conformal radiation therapy (3DCRT) to two-dimensional RT regarding both tumor control and overall survival in NSCLC [2–4]. Nowadays, 3DCRT is considered the standard RT technique for NSCLC. This benefit that resulted from technique development motivated us to investigate whether there is further advantage of an even more advanced RT technique, such as intensity-modulated radiation therapy (IMRT).
Characterized by the improved dose conformality to tumor target and reduced normal tissue exposure, IMRT has been used widely in multiple solid tumors, such as head and neck and prostate cancer. However, multiple theoretical concerns have been raised with respect to the application of IMRT in lung cancer, such as the increased low-dose volume, interplay variation between multifields-based dose delivery and tumor motion, the reduced dose rate because of the prolonged dose delivery time within each fraction, etc. [5, 6]. Nevertheless, IMRT has still been increasingly implemented in lung cancer despite these concerns [7].
There are a lack of consistent results concerning the comparative effectiveness of IMRT versus 3DCRT in patients with LA-NSCLC. In this study, we aimed to compare the clinical outcomes, as well as radiation-related toxicities, between the patients with LA-NSCLC receiving 3DCRT and IMRT from a single academic cancer center. Furthermore, we sought to explore the subgroups of patients who may be more likely to gain benefit from IMRT.
Methods
Study Population
Patients with histologically proven stage III NSCLC (American Joint Committee on Cancer, 6th edition) and receiving 3DCRT or IMRT at our center between 2002 and 2010 were included. Patients were excluded if the radiation dose was <50 Gy or if they had received prior thoracic RT or surgery. Age, gender, pre-RT Karnofsky performance status (KPS), smoking status, history of weight loss, pathology, staging, positron emission tomography (PET) scan, treatment modality, RT technique, and radiation dose were retrospectively collected from the chart records. Chronologically, IMRT was used increasingly over the study period, with all IMRT patients treated after 2004. Respiratory symptom, swallow status, and corresponding imaging demonstrations during RT and within the first year after RT ended were also collected for the evaluation of radiation-related lung and esophagus toxicities. This study was approved by the local institutional review board.
Treatment Strategy
Four-dimensional computed tomography (CT) simulation was recommended, but not mandatorily used during the study period. A “forward planning” approach was used for 3DCRT by adjusting beam weight or angle with or without beam modifiers to conform to the target volume. With regard to IMRT planning, an “inverse planning” approach could achieve even greater conformality and spare nearby critical normal tissues by optimally modulating the individual segments. The dominant chemotherapy regimens concurrent with RT included etoposide/cisplatin and paclitaxel/carboplatin. Platinum-based doublet agents regimen were generally used for the sequential chemotherapy, including vinorelbine, paclitaxel, gemcitabine, pemetrexed, etc. In addition, three patients received tyrosine kinase inhibitor (TKI) after chest RT with unknown epidermal growth factor receptor mutation status.
Definition of Study Endpoints
Overall survival (OS), local regional progression-free survival (LRPFS), and distant metastasis-free survival (DMFS) were defined as the time from diagnosis until the first occurrence of specific event: death, local-regional recurrence, or distant metastasis, respectively. Progression-free survival (PFS) was defined as the duration between the cancer diagnosis and the date of any progression or cancer-related death. Patients without specific site of progression were censored at the date of last follow-up or non-cancer-related death. Radiation-induced lung toxicity (RILT) and radiation-induced esophagus toxicity (RIET) were assessed with Common Terminology Criteria for Adverse Events 3.0 criteria.
Statistical Analysis
The chi-square test was adopted for dichotomous data comparison between groups. Continuous variables were presented as mean ± SD and were compared by using the Mann-Whitney U test. The Kaplan-Meier method was used to estimate survival time and follow-up time, and the log-rank test was performed to examine the significance of difference. Cox proportional hazard regression model with backward selection was used to identify factors associated with survival variables and to calculate hazard ratios (HRs). Propensity score matching (PSM) was conducted to balance the confounding variables between 3DCRT and IMRT groups. A 1:1 nearest-neighbor match approach without replacement using a maximum caliper of 20% of the SD of the study population propensity score was performed after randomizing the order of patients in the database. All tests were two-sided, and p ≤ .05 was considered statistically significant. PSM and forest plots of subgroup analysis were performed with Stata 12.0 and other analyses with SPSS (Version 22.0).
Results
Patient Characteristics
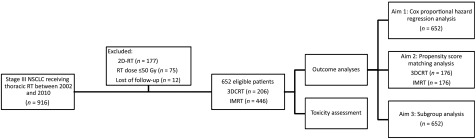
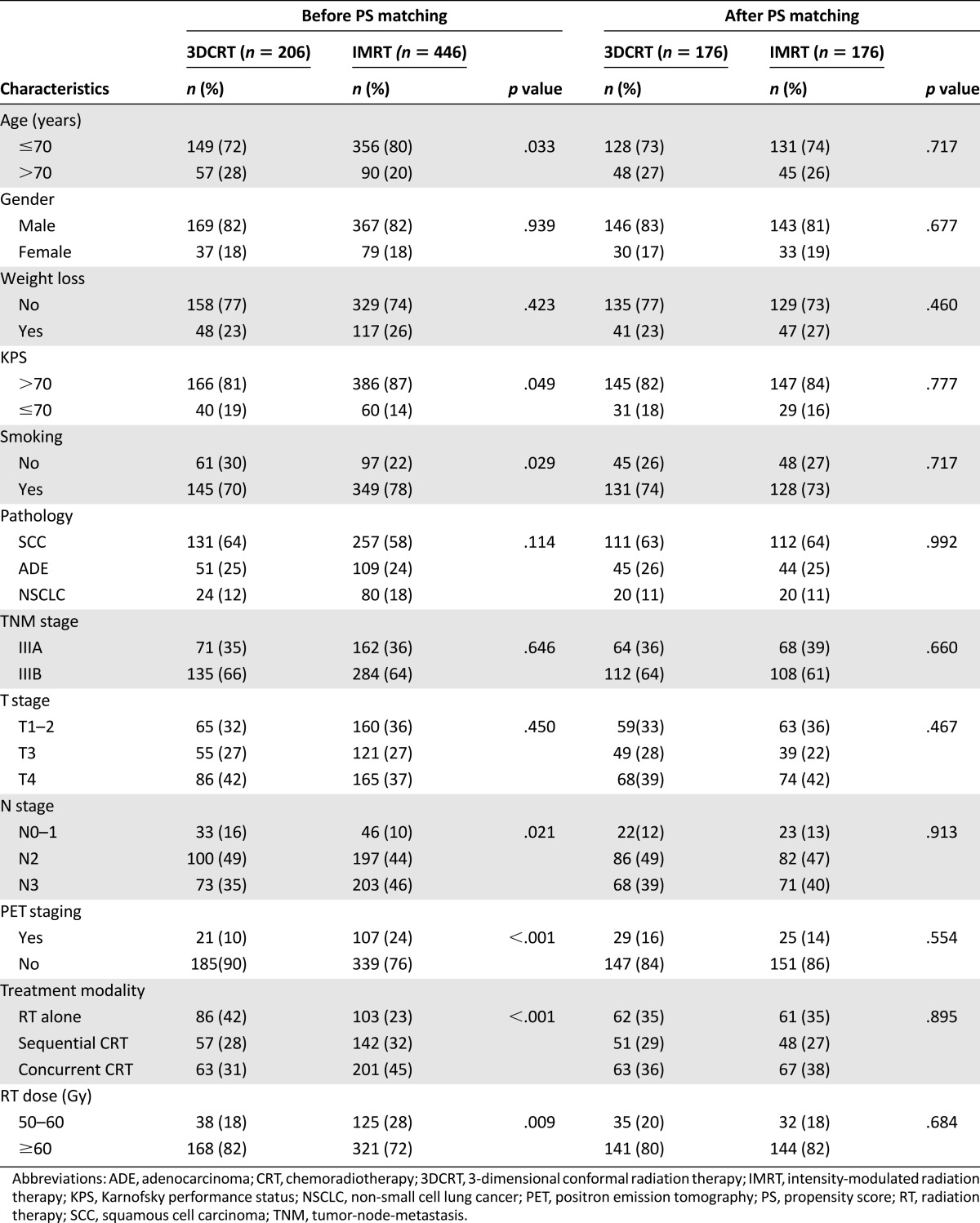
The design of study and composition of patient cohort are illustrated in Figure 1. Between January 2002 and December 2010, a total of 916 patients with stage III NSCLC received chest radiotherapy in our center, and 652 patients, including 206 with 3DCRT and 446 with IMRT, ultimately met the eligible criteria of this study. The median age of the study population was 62 (range: 25–88), and median RT dose was 60.0 Gy (range: 50.0–76.6 Gy). Detailed patient and treatment characteristics of two technique groups are shown in Table 1. IMRT group had a higher proportion of patients who were smokers, younger than 70, with better KPS, and with N3 disease. Furthermore, patients treated with IMRT were more likely to receive PET-based staging, concurrent chemoradiotherapy, and relatively lower RT dose. No significant difference was observed between the two cohorts regarding the distribution of gender, weight loss, pathological classification, overall stage, and T stage.
Figure 1.
Study design and formulation of patient cohort.
Abbreviations: 3DCRT, three-dimensional conformal radiation therapy; 2D-RT, two-dimensional radiation therapy; IMRT, intensity-modulated radiation therapy; NSCLC, non-small cell lung cancer; RT, radiation therapy.
Table 1.
Patient and treatment characteristics
Impact of RT Technique on Survival Among the Overall Cohort
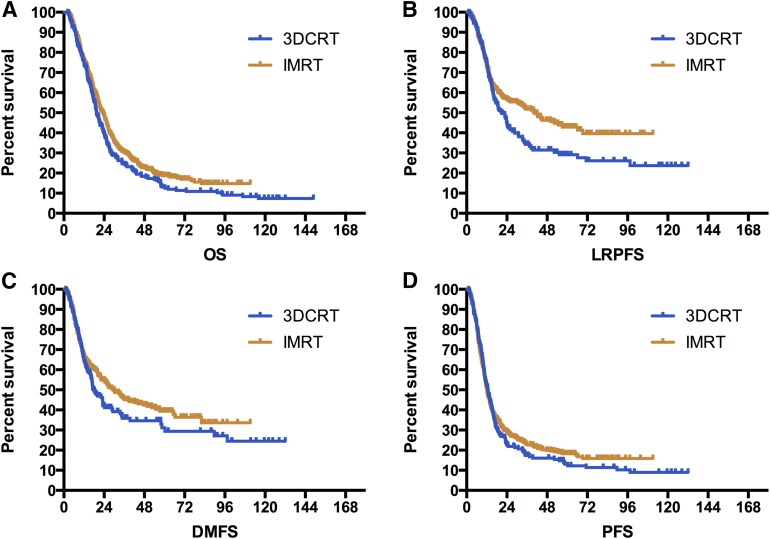
The median follow-up time was 72.1 months for overall patients. The median OS, LRPFS, DMFS, and PFS for the overall study population was 21.5, 30.8, 24.6, and 12.1 months, respectively, with the 5-year rate of 17%, 39%, 37%, and 17%. Univariate analysis identified a significant improvement of OS (HR = 0.83, 95% confidence interval [CI]: 0.70–0.99; median: 19.4 vs. 23.3 months; 5-year rate: 13% vs. 19%, p = .043) and LRPFS (HR = 0.75, 95% CI: 0.60–0.95; median: 21.0 vs. 40.5 months; 5-year rate: 29% vs. 43%, p = .017) in patients receiving IMRT. We also observed a DMFS benefit with a trend approaching significance for patients treated with IMRT (HR = 0.82, 95% CI: 0.65–1.03; median: 17.9 vs. 29.4 months; 5-year rate: 31% vs. 39%, p = .089). There was no difference in PFS between two techniques (HR = 0.92, 95% CI: 0.77–1.11; median: 12.8 vs. 12.0 months; 5-year rate: 13% vs. 18%, p = .397). The comparative survival curves for survival variables are shown in Figure 2.
Figure 2.
Survival curves of overall survival, local-regional progression-free survival, distant metastasis-free survival, and progression-free survival stratified by RT technique.
Abbreviations: 3DCRT, three-dimensional conformal radiation therapy; DMFS, distant metastasis-free survival; IMRT, intensity-modulated radiation therapy; LRPFS, local-regional progression-free survival; OS, overall survival; PFS, progression-free survival.
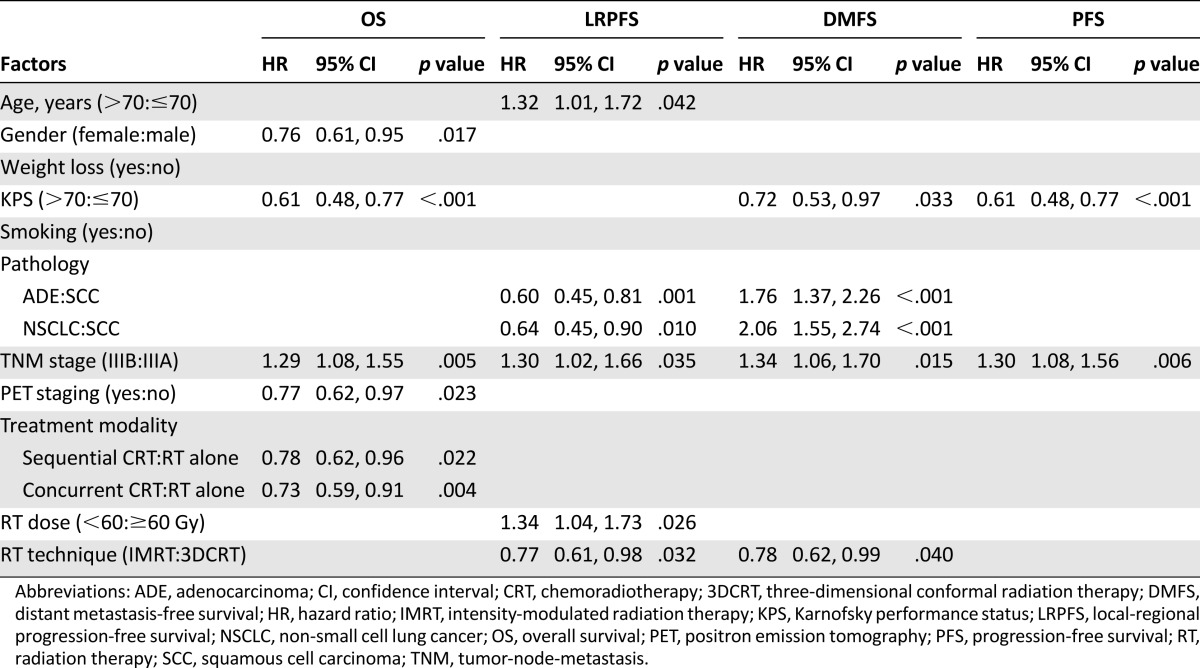
For multivariate analyses, independent factors associated with OS, LRPF, DMFS, and PFS are listed in Table 2. Unsurprisingly, tumor stage demonstrated significant association with all survival variables, with obvious outcome superiority in patients carrying IIIA diseases. Utilization of IMRT remained independently associated with improved LRPFS (HR = 0.77, 95% CI: 0.61–0.98, p = .032) and DMFS (HR = 0.78, 95% CI: 0.62–0.99, p = .040). However, IMRT failed to maintain its OS benefit and still had no correlation with PFS after adjusting for confounders. Besides tumor stage, female gender, better KPS, combined modality of chemoradiotherapy, and PET scan-based staging were also independently associated with improved OS. In addition to tumor stage and implementation of IMRT, younger age, nonsquamous cell carcinoma, and RT dose ≥ 60Gy were favorable predictors for LRPFS. In terms of DMFS, squamous cell carcinoma and KPS > 70 served as protective factors for better control of distant disease. Only tumor stage of IIIA and KPS > 70 were associated with improved PFS.
Table 2.
Multivariate Cox proportional hazard regression in overall study patients
Propensity Score-Matched Analysis
Factors used for propensity score matching consisted of age, gender, weight loss, KPS, smoking, pathology, overall stage, T stage, N stage, PET staging, treatment modality, and RT dose. Finally, the propensity score-matched cohorts included 176 patients for each technique group, with all patient and treatment variables being well balanced (Table 1). Consistent with the results in the overall study population, use of IMRT was correlated with significant improvement on LRPFS (HR = 0.71, 95% CI: 0.52–0.97, p = .029). However, we did not observe the benefit on OS (HR = 0.91, 95% CI: 0.72–1.14, p = .398), DMFS (HR = 0.87, 95% CI: 0.65–1.17, p = .350), or PFS (HR = 0.96, 95% CI: 0.76–1.22, p = .764) in favor of IMRT. Multivariate analysis based on the PSM cohort further confirmed that the use of IMRT was independently correlated with improved LRPFS (HR = 0.71, 95% CI: 0.52–0.96, p = .028), whereas no significant association was found between RT technique and other survival variables (p > .05).
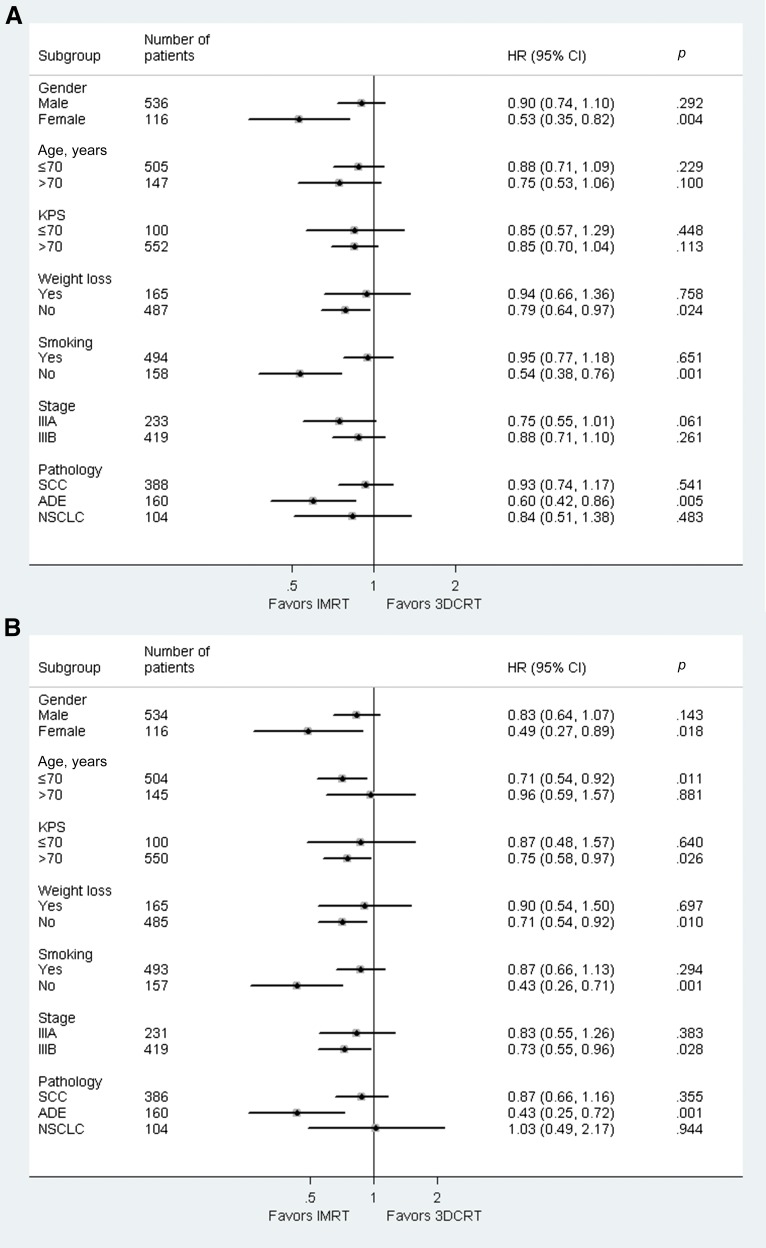
Subgroups Analysis Stratified by Clinical Factors
A subgroup analysis was undertaken to assess whether there was differential effect of IMRT in predefined subgroups of patients (Fig. 3A for OS and Fig. 3B for LRPFS). IMRT was differentially more effective on both OS and LRPFS among patients who were female, had adenocarcinoma, were nonsmokers, and were without weight loss. There was no clear evidence that IMRT could provide significant OS benefit in any group of patients stratified by age, KPS, or overall stage. With respect to LRPFS, the advantage of IMRT was also statistically significant in subgroups with age ≤ 70, KPS > 70, and IIIB diseases (p < .05).
Figure 3.
Subgroup analyses for overall survival (A) and local-regional progression-free survival (B).
Abbreviations: ADE, adenocarcinoma; CI, confidence interval; 3DCRT, three-dimensional conformal radiation therapy; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; KPS, Karnofsky performance status; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma.
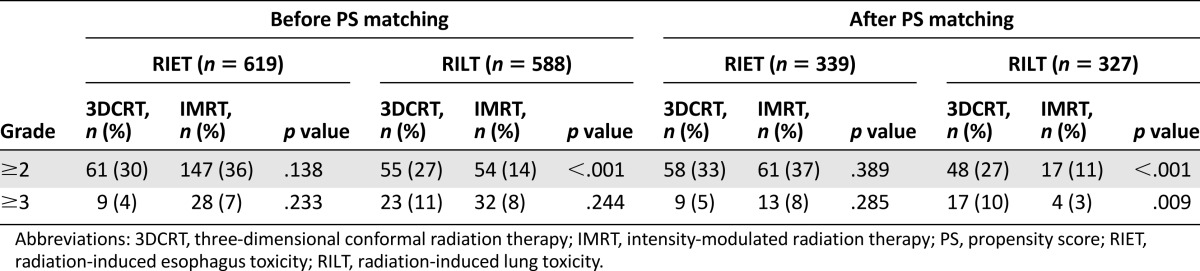
Toxicity Evaluation
A total of 619 patients (206 with 3DCRT vs. 413 with IMRT) and 588 patients (204 with 3DCRT vs. 384 with IMRT) were available for RIET and RILT assessment, respectively. Accordingly, 339 patients (176 with 3DCRT vs. 163 with IMRT) and 327 patients (175 with 3DCRT vs. 152 with IMRT) were eligible for toxicity evaluation in PSM-adjusted patients. The incidence of RIET and RILT before and after PS matching are listed in Table 3, showing that utilization of IMRT was correlated with significantly decreased occurrence of grade ≥ 2 RILT. In terms of grade ≥ 3 RILT, there was no differential incidence between two techniques based on the nonselected patients, whereas a significantly lower incidence with IMRT between PS-matched cohorts. No difference of RIET rate was observed between patients treated with IMRT and 3DCRT, regardless of toxicity grade. Preliminary dosimetric analysis showed similar percentage of volume receiving at least 20 Gy (V20) of normal lung between 3DCRT and IMRT groups, irrespective of before (24.9% ± 6.4% vs. 25.0% ± 4.5%, p = .992) or after (25.3% ± 6.1% vs. 25.2% ± 4.3%, p = .815) PS matching.
Table 3.
Incidence of radiation-related esophagus and lung toxicities
Discussion
In this retrospective study of LA-NSCLC patients treated with definitive RT, we found that patients receiving IMRT could gain a significantly improved local-regional progression-free survival and possibly longer distant metastasis-free survival compared with 3DCRT. There was no clear evidence of prolonged OS or PFS resulting from the advanced technique of IMRT. In addition, subgroup analyses indicated that patients who were female, nonsmokers, with pathological subtype of adenocarcinoma, or without weight loss may be more likely to benefit from IMRT regarding both OS and LRPFS. Meanwhile, the implementation of IMRT was correlated with reduction of radiation-induced lung toxicity and similar incidence of esophagus toxicity.
IMRT is a type conformal radiotherapy with a computer-aided optimization process to assign higher dose of radiation to tumor target, while better sparing the surrounding normal structures [8, 9]. However, whether IMRT has superiority to 3DCRT in lung cancer is still a matter of debate. In the U.S., IMRT comprised a slowly increasing proportion of conformal thoracic radiation for NSCLC, rising from 3.0% in 2002 to 26.8% in 2009, and 3DCRT remained the predominant radiotherapy modality [7]. The widespread use of IMRT in lung cancer is limited by several concerns [5, 6]. First, the increased conformality in dose distribution causes IMRT treatments to be much more sensitive to geometric and spatial changes of target volumes than the 3DCRT approach [10]. In addition, because IMRT is generally accomplished through multiple beamlets, only a portion of target is irradiated at a particular time. This interplay of target moving and leaf motion may lead to the dosimetric uncertainty [9]. Third, larger volumes of normal lung receiving low-dose irradiation may cause the potential of higher risk of radiation-related pulmonary toxicity and secondary malignancy in long-term survivors [9, 11, 12]. Last, the lower dose rate of IMRT caused by longer dose delivery time may be less lethal to cancer cells [5].
Despite theoretical concerns regarding the use of IMRT, clinical outcomes and accompanied normal tissue toxicities are essential factors in determining whether this approach can be used in lung cancer. To the best of our knowledge, five retrospective studies so far have reported the comparative effectiveness of IMRT versus 3DCRT with inconsistent results, and no randomized data have been reported yet [7, 9, 13–15]. MD Anderson Cancer Center first reported the comparative survival results between two techniques in treating LA-NSCLC. Multivariate analysis revealed notably improved overall survival (HR = 0.64) and similar local and distant control in patients receiving IMRT when compared to those with 3DCRT. Furthermore, grade ≥ 3 of lung toxicity was significantly reduced in patients treated with IMRT [9]. However, the follow-up time was relatively short, which may decrease the reliability of the difference between IMRT and 3DCRT. In 2014, two Surveillance, Epidemiology, and End Results (SEER) data-based studies both demonstrated similar OS and toxicity profile between two RT modalities. However, the authors admitted the limitation that pulmonary toxicity is difficult to ascertain from the SEER database because events are identified from diagnostic codes, which are less reliable than clinical records [7, 13]. RTOG 0617 included approximately one-half of patients treated with IMRT and others with 3DCRT. The results showed that, although it was more likely to be used to treat larger and less favorable tumors, IMRT was associated with a reduced risk of severe pneumonitis and similar OS and PFS as compared with 3DCRT [14]. A recently published retrospective study consisting of 145 patients with NSCLC observed similar local control and overall survival between cohorts treated with 3DCRT and IMRT, as well as a trend toward a lower rate of grade 2 or higher pneumonitis [15]. In summary, based on current evidence, IMRT-related survival outcome is at least as good as that which can be achieved with 3DCRT in LA-NSCLC without compromise of toxicity. However, the comparable survival and toxicity results cannot support the recommendation of IMRT as a surrogate for 3DCRT in LA-NSCLC from a cost-effectiveness point of view. Currently, there is still a lack of strong data regarding whether IMRT could improve clinical outcome or radiation-related toxicity in LA-NSCLC.
To the best of our knowledge, the present study consisted of the largest number of patients from a single institution with integrated patient, disease, treatment, and follow-up data, demonstrating comparable survival data to the contemporary report from MD Anderson Cancer Center (median OS of 21.6 months and 3-year OS rate of 30%) [16]. Controlling for patient and treatment characteristics, the Cox proportional hazard model demonstrated an advantageous LRPFS of IMRT compared with that of 3DCRT, which was further confirmed by the PSM model. Under the circumstances that trimodality therapy is being extensively investigated for treatment of LA-NSCLC, the relative additional benefit of surgery may be mitigated by the improved LRPFS from modern RT technique of IMRT [4, 17]. The intrinsic mechanism for the improved LRPFS is equivocal. One logical reason may be the capability of RT dose escalation with IMRT. However, in our study, RT dose was not higher in the IMRT group than what was delivered with conventional 3DCRT, and such benefits persisted after adjusting for dose factor with the Cox and PSM models. Besides LRPFS, the Cox model also suggested that IMRT might be an independent predictor for improved DMFS, whereas such benefit failed to be supported by the PSM model. Similar to the previous reports, IMRT did no better than 3DCRT with respect to OS or PFS [14].
In addition, we performed subgroup analysis to identify the appropriate candidates that may gain more significant benefit from IMRT and found some interesting hints. We found that the advantageous effect of IMRT on LRPFS and OS was consistently greater in patients with female gender, adenocarcinoma, no weight loss, and no smoking history compared with their counterparts, most of whom were well-known favorable prognostic factors [18, 19]. Nevertheless, given the retrospective nature of this study and the fact that subgroup analyses were actually undertaken based on univariate analysis, the inference from this exploratory analysis should be interpreted cautiously.
Consistent with the previous reports [9, 14, 20], a significant reduction of grade ≥ 2 pulmonary toxicity for the IMRT group was observed among both overall study population and PS-matched cohorts. However, unlike the previous reports showing a significantly decreased V20 for IMRT [8, 9, 21], our dosimetric data did not present a remarkable V20 reduction in the IMRT group as expected. This may be explained in part by the fact that there was higher proportion of N3 disease in IMRT group, which might consequently cause larger target volume. Nevertheless, because our study was not designed primarily to assess toxicities, detailed dosimetric analysis on toxicity was not performed in this study, although it will be further explored in another paper (unpublished data). With respect to esophagus toxicity, we identified no significant difference between RT techniques. A possible explanation of the similar episodes of esophagus toxicity may be that a higher proportion of concurrent chemoradiotherapy in the IMRT cohort mitigated the dosimetric advantage of IMRT.
Besides the inherent limitations of a retrospective study, we have to admit that the time-trend exists regarding the evolution of radiotherapy technique, with 3DCRT mostly used in the early years and IMRT implemented in later years. During this time span, changes in target identification, advancements in tumor motion control, and better management of treatment-related toxicities might also contribute to the outcome improvement of LA-NSCLC.
Conclusion
Despite multiple theoretical concerns regarding the use of IMRT in lung cancer, our data from a high-volume academic institution with abundant expertise in IMRT implementation suggest that IMRT is able to confer superior local-regional tumor control and similar overall survival compared with 3DCRT, along with a reduction of pulmonary toxicity, emphasizing the importance of modern IMRT technique in treating LA-NSCLC.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This study was supported by National Natural Science Foundation of China Grants 81272616 (to L.W.) and 81541157 (to J.W.).
This work was presented in part as a poster presentation at the 16th World Conference on Lung Cancer, Denver, Colorado, September 6–9, 2015.
Author Contributions
Conception/Design: Jingbo Wang, Luhua Wang
Provision of study material or patients: Zongmei Zhou, Jun Liang, Qinfu Feng, Zefen Xiao, Zhouguang Hui, Xiaozhen Wang, Jima Lv, Dongfu Chen, Hongxing Zhang, Luhua Wang
Collection and/or assembly of data: Jingbo Wang, Zongmei Zhou, Jun Liang, Qinfu Feng, Zefen Xiao, Zhouguang Hui, Xiaozhen Wang, Jima Lv, Dongfu Chen, Hongxing Zhang, Zhe Ji, Jianzhong Cao, Lipin Liu, Wei Jiang, Yu Men, Cai Xu
Data analysis and interpretation: Jingbo Wang, Jiangrong Dai, Weibo Yin, Luhua Wang
Manuscript writing: Jingbo Wang, Luhua Wang
Final approval of manuscript: Jingbo Wang, Zongmei Zhou, Jun Liang, Qinfu Feng, Zefen Xiao, Zhouguang Hui, Xiaozhen Wang, Jima Lv, Dongfu Chen, Hongxing Zhang, Zhe Ji, Jianzhong Cao, Lipin Liu, Wei Jiang, Yu Men, Cai Xu, Jiangrong Dai, Weibo Yin, Luhua Wang
Disclosures
The authors indicated no financial relationships.
References
- 1.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 2.Fang LC, Komaki R, Allen P, et al. Comparison of outcomes for patients with medically inoperable stage I non-small-cell lung cancer treated with two-dimensional vs. three-dimensional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:108–116. doi: 10.1016/j.ijrobp.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Cao JZ, Ou GF, Liang J, et al. Therapeutic efficacy of three-dimensional conformal radiation therapy for patients with locally advanced non-small cell lung cancer [in Chinese] Zhonghua Zhong Liu Za Zhi. 2011;33:529–534. [PubMed] [Google Scholar]
- 4.Sher DJ, Koshy M, Liptay MJ, et al. Influence of conformal radiotherapy technique on survival after chemoradiotherapy for patients with stage III non-small cell lung cancer in the National Cancer Data Base. Cancer. 2014;120:2060–2068. doi: 10.1002/cncr.28677. [DOI] [PubMed] [Google Scholar]
- 5.Chang JY. Intensity-modulated radiotherapy, not 3 dimensional conformal, is the preferred technique for treating locally advanced lung cancer. Semin Radiat Oncol. 2015;25:110–116. doi: 10.1016/j.semradonc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Price A. Intensity-modulated radiotherapy, not 3 dimensional conformal, is the preferred technique for treating locally advanced disease with high-dose radiotherapy: The argument against. Semin Radiat Oncol. 2015;25:117–121. doi: 10.1016/j.semradonc.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Chen AB, Li L, Cronin A, et al. Comparative effectiveness of intensity-modulated versus 3D conformal radiation therapy among medicare patients with stage III lung cancer. J Thorac Oncol. 2014;9:1788–1795. doi: 10.1097/JTO.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 8.Liu HH, Wang X, Dong L, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1268–1279. doi: 10.1016/j.ijrobp.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 9.Liao ZX, Komaki RR, Thames HD, Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz M, Van der Geer J, Van Herk M, et al. Impact of geometrical uncertainties on 3D CRT and IMRT dose distributions for lung cancer treatment. Int J Radiat Oncol Biol Phys. 2006;65:1260–1269. doi: 10.1016/j.ijrobp.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proc Natl Acad Sci USA. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Harris JP, Murphy JD, Hanlon AL, et al. A population-based comparative effectiveness study of radiation therapy techniques in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:872–884. doi: 10.1016/j.ijrobp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Chun SG, Hu C, Choy H, et al. Comparison of 3-D conformal and intensity modulated radiation therapy outcomes for locally advanced non-small cell lung cancer in NRG Oncology/RTOG 0617. Int J Radiat Oncol Biol Phys. 2015;93:S1–S2. [Google Scholar]
- 15.Ling DC, Hess CB, Chen AM, et al. Comparison of toxicity between intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy for locally advanced non-small-cell lung cancer. Clin Lung Cancer. 2016;17:18–23. doi: 10.1016/j.cllc.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang ZQ, Yang K, Komaki R, et al. Long-term clinical outcome of intensity-modulated radiotherapy for inoperable non-small cell lung cancer: The MD Anderson experience. Int J Radiat Oncol Biol Phys. 2012;83:332–339. doi: 10.1016/j.ijrobp.2011.06.1963. [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65:25–32. [PubMed] [Google Scholar]
- 19.Werner-Wasik M, Scott C, Cox JD, et al. Recursive partitioning analysis of 1999 Radiation Therapy Oncology Group (RTOG) patients with locally-advanced non-small-cell lung cancer (LA-NSCLC): Identification of five groups with different survival. Int J Radiat Oncol Biol Phys. 2000;48:1475–1482. doi: 10.1016/s0360-3016(00)00801-4. [DOI] [PubMed] [Google Scholar]
- 20.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer [published correction appears in Int J Radiat Oncol Biol Phys 2004;59:921] Int J Radiat Oncol Biol Phys. 2004;58:1258–1267. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]