This article discusses N-terminal domain (NTD) inhibition as a novel concept in the field of androgen receptor (AR)-directed therapies for prostate cancer. AR NTD-targeting agents have the potential to overcome shortcomings of current hormonal therapies by inhibiting all forms of AR-mediated transcriptional activity and, as a result, may affect a broader AR population, including mutational and splice variant ARs.
Keywords: EPI-506, Androgen receptor, N-terminal domain, Prostate cancer
Abstract
Despite the recent approval and widespread use of abiraterone acetate and enzalutamide for the treatment of castration-resistant prostate cancer (CRPC), this disease still poses significant management challenges because of various tumor escape mechanisms, including those that allow androgen receptor (AR) signaling to remain active. These AR-related resistance mechanisms include AR gene amplification or overexpression, constitutively active ligand-independent AR splice variants, and gain-of-function mutations involving the AR ligand-binding domain (LBD), among others. Therefore, the development of AR-targeted therapies that function independently of the LBD represents an unmet medical need and has the potential to overcome many of these resistance mechanisms. This article discusses N-terminal domain (NTD) inhibition as a novel concept in the field of AR-directed therapies for prostate cancer. AR NTD-targeting agents have the potential to overcome shortcomings of current hormonal therapies by inhibiting all forms of AR-mediated transcriptional activity, and as a result, may affect a broader AR population including mutational and splice variant ARs. Indeed, the first clinical trial of an AR NTD inhibitor is now underway.
Implications for Practice:
Because of emerging resistance mechanisms that involve the ligand-binding domain of the androgen receptor (AR), there is currently no effective treatment addressing tumor escape mechanisms related to current AR-targeted therapies. Many patients still demonstrate limited clinical response to current hormonal agents, and castration-resistant prostate cancer remains a lethal disease. Intense research efforts are under way to develop therapies to target resistance mechanisms, including those directed at other parts of the AR molecule. A novel small-molecule agent, EPI-506, represents a new pharmaceutical class, AR N-terminal domain inhibitors, and shows preclinical promise to overcome many known resistance mechanisms related to novel hormonal therapies.
Introduction
Prostate cancer is the second most prevalent cancer among men in the United States, leading to approximately 30,000 deaths annually [1]. The course of prostate cancer from diagnosis to death is usually categorized by clinical states based on extent of disease, hormonal status (i.e., castration resistance), and the presence or absence of detectable metastases on radiographic imaging [2]. Initial growth of prostate cancer depends highly on androgen signaling, which mediates its effects through the androgen receptor (AR), a transcription factor that regulates the expression of hundreds of genes including those involved in tumor cell growth and proliferation [3, 4]. Because of the dependency of the disease on androgen, androgen deprivation therapy (ADT) has been a mainstay of prostate cancer therapy for decades [5]. Although ADT can delay prostate cancer progression for several years, this treatment modality eventually becomes ineffective as patients develop castration-resistant prostate cancer (CRPC).
The management of patients with CRPC has evolved rapidly over the past 5 years with the advent of next-generation hormonal agents, such as abiraterone acetate (Zytiga) and enzalutamide (Xtandi). Despite these advances, additional treatment options are still needed to improve clinical outcomes and prolong survival of patients with CRPC, particularly those who have failed existing treatments or those who have contraindications or other limitations precluding the use of currently available drugs. Most patients with CRPC who fail current treatment options experience continued disease progression and may develop complications such as urinary obstruction and worsening pain, leading to substantial morbidity and limited survival rates. The development of resistance to current hormonal therapies and their potential underlying biology have been increasingly described [6–12]. In light of these evolving resistance mechanisms, CRPC remains a lethal disease with a particularly high unmet need in patients for whom existing treatment options are not effective.
Current Treatments for CRPC
Before 2011, the mainstay of treatment for CRPC was docetaxel-based chemotherapy. Since the approval of abiraterone and enzalutamide for the treatment of CRPC, the reported efficacy has been considerable; however, treatment failures are noted frequently with both agents. The vast majority of patients with CRPC demonstrate primary resistance (patients who do not respond to therapy upfront) or acquired resistance (patients who initially respond to therapy but then relapse) to these agents, with a gain in median overall survival of less than 6 months at the end of the treatment spectrum compared with standard of care [13–16]. It is estimated that approximately one-quarter of patients treated with abiraterone or enzalutamide will show primary resistance to these agents [13, 15–17]. Moreover, the development of acquired resistance will occur in nearly all patients with CRPC, even those who initially benefit from hormonal therapy.
The optimal sequence of therapies to maximize the clinical benefit for patients with CRPC remains undetermined. Clinical trials investigating the efficacy of chemotherapy after novel hormonal therapy, the efficacy of sequential or parallel use of novel hormonal therapy after chemotherapy, the efficacy of sequential use of novel hormonal therapies, and the efficacy of subsequent treatment after first-line novel hormonal therapy have not revealed definite answers [6, 9, 18–25]. Two randomized phase III trials (Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy [STAMPEDE] and ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer [CHAARTED]) support the upfront use of chemotherapy with ADT in men with hormone-sensitive metastatic prostate cancer [26, 27], particularly in patients with high-volume disease, which may impact the use of novel AR-targeted therapy in a subset of patients. Other studies exploring concomitant use of chemotherapy and ADT (GETUG-AFU-15), however, did not show a survival benefit [28]. In addition, a recent review suggests that the optimal sequencing of agents in CRPC is unclear because of the absence of robust surrogate measures of survival and the lack of predictive biomarkers [29]. Until there is a greater biological understanding of which patients may benefit from upfront use of chemotherapy, the impact on the landscape of using AR-directed therapies is largely unknown and remains to be evaluated.
CRPC Progression and Restored AR Signaling
The AR is structurally composed of an androgen-independent N-terminal domain (NTD), a DNA-binding domain (DBD), and an androgen-dependent ligand-binding domain (LBD). Androgens such as testosterone and dihydrotestosterone bind to the AR LBD, resulting in conformational changes and posttranslational modifications, dimerization, nuclear translocation, and ultimately, binding to the regulatory regions of the DNA of target genes, known as androgen response elements [30]. The transcriptional activity of the AR is also governed by complex epigenetic mechanisms involving coactivators and corepressors that help localize the AR to chromatin.
The AR signaling pathway remains essential for CRPC progression, and under conditions of androgen depletion, multiple mechanisms might lead to reactivation or restoration of AR signaling [31, 32]. Amplification of the AR gene is a mechanism that has been frequently observed clinically and is predominantly seen in response to treatment with ADT. AR overexpression and gene amplification have been reported to occur rarely in untreated primary prostate cancers, with observed frequency of 0%–5% [33–37], but the frequency increases significantly to 20%–52% in ADT-resistant populations [33–42].
Intracrine and paracrine androgen production has also been shown to contribute to continued AR stimulation in the castration-resistant state [43]. Studies evaluating tissue biopsies before and after initiation of abiraterone or enzalutamide have demonstrated increased levels of testosterone as a compensatory mechanism in the tumor tissue of men with CRPC [44–47]. This may result from conversion of weak androgens to potent androgens or de novo production of androgens within the tumor itself [48, 49].
AR splice variants have emerged as another potential mechanism associated with resistance, especially in the context of treatment with new hormonal agents, such as abiraterone and enzalutamide [4, 50–52]. The protein products of AR splice variants have an NTD that is required for transactivation, but have a truncated C-terminal domain in which the LBD is absent, resulting in ligand-independent constitutive activation [53–55]. Antiandrogen therapies are ineffective at inhibiting AR splice variants, because these target the LBD, which is truncated from the AR protein. The presence of AR splice variants, such as AR-V7 and AR-V567es, has been correlated with both primary and acquired resistance to antiandrogens and has been linked to more rapid disease recurrence, poor prognosis, and shorter survival [8, 54–56]. Of these, AR-V7 may be the most important, has been implicated in resistance to abiraterone and enzalutamide in men with advanced prostate cancer [8, 11, 57, 58], and may play a role in partial resistance to docetaxel as well [59].
Point mutations in the AR have been found more commonly in CRPC compared with primary or hormone-sensitive tumors [60, 61], and some have been shown to confer agonist properties and cross-resistance among antiandrogens [62, 63]. A recent prospective study conducting sequencing of bone and soft tissue tumor biopsies from a cohort of 150 patients with CRPC showed that AR mutations were enriched in CRPC compared with primary prostate cancer and that the majority of these mutations occurred in the LBD [61]. These mutations can increase the ligand-binding affinity of the AR and cause signaling hypersensitivity [64]. These gain-of-function mutations can also cause binding flexibility in the LBD, allowing the AR to become activated by adrenal androgens, progesterone, glucocorticoids, estrogens, and antiandrogens [62, 63, 65–71]. In view of the persistent role of the AR in CRPC, there is a high need for novel antagonists that address the adaptive AR aberrations that emerge following current hormonal therapies.
Current Therapies Target the AR C-Terminus
Current inhibitors of the AR function by lowering levels of circulating or intratumoral androgen or by preventing androgen binding to the AR, all of which depend on an intact C-terminal LBD. Abiraterone inhibits CYP17, a critical enzyme in the synthesis of testosterone, and has been shown to block androgen biosynthesis by the adrenal glands and testes and within the tumor. Treatment with abiraterone reduces serum testosterone levels from 20–50 ng/dL with surgical castration or gonadotropin-releasing hormone analogs alone to 1–2 ng/dL, leading to a “super-castration” state. In addition to decreases seen in the serum, CYP17 inhibition also leads to decreased intratumoral testosterone synthesis within the prostate cancer cells themselves [72], ultimately leading to an inhibition of AR activity. Enzalutamide is a next-generation AR antagonist with significantly increased affinity for the AR compared with previous AR antagonists such as bicalutamide. Furthermore, enzalutamide is reported to inhibit nuclear translocation and coactivator recruitment of the ligand-receptor complex, leading to effective inhibition of AR signaling [73].
Despite their impressive efficacy outcomes, resistance to abiraterone and enzalutamide occurs frequently [13–16], with most patients demonstrating primary or acquired resistance through various AR-dependent and -independent mechanisms. As a result, a number of new-generation CYP17 inhibitor and AR antagonist agents are in clinical development (Table 1). These agents have been shown to exhibit increased affinity to the AR, greater potency, decreased agonist properties, and multiple inhibitory functions compared with their predecessors [74–79]. However, these third-generation agents may potentially face the same issue of cross-resistance conferred by mutations in the LBD (either existing or yet unknown). In addition, these newer agents have yet to demonstrate clinical effectiveness against AR splice variants. In light of the current issues facing AR inhibitors that target the LBD, development of novel class of agents that target other domains of the AR is necessary. Agents targeting the AR NTD and DBD are currently in development, in addition to agents that can induce degradation of the AR protein (Table 1).
Table 1.
Investigational agents with potential activity against mechanisms of resistance in CRPC
The AR N-Terminal Domain as a Therapeutic Target
All current therapies that target the AR rely on the presence of its LBD. However, it is the NTD of the AR that harbors the critical region for AR transcriptional activity. Within the NTD lies the activation function-1 (AF1) region, which is essential for AR transactivation [80–82]. Deletions of this region render the AR transcriptionally inactive [80–82]. The AR NTD contains a high degree of intrinsic disorder because of few α-helices and β-sheets and, therefore, has been difficult to target using structure-based drug design [83]. However, in spite of these challenges, efforts to develop drugs that target the AR NTD are ongoing and have the potential to overcome shortcomings of current LBD-targeting therapies. A potential pharmacologic consequence of AR NTD inhibitors is the ability to affect a broader AR population. Because the NTD is required for all AR transcriptional activity and is present in all forms of the AR, targeting this critical region would be expected to inhibit the activity of resistance-related AR splice variants as well as AR species harboring gain-of-function LBD mutations. This is in contrast to current therapies, which can affect only AR populations that possess an intact LBD.
Recent efforts to develop drugs that target the AR NTD have yielded several compounds that are in preclinical or early clinical development (Table 1). The use of bispecific antibodies (bsAbs) to simultaneously bind to two different targets has shown promise, with at least two US Food and Drug Administration-approved agents to date [84]. 3E10-AR441 is a bsAb in preclinical development for CRPC that functions by penetrating prostate cancer cells via its affinity for DNA and, at the same time, binding to the AR NTD to inhibit AR signaling [85]. In vitro treatment of LNCaP prostate cancer cells with 3E10-AR441 demonstrated nuclear accumulation and target engagement of the bsAb. Immunoprecipitation assays in VCaP and 22Rv1 prostate cancer cell lines showed that 3E10-AR441 was able to bind full-length AR as well as splice-variant AR lacking the LBD (AR-V7) [85]. In addition, 3E10-AR441 was shown to block AR signaling in both reporter gene-based assays and assays that monitor endogenous levels of prostate-specific antigen (PSA). To date, no information on the impact of this agent on tumor growth has been released; however, the current data indicate the potential of 3E10-AR441 bsAb as a therapeutic agent that targets the AR in a manner that does not rely on the LBD.
Sintokamides have also been shown to display inhibitory activity against the AR NTD. These chlorinated peptides, isolated from the marine sponge Dysidea sp., were identified through a screening of marine natural extracts and show inhibition of AR activity as measured by reporter gene-based assays [86]. In addition, sintokamides effectively blocked proliferation of LNCaP prostate cancer cells but not PC3 prostate cancer cells, which do not express AR, indicating that the inhibitory effect of sintokamides on cell proliferation was likely caused by its effect on the AR and not via cell cytotoxicity. Further characterization of sintokamides will be useful in assessing these agents as a potential AR-targeted therapy.
Agents directed at preventing the AR NTD from properly initiating transcription are also currently being explored. At least four compounds (GSK525762, GS-5829, OTX015, and JQ1) are in development for CRPC that target bromodomain-containing protein 4 (BRD4), a member of the bromodomain extraterminal (BET) family of proteins. BRD4 is a coregulator of the AR and interacts with the AR NTD to facilitate transcriptional activity [87]. BRD4 inhibitors have been shown to block BRD4-AR interactions and prevent binding of both full-length and splice variant AR to chromatin, thereby impairing transcription of downstream genes [87, 88]. BRD4 inhibition also induced apoptosis and cell-cycle arrest in AR-driven prostate cancer cell lines (VCaP, LNCaP, and 22Rv1), but not in cell lines that are negative for AR signaling (PC3, DU145) [87]. In vivo, BRD4 blockade was shown to significantly reduce tumor volume and weight in VCaP xenograft mice compared with enzalutamide. In addition, BRD4 inhibitors can suppress the transcription of c-Myc, a key mediator of ligand-independent prostate cancer growth and a ligand-independent AR target gene [89, 90]. Together, these data show the potential for BET protein inhibitors as novel treatments for CRPC that function by blocking BRD4 contact with the AR NTD and do not depend on AR-ligand interactions.
EPI Compounds: Direct AR NTD Inhibitors
The EPI family of compounds was originally discovered from a marine sponge extract. They bind specifically to Tau-5 within the AF1 region of the AR NTD and have been shown to block protein-protein interactions of the AF1 region with CREB-binding protein and the large subunit of the transcription factor TFIIF (RAP74) [91, 92]. In vitro experiments using LNCaP prostate cancer cells that endogenously express AR show that treatment with EPI blocked AR-driven transcriptional activity both in the presence and absence of androgen [91, 93]. In addition to inhibition of full-length AR, EPI was shown to inhibit transcriptional activity of AR splice variants [93, 94]. In LNCaP cells transfected with a plasmid to overexpress AR-V567es, EPI treatment resulted in inhibition of constitutive AR-V567es-driven transcriptional activity [93]. By contrast, bicalutamide and enzalutamide were unable to inhibit transcriptional activity driven by AR-V567es [93]; bicalutamide and enzalutamide target the LBD, which is absent from AR splice variants.
In response to androgen, full-length AR regulates the transcription of well-characterized target genes including PSA, FKBP5, TMPRSS2, and NKX3.1 [4]. The transcriptome of AR splice variants may have some overlap with that of full-length AR, but splice variants also regulate expression of a distinct set of genes [4]. AR splice variants, such as AR-V7, preferentially increase the expression levels of genes such as UBE2C, CDC20, CYCLINA2, and AKT1 [4]. Consistent with upstream blockade of both full-length and splice variant AR transcriptional activities, EPI treatment inhibits gene expression that is regulated by both full-length and AR-V7 in LNCaP95 and VCaP cells, whereas enzalutamide and bicalutamide had no effect, respectively [93, 94]. Notably, both LNCaP95 cells and VCaP cells endogenously express the full-length AR and AR-V7 protein [4]. An adaptive shift to AR-V7 signaling is suggested to occur in androgen-depleted environments and with antiandrogen treatment [4]. Thus, LNCaP95 and VCaP cells represent an important population of mixed full-length and splice variant ARs that may be reflective of human CRPC.
Consistent with targeting the AR NTD without reliance on the LBD for AR inhibition, EPI did not compete with androgen in a competitive ligand-binding assay [91]. Increasing concentrations of unlabeled synthetic androgen, bicalutamide, and EPI were used to compete with fluorescent-labeled androgen for binding to the AR LBD. Increasing concentrations of both synthetic androgen and bicalutamide displaced the fluorescent-labeled androgen and competed for the ligand-binding pocket. By contrast, EPI did not affect binding of the fluorescent-labeled androgen, regardless of androgen concentration [91]. In another study, elevated androgen levels were shown to compete for, and reverse, the inhibitory effect of enzalutamide [73]. Thus, the reversible binding of antiandrogens to the AR may indicate the reason for their possible failure when intratumoral androgen becomes elevated in CRPC. In contrast, EPI neither targets the AR LBD nor does it compete for binding to the LBD. Thus, EPI compounds possess a unique mechanism of action and do not depend on the presence of the LBD.
The potential therapeutic benefits of EPI have been demonstrated using a variety of human prostate cancer cell lines and xenograft models in castrated male mice. The EPI compounds have been shown to block AR-dependent proliferation of human prostate cancer cells, but have no effect on the viability of cells that do not rely on AR signaling for growth and survival [91, 93]. EPI was shown to block growth of tumor xenografts that express full-length AR as well as xenografts that express both full-length and splice variant AR in castrated male mice [91, 93, 94]. In contrast, EPI had no effect on PC3 prostate cancer xenografts [91] that are insensitive to androgen and do not express functional AR. Importantly, EPI blocked tumor growth of enzalutamide-resistant LNCaP95 xenograft tumors [94], demonstrating an efficacy potential that may be superior to that of antiandrogens. In addition, EPI was shown to inhibit other clinically relevant resistance mechanisms, such as gain-of-function point mutations in the AR and overexpressed transcriptional coactivators [94], further supporting its capability to target a broad range of AR-dependent drivers of tumor growth. Furthermore, in an exploratory toxicology assessment in mice, no toxicity was observed in animals treated systemically with EPI: no loss of body weight, no changes in behavior, and no pathologic changes in the histology of internal organs [91, 93]. Based on specificity of this agent to its target, apparent lack of toxicity, and antitumor activity in preclinical models, these data suggest that EPI is a promising anticancer agent in CRPC.
Clinical Development of EPI-506
EPI-506 is a novel small-molecule potent inhibitor of the AR NTD that is currently under investigation for the treatment of metastatic CRPC (mCRPC). EPI-506 is related to the EPI compound family originally discovered by functional assay screening of marine sponge extracts [91] and is the first AR NTD inhibitor to enter human clinical development. The discovery compound was EPI-001, which is a mixture of four stereoisomers, each of which has the same chemical constitution, but different spatial orientation of its constituent atoms. The most potent stereoisomer is EPI-002. EPI-506, the clinical candidate with desired pharmaceutical properties, is a prodrug of EPI-002.
The EPI compounds have important mechanistic differences from current AR-targeted therapies in that EPI directly inhibits the essential function of the AR, which is its transcriptional activity. By targeting the NTD, EPI has the ability to inhibit AR splice variants and LBD-mutant ARs that have been implicated in resistance to current therapies (Fig. 1). The unique mechanism of action for EPI-506 suggests that EPI-506 and other potential NTD inhibitors may have a different pharmacological action. Although there are several investigational agents under development for patients with CRPC who are failing abiraterone and/or enzalutamide, many of these agents are LBD-targeting drugs that have similar mechanisms of action to abiraterone and enzalutamide and will potentially face the same issues of cross-resistance conferred by AR splice variants and AR LBD mutations. By comparison, EPI-506 is anticipated to overcome these resistance mechanisms and may be effective in CRPC driven by both canonical and aberrant AR signaling by targeting the NTD common to full-length, splice variant, and LBD-mutated AR isoforms.
Figure 1.
Targeting the androgen receptor: N-terminal domain versus ligand-binding domain. EPI’s unique mechanism of action confers its ability to affect a broader AR population, including mutational and splice variant ARs implicated in resistant metastatic castration-resistant prostate cancer. (A) EPI targets the AR N-terminal domain, a region critical for AR transactivation, whereas current AR-directed therapies target the LBD and prevent androgens from binding. (B) EPI inhibits activity of constitutively active, truncated AR splice variants that lack the LBD. In contrast, current AR-directed therapies can only affect AR populations that have an intact LBD. (C) Mutations in the LBD have been shown to confer agonist activity to antiandrogens. EPI inhibition occurs despite the presence of these gain-of-function mutations in the AR.
Abbreviations: AR, androgen receptor; LBD, ligand-binding domain.
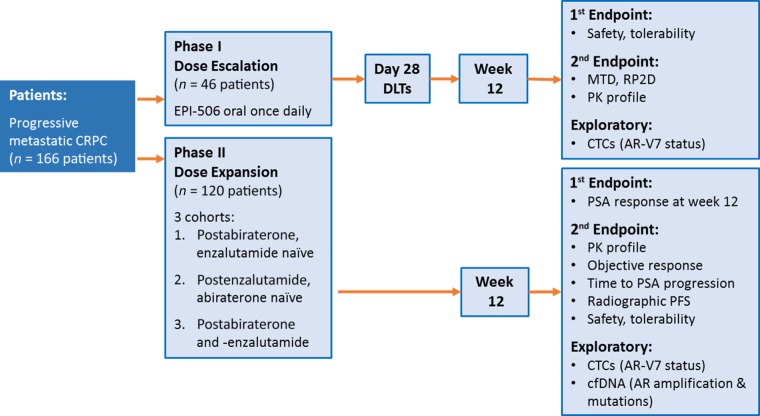
A phase I/II study of EPI-506 is currently ongoing in men with mCRPC with progression after enzalutamide and/or abiraterone (NCT02606123). This open-label, single-arm study will evaluate the benefit of 12-week once-a-day oral dosing with EPI-506, after establishing the safety, pharmacokinetics, and optimal dose of EPI-506 in single- and multiple-dose escalations. The phase I portion of the study will follow an adaptive 3 + 3 dose escalation design. The phase II portion of the study will evaluate activity in 3 patient populations: postenzalutamide but abiraterone-naïve patients with mCRPC, postabiraterone but enzalutamide-naïve patients with mCRPC, and postenzalutamide and -abiraterone patients with mCRPC (Fig. 2). The planned total enrollment is approximately 166 patients. Inclusion criteria include mCRPC with progression of disease after one or more lines of hormonal therapy or taxane chemotherapy, and progression of disease while under treatment with enzalutamide or abiraterone. Exclusion criteria include hematologic, hepatic, or renal insufficiency. Primary endpoints include PSA response rate at week 12, defined as a ≥50% PSA decrease from baseline. Secondary endpoints include pharmacokinetics, objective Response Evaluation Criteria in Solid Tumors (RECIST) response rate, time to PSA progression, radiographic progression-free survival, and safety/tolerability. Exploratory endpoints include evaluation of biomarkers of AR-driven treatment resistance, including AR splice variants and AR LBD mutations, with circulating tumor cell-based and plasma-derived cell-free DNA-based methodologies [62, 95]. Information gathered will be used to evaluate the clinical activity of EPI-506 in the context of known AR resistance mechanisms. This clinical study will be the first to evaluate the novel AR NTD inhibitor EPI-506 in men with mCRPC who have failed enzalutamide and/or abiraterone. EPI-506 is the first agent with the potential to inhibit both canonical and variant-mediated AR signaling.
Figure 2.
Phase I/II study of EPI-506: clinical trial design. This open-label, single-arm phase I/II study will evaluate the benefit of 12-week once-daily dosing with EPI-506. The phase I portion of the study will establish the safety, pharmacokinetics, and optimal dose of EPI-506. The phase II portion of the study will evaluate activity in three patient populations: postabiraterone but enzalutamide-naïve patients with metastatic CRPC, postenzalutamide but abiraterone-naïve patients with metastatic CRPC, and postabiraterone and -enzalutamide patients with metastatic CRPC. This study will be the first to evaluate the novel AR N-terminal domain inhibitor EPI-506 in men with metastatic CRPC who have failed enzalutamide and/or abiraterone.
Abbreviations: AR, androgen receptor; cfDNA, cell-free DNA; CRPC, castration-resistant prostate cancer; CTC, circulating tumor cell; DLT, dose-limiting toxicity; MTD, maximum tolerated dose; PFS, progression-free survival; PK, pharmacokinetics; PSA, prostate-specific antigen; RP2D, recommended phase II dose.
Conclusion and Future Perspectives
Despite significant recent advances in the treatment of CRPC, many patients demonstrate limited clinical response or develop secondary progression despite treatment with currently available next-generation drugs. Given the heterogeneity of mechanisms that may contribute to progression of CRPC, ongoing and future trials should consider approaches to optimize delivery of care to CRPC patients who are most likely to benefit. One such approach is to incorporate the longitudinal tracking of disease (both genotypic and phenotypic) so that a change in therapy may be triggered at the time actionable biomarkers are detected. Several trials evaluating the clinical utility of AR-V7 as a putative biomarker for informing treatment decisions in CRPC are ongoing and are summarized in a recent review [96]. Most recently, Scher et al. reported on the clinical validation of AR-V7 protein as a treatment-specific biomarker that may be associated with superior survival on taxane therapy over AR-directed therapy in men with AR-V7+ mCRPC [97]. Such biomarker-driven studies not only will help direct caregivers to using the right treatment at the right time, but also will reduce the time, burden, cost, and unnecessary toxicities experienced by patients undergoing ineffective therapies [98].
Among the multiple pathways that drive CRPC, the heterogeneity within AR-driven CRPC alone is extensive. To this end, intense research efforts are underway to target other parts of the AR molecule, namely the AR NTD and AR DBD, and to degrade the AR protein itself. As the focus of this article is on the AR NTD as a novel therapeutic approach, one future challenge will be to link potential therapeutic responses to NTD inhibitors with integrated molecular characterization of the AR-ome from liquid biopsies [99], which could possibly lead to a promising, precise, oncology-based approach to CRPC treatment. The ongoing phase I/II clinical trial of the first AR NTD inhibitor, EPI-506, includes an evaluation of EPI-506 in the context of known AR resistance mechanisms, including AR-V7, using liquid biopsies. The results generated from this study will be timely and provide proof-of-concept that targeting the AR NTD may provide a means to overcome resistance pathways driven by the AR, or that broader therapeutic approaches, such as combinations of targeted AR agents, are needed to adequately address the heterogeneity of AR-driven CRPC.
Acknowledgments
Emmanuel S. Antonarakis has received funding from the Prostate Cancer Foundation and the Patrick C. Walsh Fund and National Institutes of Health Grants R01 CA185297 and P30 CA006973. Jun Luo is currently funded by a Prostate Cancer Foundation grant, NIH Grant R01 CA185297, and U.S. Department of Defense Prostate Cancer Research Program Grants W81XWH-13-2-0093 and W81XWH-15-2-0050.
Author Contributions
Conception/Design: Emmanuel S. Antonarakis, Chandtip Chandhasin, Frank Perabo
Manuscript writing: Emmanuel S. Antonarakis, Chandtip Chandhasin, Erica Osbourne, Jun Luo, Marianne D. Sadar, Frank Perabo
Final approval of manuscript: Emmanuel S. Antonarakis, Chandtip Chandhasin, Erica Osbourne, Jun Luo, Marianne D. Sadar, Frank Perabo
Disclosures
Emmanuel S. Antonarakis: Janssen, Astellas, Sanofi, Dendreon, ESSA, Medivation (C/A), Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai (RF), Tokai (IP); Chandtip Chandhasin: ESSA Phamaceuticals Corp. (E); Erica Osbourne: ESSA Pharmaceuticals Corp. (E); Jun Luo: Tokai, Sun Pharma (C/A), Mirati, Gilead, Sanofi, Orion, Astellas (RF), A&G, Tokai (IP); Marianne D. Sadar: ESSA Pharma Inc. (C/A, RF, E, OI, IP); Frank Parebo: Essa Pharmaceuticals Corp. (E, OI).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.American Cancer Society. Cancer Facts and Figures, 2015. Available at http://www.cancer.org/Research/CancerFactsStatistics/cancerfactsfigures2015/cancer-facts-and-figures-2015. Accessed April 1, 2016.
- 2.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huggins C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann Surg. 1942;115:1192–1200. doi: 10.1097/00000658-194206000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng HH, Gulati R, Azad A, et al. Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis. 2015;18:122–127. doi: 10.1038/pcan.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianchini D, Lorente D, Rodriguez-Vida A, et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur J Cancer. 2014;50:78–84. doi: 10.1016/j.ejca.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azad AA, Eigl BJ, Murray RN, et al. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol. 2015;67:23–29. doi: 10.1016/j.eururo.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen FB, Røder MA, Rathenborg P, et al. Enzalutamide treatment in patients with metastatic castration-resistant prostate cancer progressing after chemotherapy and abiraterone acetate. Scand J Urol. 2014;48:268–275. doi: 10.3109/21681805.2013.860189. [DOI] [PubMed] [Google Scholar]
- 11.Yu Z, Chen S, Sowalsky AG, et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20:1590–1600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azad AA, Leibowitz-Amit R, Eigl BJ, et al. A retrospective, Canadian multi-center study examining the impact of prior response to abiraterone acetate on efficacy of docetaxel in metastatic castration-resistant prostate cancer. Prostate. 2014;74:1544–1550. doi: 10.1002/pros.22872. [DOI] [PubMed] [Google Scholar]
- 13.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138–148. doi: 10.1056/NEJMoa1209096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 18.Maughan BL, Xhou XC, Suzman DL, et al. Optimal sequencing of docetaxel and abiraterone in men with metastatic castration-resistant prostate cancer. Prostate. 2015;75:1814–1820. doi: 10.1002/pros.23064. [DOI] [PubMed] [Google Scholar]
- 19.Nadal R, Tsai HL, Sinibaldi VJ, et al. Prognostic factors for clinical outcomes in patients with metastatic castration resistant prostate cancer treated with sequential novel androgen receptor-directed therapies. Prostate. 2016;76:512–520. doi: 10.1002/pros.23141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadal R, Zhang Z, Rahman H, et al. Clinical activity of enzalutamide in Docetaxel-naïve and Docetaxel-pretreated patients with metastatic castration-resistant prostate cancer. Prostate. 2014;74:1560–1568. doi: 10.1002/pros.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schweizer MT, Zhou XC, Wang H, et al. The influence of prior abiraterone treatment on the clinical activity of docetaxel in men with metastatic castration-resistant prostate cancer. Eur Urol. 2014;66:646–652. doi: 10.1016/j.eururo.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzman DL, Luber B, Schweizer MT, et al. Clinical activity of enzalutamide versus docetaxel in men with castration-resistant prostate cancer progressing after abiraterone. Prostate. 2014;74:1278–1285. doi: 10.1002/pros.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24:1807–1812. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 24.Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24:1802–1807. doi: 10.1093/annonc/mdt138. [DOI] [PubMed] [Google Scholar]
- 25.Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65:30–36. doi: 10.1016/j.eururo.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 26.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 29.Lorente D, Mateo J, Perez-Lopez R, et al. Sequencing of agents in castration-resistant prostate cancer. Lancet Oncol. 2015;16:e279–e292. doi: 10.1016/S1470-2045(15)70033-1. [DOI] [PubMed] [Google Scholar]
- 30.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silberstein JL, Taylor MN, Antonarakis ES. Novel insights into molecular indicators of response and resistance to modern androgen-axis therapies in prostate cancer. Curr Urol Rep. 2016;17:29. doi: 10.1007/s11934-016-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701–711. doi: 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bubendorf L, Kononen J, Koivisto P, et al. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999;59:803–806. [PubMed] [Google Scholar]
- 34.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 35.Miyoshi Y, Uemura H, Fujinami K, et al. Fluorescence in situ hybridization evaluation of c-myc and androgen receptor gene amplification and chromosomal anomalies in prostate cancer in Japanese patients. Prostate. 2000;43:225–232. doi: 10.1002/(sici)1097-0045(20000515)43:3<225::aid-pros9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Visakorpi T, Hyytinen E, Koivisto P, et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 37.Visakorpi T, Kallioniemi AH, Syvänen AC, et al. Genetic changes in primary and recurrent prostate cancer by comparative genomic hybridization. Cancer Res. 1995;55:342–347. [PubMed] [Google Scholar]
- 38.Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–677. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linja MJ, Savinainen KJ, Saramäki OR, et al. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 41.Qu X, Randhawa G, Friedman C, et al. A novel four-color fluorescence in situ hybridization assay for the detection of TMPRSS2 and ERG rearrangements in prostate cancer. Cancer Genet. 2013;206:1–11. doi: 10.1016/j.cancergen.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 2012;30:637–643. doi: 10.1200/JCO.2010.33.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:53–60. doi: 10.1016/j.eururo.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 47.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knuuttila M, Yatkin E, Kallio J, et al. Castration induces up-regulation of intratumoral androgen biosynthesis and androgen receptor expression in an orthotopic VCaP human prostate cancer xenograft model. Am J Pathol. 2014;184:2163–2173. doi: 10.1016/j.ajpath.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 50.Nakazawa M, Antonarakis ES, Luo J. Androgen receptor splice variants in the era of enzalutamide and abiraterone. Horm Cancer. 2014;5:265–273. doi: 10.1007/s12672-014-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Chan SC, Brand LJ, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mostaghel EA, Marck BT, Plymate SR, et al. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–5925. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo Z, Qiu Y. A new trick of an old molecule: Androgen receptor splice variants taking the stage?! Int J Biol Sci. 2011;7:815–822. doi: 10.7150/ijbs.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hörnberg E, Ylitalo EB, Crnalic S, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakazawa M, Lu C, Chen Y, et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26:1859–1865. doi: 10.1093/annonc/mdv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinestel J, Luedeke M, Arndt A, et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. 2015 doi: 10.18632/oncotarget.3925. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antonarakis ES, Lu C, Luber B, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koochekpour S. Androgen receptor signaling and mutations in prostate cancer. Asian J Androl. 2010;12:639–657. doi: 10.1038/aja.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: Biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21:2315–2324. doi: 10.1158/1078-0432.CCR-14-2666. [DOI] [PubMed] [Google Scholar]
- 63.Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3:1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 64.Gregory CW, Johnson RT, Jr, Mohler JL, et al. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001;61:2892–2898. [PubMed] [Google Scholar]
- 65.Chen G, Wang X, Zhang S, et al. Androgen receptor mutants detected in recurrent prostate cancer exhibit diverse functional characteristics. Prostate. 2005;63:395–406. doi: 10.1002/pros.20191. [DOI] [PubMed] [Google Scholar]
- 66.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 67.Taplin ME, Bubley GJ, Shuster TD, et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 68.Eisermann K, Wang D, Jing Y, et al. Androgen receptor gene mutation, rearrangement, polymorphism. Transl Androl Urol. 2013;2:137–147. doi: 10.3978/j.issn.2223-4683.2013.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hay CW, McEwan IJ. The impact of point mutations in the human androgen receptor: classification of mutations on the basis of transcriptional activity. PLoS One. 2012;7:e32514. doi: 10.1371/journal.pone.0032514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen EJ, Sowalsky AG, Gao S, et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin Cancer Res. 2015;21:1273–1280. doi: 10.1158/1078-0432.CCR-14-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7:312re10. doi: 10.1126/scitranslmed.aac9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bradbury RH, Acton DG, Broadbent NL, et al. Discovery of AZD3514, a small-molecule androgen receptor downregulator for treatment of advanced prostate cancer. Bioorg Med Chem Lett. 2013;23:1945–1948. doi: 10.1016/j.bmcl.2013.02.056. [DOI] [PubMed] [Google Scholar]
- 75.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72:1494–1503. doi: 10.1158/0008-5472.CAN-11-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loddick SA, Ross SJ, Thomason AG, et al. AZD3514: A small molecule that modulates androgen receptor signaling and function in vitro and in vivo. Mol Cancer Ther. 2013;12:1715–1727. doi: 10.1158/1535-7163.MCT-12-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moilanen AM, Riikonen R, Oksala R, et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci Rep. 2015;5:12007. doi: 10.1038/srep12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Njar VC, Brodie AM. Discovery and development of Galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancer. J Med Chem. 2015;58:2077–2087. doi: 10.1021/jm501239f. [DOI] [PubMed] [Google Scholar]
- 79.Toren PJ, Kim S, Pham S, et al. Anticancer activity of a novel selective CYP17A1 inhibitor in preclinical models of castrate-resistant prostate cancer. Mol Cancer Ther. 2015;14:59–69. doi: 10.1158/1535-7163.MCT-14-0521. [DOI] [PubMed] [Google Scholar]
- 80.Jenster G, van der Korput HA, van Vroonhoven C, et al. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991;5:1396–1404. doi: 10.1210/mend-5-10-1396. [DOI] [PubMed] [Google Scholar]
- 81.Jenster G, van der Korput HA, Trapman J, et al. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995;270:7341–7346. doi: 10.1074/jbc.270.13.7341. [DOI] [PubMed] [Google Scholar]
- 82.Simental JA, Sar M, Lane MV, et al. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J Biol Chem. 1991;266:510–518. [PubMed] [Google Scholar]
- 83.McEwan IJ. Intrinsic disorder in the androgen receptor: Identification, characterisation and drugability. Mol Biosyst. 2012;8:82–90. doi: 10.1039/c1mb05249g. [DOI] [PubMed] [Google Scholar]
- 84.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 85.Goicochea NL, Garnovskaya M, Blanton M, et al. Cell-penetrating bispecific antibodies for targeting androgen receptor signaling in advanced prostate cancer. Cancer Res. 2015;75(suppl 15) 642a. [Google Scholar]
- 86.Sadar MD, Williams DE, Mawji NR, et al. Sintokamides A to E, chlorinated peptides from the sponge Dysidea sp. that inhibit transactivation of the N-terminus of the androgen receptor in prostate cancer cells. Org Lett. 2008;10:4947–4950. doi: 10.1021/ol802021w. [DOI] [PubMed] [Google Scholar]
- 87.Asangani IA, Dommeti VL, Wang X, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014;510:278–282. doi: 10.1038/nature13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan SC, Selth LA, Li Y, et al. Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015;43:5880–5897. doi: 10.1093/nar/gkv262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wyce A, Degenhardt Y, Bai Y, et al. Inhibition of BET bromodomain proteins as a therapeutic approach in prostate cancer. Oncotarget. 2013;4:2419–2429. doi: 10.18632/oncotarget.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gao L, Schwartzman J, Gibbs A, et al. Androgen receptor promotes ligand-independent prostate cancer progression through c-Myc upregulation. PLoS One. 2013;8:e63563. doi: 10.1371/journal.pone.0063563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andersen RJ, Mawji NR, Wang J, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–546. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- De Mol E, Fenwick RB, Phang CT et al. EPI-001, a compound active against castration-resistant prostate cancer, targets transactivation Unit 5 of the androgen receptor. ACS Chem Biol 2016 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 93.Myung JK, Banuelos CA, Fernandez JG, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123:2948–2960. doi: 10.1172/JCI66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang YC, Banuelos CA, Mawji NR, et al. Targeting androgen receptor activation function-1 with EPI to overcome resistance mechanisms in castration-resistant prostate cancer. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2901. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kelvin J, Lu D, Packer D, et al. Single cell analysis of AR N-terminal, AR C-terminal, and the ARv7 splice variant in the CTCs of metastatic castration resistant prostate cancer (mCRPC) patients. Cancer Res. 2015;75(suppl 15):1588a. [Google Scholar]
- 96.Antonarakis ES, Armstrong AJ, Dehm SM, et al. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis. 2016;19:231–241. doi: 10.1038/pcan.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1828. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowski MC, Frick KD, Eshleman JR et al. Cost-savings analysis of AR-V7 testing in patients with metastatic castration-resistant prostate cancer eligible for treatment with abiraterone or enzalutamide. Prostate 2016 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 99.Schweizer MT, Antonarakis ES. Liquid biopsy: Clues on prostate cancer drug resistance. Sci Transl Med. 2015;7:312fs45. doi: 10.1126/scitranslmed.aad4008. [DOI] [PubMed] [Google Scholar]