Abstract
Brandi et al. clarify data about the localization of human equilibrative nucleoside transporter 1 in cancer patients receiving gemcitabine-based chemotherapy. They discuss methodology, differences between antibodies, and recommendations for further study.
We are very pleased about the interest of Meijer et al. [1] in our article recently published on The Oncologist [2]. This is the first study intended for the evaluation of the putative predictive role of human equilibrative nucleoside transporter 1 (hENT-1) localization in cancer patients receiving gemcitabine-based chemotherapy. The results obtained in our retrospective study strongly suggest that the localization of hENT-1 on the tumor cell membrane may play a central role in the response to this drug in cholangiocarcinoma (CC) patients undergoing adjuvant gemcitabine.
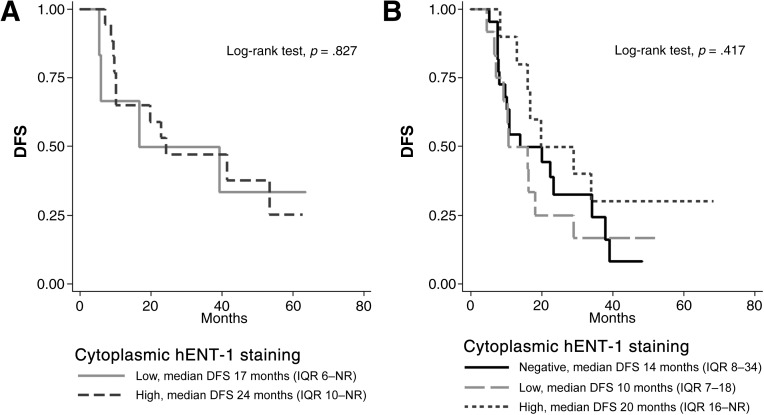
To further support the hypothesis that hENT-1 localization (and not the amount of its protein expression) is the most prominent factor in modulating the response to such adjuvant treatment, here we present an additional descriptive analysis of disease-free survival (DFS) in the same CC patient population of our previous study [2], stratified according to membrane hENT-1 immunoreactivity and cytoplasmic staining intensity (Fig. 1A, 1B). Among patients with positive membrane hENT-1 staining (Fig. 1A), we observed that DFS was not affected by the intensity of cytoplasmic staining (high vs. low). As to subjects with negative membrane hENT-1 staining (Fig. 1B), patients with high cytoplasmic staining showed a slightly longer DFS than those with negative or low staining, although this finding was not supported by statistical significance (p-values of log-rank test for pairwise comparisons always >0.1). On balance, we believe that our findings show that membrane hENT-1 staining is the most important factor predictive of response to gemcitabine; perhaps, among patients with negative membrane staining, the amount of hENT-1 present in the cytoplasm could still play a minor role, but larger study populations are needed to confirm or rule out this hypothesis.
Figure 1.
DFS of 71 CC patients who received adjuvant gemcitabine chemotherapy after surgical resection, stratified according to membrane hENT-1 immunoreactivity (positive and negative) and cytoplasmic hENT-1 staining intensity (negative, low, or high). (A): Positive membrane hENT-1 staining. (B): Negative membrane hENT-1 staining.
Abbreviations: CC, cholangiocarcinoma; DFS, disease-free survival; hENT-1, human equilibrative nucleoside transporter 1; IQR, interquartile range; NR, not reached.
Furthermore, a major issue raised by Meijer et al. is the current lack of standard techniques and antibodies for hENT-1 detection in cancer tissues, a condition that may be responsible for the controversial results obtained in both retrospective and prospective studies. In our study, we used immunohistochemistry (IHC) analysis, because it represents the most suitable methodology for our purpose, namely the assessment of hENT-1 intracellular localization in tumor tissue. Notably, compared to staining intensity evaluation (as reported in previous studies), the assessment of protein localization in tissue samples associates with a minor risk of possible bias based on pathologist experience (this parameter being less susceptible to individual interpretation), thus representing an easier and more reproducible biomarker to be validated and used in clinical practice.
An open question is still the use of the most suitable antibody when assessing hENT-1 staining in tumor tissue samples. The rabbit polyclonal hENT-1 antibody used in our study differs from the mouse monoclonal antibody used in previous studies. Compared to monoclonal antibodies, polyclonal antibodies are less specific but more sensitive, because they are able to recognize different epitopes of the same antigen. Because hENT-1 is a transmembrane nucleoside transporter, proper localization on the cell membrane requires correct protein processing, trafficking, and folding [3]. The mechanisms underlying these processes are still poorly understood, but it could be hypothesized that during these processes the epitope recognized by the mouse monoclonal antibody when hENT-1 is localized in the cytoplasm could become no more accessible for binding when this transporter translocates to the cell membrane. Conversely, the ability of polyclonal antibodies to bind different epitopes could allow hENT-1 detection when it is also localized on the cell membrane, as occurred in our study.
In summary, the different sensibility in recognizing the epitopes of the same antigen between mouse monoclonal and rabbit polyclonal antibodies calls for additional studies comparing hENT-1 staining by IHC in the same tissue samples with the two types of antibodies.
These studies are needed even more in light of the functional role of hENT-1 in cells (i.e., intracellular uptake of nucleosides required for DNA synthesis), to better elucidate not only the predictive role, but also the potential prognostic role of hENT-1 in cholangiocarcinoma and other malignancies.
Acknowledgments
The Gruppo Italiano Colangiocarcinoma (G.I.CO.) members are Giovanni Brandi, Giuseppe Aprile, Stefano Cereda, Lorenzo Fornaro, Francesco Leone, Sara Lonardi, Daniele Santini, Nicola Silvestris, and Enrico Vasile.
Contributor Information
Collaborators: Giovanni Brandi, Giuseppe Aprile, Stefano Cereda, Lorenzo Fornaro, Francesco Leone, Sara Lonardi, Daniele Santini, Nicola Silvestris, and Enrico Vasile
Disclosures
The authors indicated no financial relationships.
References
- 1.Meijer LL, Puik JR, Peters GJ, et al. hENT-1 expression and localization predict outcome after adjuvant gemcitabine in resected cholangiocarcinoma patients. The Oncologist. 2016;21:e4. doi: 10.1634/theoncologist.2016-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandi G, Deserti M, Vasuri F, et al. Membrane localization of human equilibrative nucleoside transporter 1 in tumor cells may predict response to adjuvant gemcitabine in resected cholangiocarcinoma patients. The Oncologist. 2016;21:600–607. doi: 10.1634/theoncologist.2015-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nivillac NM, Bacani J, Coe IR. The life cycle of human equilibrative nucleoside transporter 1: From ER export to degradation. Exp Cell Res. 2011;317:1567–1579. doi: 10.1016/j.yexcr.2011.03.008. [DOI] [PubMed] [Google Scholar]