Skin reactions are the most common adverse events attributable to epidermal growth factor receptor (EGFR) inhibitors. This review leads to the recommendation of prophylactic—rather than reactive—management of skin reactions for all patients receiving EGFR inhibitors because appropriate prophylaxis could effectively reduce the severity of skin reactions.
Keywords: EGFR inhibitors, Skin reactions, Prophylactic care, Algorithms
Abstract
Inhibition of the epidermal growth factor receptor (EGFR) is an established treatment that extends patient survival across a variety of tumor types. EGFR inhibitors fall into two main categories: anti-EGFR monoclonal antibodies, such as cetuximab and panitumumab, and first-generation tyrosine kinase inhibitors, such as afatinib, gefitinib, and erlotinib. Skin reactions are the most common EGFR inhibitor-attributable adverse event, resulting in papulopustular (acneiform) eruptions that can be painful and debilitating, and which may potentially have a negative impact on patients’ quality of life and social functioning, as well as a negative impact on treatment duration. Shortened treatment duration can, in turn, compromise antineoplastic efficacy. Similarly, appropriate management of skin reactions is dependent on their accurate grading; however, conventional means for grading skin reactions are inadequate, particularly within the context of clinical trials. Treating a skin reaction only once it occurs (reactive treatment strategies) may not be the most effective management approach; instead, prophylactic approaches may be preferable. Indeed, we support the viewpoint that prophylactic management of skin reactions should be recommended for all patients treated with EGFR inhibitors. Appropriate prophylactic management could effectively reduce the severity of skin reactions in patients treated with EGFR inhibitors and therefore has the potential to directly benefit patients and improve drug adherence. Accordingly, here we review published and still-emerging data, and provide practical and evidence-based recommendations and algorithms regarding the optimal prophylactic management of EGFR inhibitor-attributable skin reactions.
Implications for Practice:
Epidermal growth factor receptor (EGFR) inhibitors extend patient survival across a variety of tumor types. The most common EGFR inhibitor-attributable adverse events are skin reactions. Prophylactic—rather than reactive—management of skin reactions for all patients receiving EGFR inhibitors should be recommended because appropriate prophylaxis could effectively reduce the severity of skin reactions; thus, the derivation of highly effective prophylactic strategies has the potential to directly benefit patients. Accordingly, a review of the available data leads to practical and evidence-based recommendations and algorithms regarding the optimal prophylactic management of EGFR inhibitor-attributable skin reactions.
Introduction
Inhibition of the epidermal growth factor receptor (EGFR) has proven to be an effective treatment option in a variety of cancers, including metastatic colorectal cancer (mCRC) [1], squamous cell carcinoma of the head and neck (SCCHN) [2], non-small cell lung cancer (NSCLC) [3], and pancreatic cancer [4], leading to increased overall survival. The purpose of this review is to provide practicing oncologists with a concise, up-to-date, and accessible set of recommendations for prophylactically managing skin reactions resulting from EGFR inhibitor-based therapy. By summarizing key findings on the topic and compiling them into an updated, convenient algorithm, we endeavor to benefit patients via facilitating improved clinical management of their EGFR inhibitor-attributable skin reactions. Broadly speaking, EGFR inhibitors can be categorized as either monoclonal antibodies (mAbs) or tyrosine kinase inhibitors (TKIs). We will focus on those mAbs (cetuximab and panitumumab) and first-generation TKIs (afatinib, erlotinib, and gefitinib) for which data regarding prophylactic management of their associated skin reactions are already available. Thus, although we appreciate the clinical importance of the mAb necitumumab and the TKIs lapatinib and osimertinib, these newer EGFR inhibitors will not be specifically discussed here.
mAbs, such as cetuximab and panitumumab, function by binding to the EGFR receptor, preventing endogenous ligand binding and thereby dampening downstream signaling. Cetuximab is approved for the first- and later-line treatment of patients with RAS wild-type mCRC and locally advanced or recurrent/metastatic SCCHN [1, 2, 5]. In contrast, panitumumab is approved only for the first- and later-line treatment of patients with RAS wild-type mCRC [6], because no survival benefit has been demonstrated for patients with either locally advanced or recurrent/metastatic SCCHN [7, 8]. Although both panitumumab and cetuximab are mAbs that target EGFR, they are immunologically distinct (IgG2 vs. IgG1, respectively) and have somewhat different binding sites on the EGFR; thus, they may not be identical in their actions. Notably, in vitro studies suggest that induction of antibody-dependent cellular cytotoxicity is associated with cetuximab, but not with panitumumab.
First-generation TKIs, such as afatinib, erlotinib, and gefitinib, bind to the kinase domain of EGFR, preventing its phosphorylation and blocking downstream signaling cascades. Afatinib is approved for the first-line treatment of metastatic NSCLC [9]. Erlotinib is approved for maintenance and second-line treatment of locally advanced and metastatic NSCLC [10, 11], as well as the first-line treatment, in combination with gemcitabine, of patients with locally advanced, unresectable, or metastatic pancreatic cancer [4]. Gefitinib is approved for the first-line treatment of patients with EGFR mutation-positive metastatic NSCLC [12, 13].
Although efficacious across a wide variety of tumor types, EGFR inhibitors also possess a predictable and manageable adverse event (AE) profile. The most commonly reported AE for both EGFR-targeting mAbs and first-generation TKIs is acneiform rash, which is typically mild or moderate, but may be severe in up to 18% of patients [14, 15]. Some believe that the incidence and severity of rash may be worse in patients treated with anti-EGFR mAbs versus first-generation TKIs [16–20]; however, there are no available data to suggest that the management of EGFR inhibitor-attributable skin reactions differs notably between the various mAbs and first-generation TKIs. Although anecdotal evidence has suggested that panitumumab-associated skin reactions often occur more frequently than those attributable to cetuximab, investigators have found that the incidence of AEs of any grade and grade 3/4 AEs were similar between cetuximab- and panitumumab-treated patients with mCRC in the randomized, third-line, monotherapy ASPECCT study, the only head-to-head trial comparing panitumumab and cetuximab. Of particular interest in ASPECCT, 13% of patients treated with panitumumab and 10% of those treated with cetuximab developed grade 3/4 skin reactions [21, 22]. Furthermore, cetuximab-associated [23] and panitumumab-associated [24] skin reactions generally start within the first 3 weeks of initiating treatment for mCRC.
Some believe that the incidence and severity of rash may be worse in patients treated with anti-EGFR mAbs versus first-generation TKIs; however, there are no available data to suggest that the management of EGFR inhibitor-attributable skin reactions differs notably between the various mAbs and first-generation TKIs.
The development of skin reactions often follows a predictable time course during therapy. Patients usually experience edema and erythema during the first weeks of treatment, followed by papulopustular (acneiform) eruptions and crusting; later effects include paronychia and fissure [25, 26]. Skin reactions are transient, abating after the completion of therapy, although postinflammatory changes (erythema or pigmentation) may persist. As discussed in greater detail below, clinical pathogenesis of skin reactions is assayed according to various guidelines, each with unique strengths and weaknesses; however, a simple, straightforward, and accurate evaluation of the severity of skin reactions has not yet been established.
Because EGFR inhibitor-related skin reactions can potentially be painful and disfiguring, the occurrence of skin toxicities may negatively impact quality of life (QoL) and social functioning. For example, in one study of 283 patients who received targeted or nontargeted therapies, patients completed the Skindex-16 questionnaire, a dermatologic-specific QoL evaluation of symptoms, emotions, and function. The authors found that patients receiving targeted therapies had significantly poorer symptom, emotion, and function subscores on the Skindex-16 questionnaire, relative to those receiving nontargeted therapies [27]. Similarly, findings from the Cancer and Leukemia Group B/Southwest Oncology Group 80405 trial suggested that, although overall QoL was comparable between treatment arms, dermatologic-related QoL was worse in patients with mCRC treated with chemotherapy plus cetuximab versus patients treated with chemotherapy plus bevacizumab [28]. At present, it seems prudent to conclude that skin reactions appear to lead to a negative impact on QoL, but our methods for capturing this impact are highly variable in their effectiveness [6, 29–31]. Of interest, the development of a new Functional Assessment of Cancer Therapy questionnaire specific for EGFR inhibitor-attributable skin reactions may aid in unequivocally resolving the impact of skin reactions on patients’ QoL and social functioning [32–37].
EGFR inhibitor-related skin reactions can also potentially impact treatment duration, leading to treatment interruptions and/or early cessation of therapy. More specifically, in cases where a patient’s skin toxicity reaches grade 3 or higher on the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI CTCAE v4.0) scale, physicians can choose to lower the dose of the drug, temporarily interrupt treatment, or terminate EGFR inhibitor therapy entirely [38]. Furthermore, patients may request to discontinue treatment because of skin reactions resulting from therapy [10]. Because patients aged less than 50 years are more likely to experience a negative emotional effect from dermatological AEs, they may face a higher risk of reduced efficaciousness of EGFR inhibitors due to treatment interruptions and/or discontinuation [27, 39].
The most common treatment for EGFR inhibitor-related skin reactions is the oral or topical application of tetracycline-family antibiotics and corticosteroids, alone or in combination with moisturizers and sunscreen [40–48]. These therapeutics, which include doxycycline and minocycline, are commonly used to treat acneiform rash and help reduce its symptoms. The implementation of this care has historically only been reactive to (i.e., subsequent to) development of the skin reaction; however, as outlined in greater detail below, we espouse the viewpoint that prophylactic management of skin reactions should be recommended for all patients treated with EGFR inhibitors. Indeed, appropriate prophylactic management could effectively reduce the severity (although perhaps not incidence) of skin reactions in patients treated with EGFR inhibitors, and therefore has the potential to directly benefit patients and improve drug adherence. Reactive management of EGFR inhibitor-associated skin reactions has been reviewed extensively elsewhere [49–52] and will not be discussed here; instead, we will focus on optimal deployment of prophylactic measures to combat EGFR inhibitor-attributable skin reactions.
Materials and Methods
When developing this nonsystematic review article, we searched PubMed and American Society of Clinical Oncology and European Society for Medical Oncology meeting abstracts to identify relevant studies. The search window encompassed January 2004–January 2016; search terms included “EGFR inhibitor skin reactions,” “cetuximab skin reactions,” “panitumumab skin reactions,” “afatinib skin reactions,” “erlotinib skin reactions,” “gefitinib skin reactions,” “prophylactic care of skin reactions,” and “skin reaction algorithms.” Priority was granted to randomized clinical trials. There were no formal inclusion or exclusion criteria, and the outputs of our searches were hand curated. No unpublished material is included in this review article.
Methods for Grading Skin Reactions
A major issue that complicates the proper management of EGFR inhibitor-associated skin reactions—particularly in the clinical trial setting—is that conventional means for grading skin reactions are inadequate. One key issue concerns the need for a clear definition as to what constitutes a “skin reaction,” because many subcategories of this broad term are sometimes included, and this can vary among studies.
In this review, we will discuss three methods for grading skin reactions: the NCI CTCAE v4.0, the Multinational Association of Supportive Care in Cancer (MASCC) EGFR Inhibitor Skin Toxicity Tool (MESTT©), and a three-part system suggested by Wollenberg et al. [53]. Each of these systems will be described below, along with its limitations.
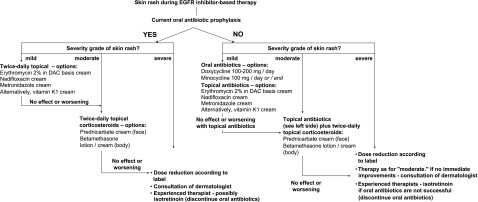
One method for grading skin reactions is the NCI CTCAE v4.0, which defines acneiform rash as a disorder characterized by an eruption of papules and pustules, typically appearing on the face, scalp, upper chest, and back. Skin reactions can be categorized as grade 1–5 per this scoring system (Table 1). A key issue with the NCI CTCAE v4.0 system is that “the rule of 9”—which relates to the percentage of the body that is affected—dictates that even locally severe rashes could potentially be scored very low on the scale (grade 1/2), because the area typically affected by rash would not reach the 30% of body surface area (BSA) required by the current guidelines to be scored as grade 3 or higher. For example, face-only rash (even if severe, disfiguring, and/or having an impact on patients’ lives) tends to be graded no higher than grade 1 (although this rash can still be categorized as grade 3 in situations in which self-care in activities of daily living is limited). Overall, although the CTCAE scale allows for the flexibility of upgrading an AE beyond the grade dictated by affected BSA guidelines, this decision is left up to the discretion of the physician, which can create inconsistencies.
Table 1.
NCI CTCAE v4.0 grading criteria
A second skin-reaction grading system is the MESTT. This method for grading skin reactions takes into consideration patient-reported data, such as QoL, and also includes time and effect on therapy dose of the experienced AEs. MESTT includes guidelines pertaining to papulopustular eruption, nail changes, erythema, pruritus, xerosis, hair changes, flushing, telangiectasia, and hyperpigmentation. However, the MESTT system is overly complex, with 17 possible evaluable measures, making it difficult for physicians to complete the evaluation in routine clinical practice, even within the context of a clinical trial.
Finally, a third method for grading skin reactions is the three-part system proposed by Wollenberg et al. [53]. This scoring system takes into account the NCI CTCAE score on the body surface, the percentage of skin involvement on the face (which is easily ascertained), and a readily accessible composite of five items of information on the most highly affected region of the body. Notably, the three-part system is well suited for clinical trials and perhaps also for routine clinical practice; for example, it is currently being evaluated alongside the NCI CTCAE v4.0 system in two phase III clinical trials (NCT01345526 and NCT01668498). Such studies may aid in further establishing the reproducibility and validity of this tool. The semiquantitative nature of the Wollenberg et al. system may be perceived as somewhat limiting, and some may find the three-part, five-component structure to be complex; however, we believe that this system—which addresses the whole body surface and skin involvement of the face, and describes the type of skin toxicity in a semiquantitative manner from merely five semiquantitative evaluations—is a comprehensive and user-friendly tool to utilize when evaluating dermatologic AEs because most components, such as color intensity or degree of pustulation, can be determined quickly via visual assessment [53].
Published Evidence Regarding Antibiotic-Based Prophylactic Management of Skin Reactions Induced by EGFR Inhibitors in Patients With Solid Tumors
A number of studies have been performed to evaluate the prophylactic management of EGFR inhibitor-related skin reactions using tetracycline-family antibiotics, often in conjunction with topical approaches [40–48]. In a variety of indications, including mCRC and NSCLC, prophylactic treatment with tetracycline-class therapeutics (doxycycline, minocycline, etc.) significantly reduced the severity of skin reactions. However, despite a reduction in their severity, it should be noted that a reduction in the incidence of all grades of skin reaction was not always observed (Table 2).
Table 2.
Relevant studies
The exceptions to this finding of reduced severity of skin reactions with prophylactic intervention were two studies performed by Jatoi et al. [40, 41]. In their original trial, Jatoi et al. noted that tetracycline may reduce the severity of skin reactions when given proactively, but their conclusions were potentially compromised by the number of patients who dropped out of the study; in a follow-up study that included new data, as well as a pooling of the previously collected prospective data alluded to above, the authors determined that tetracycline treatment did not significantly reduce the incidence or severity of skin reactions [40, 41]. This inconsistency may be attributable to imbalances stemming from the multiple tumor types simultaneously examined (lung, gastrointestinal, and other sites) compared with the single indications evaluated by the other trials cited above. Furthermore, the Jatoi et al. trial evaluated cetuximab, gefitinib, and other EGFR inhibitors, rather than focusing on a single therapeutic agent. Finally, these studies may have been confounded by the utilization of a nonoptimal dose of tetracycline.
Consequently, the preponderance of the available evidence establishes that treatment with tetracyclines reduces the severity of skin reactions when administered prophylactically.
Recommendations and Algorithms for the Prophylactic Management of Skin Reactions Induced by EGFR Inhibitors in Patients With Solid Tumors
With improved management of skin reactions, patients may experience an improved QoL and avoid treatment-related lapses in EGFR-inhibitor administration (which could, in turn, lead to decreased efficacy). Although commonly deployed, reactive management of skin reactions often occurs too late, indicating that treatment algorithms would be more successful if they incorporated prophylactic care. In a recent paper from Dascalu et al. [54], the authors evaluated the effect of prophylactic versus reactive treatment of skin reactions in patients with mCRC treated with cetuximab or panitumumab. Although overall survival was similar between the two groups, this merely underscores the importance of optimizing prophylactic intervention practices [54].
Although commonly deployed, reactive management of skin reactions often occurs too late, indicating that treatment algorithms would be more successful if they incorporated prophylactic care.
Although grading scales are important in the implementation and evaluation of clinical trials, they are less useful in the day-to-day setting, where physicians are more likely to be evaluating skin reaction severity with the sole objective of treating it appropriately. Therefore, treatment algorithms are of more practical use in clinical practice than are grading scales. Macdonald et al. previously published an algorithm [52] in which they proposed preventative care (including topical steroids, sunscreen, and systemic tetracycline antibiotics) for patients treated with cetuximab, panitumumab, erlotinib, and gefitinib. However, the authors noted that prophylactic tetracycline may not diminish papulopustular eruptions, citing Jatoi et al. [41] (the limitations of which were discussed earlier). Another study showed that preventative sunscreen use did not prevent or attenuate eruptions [55].
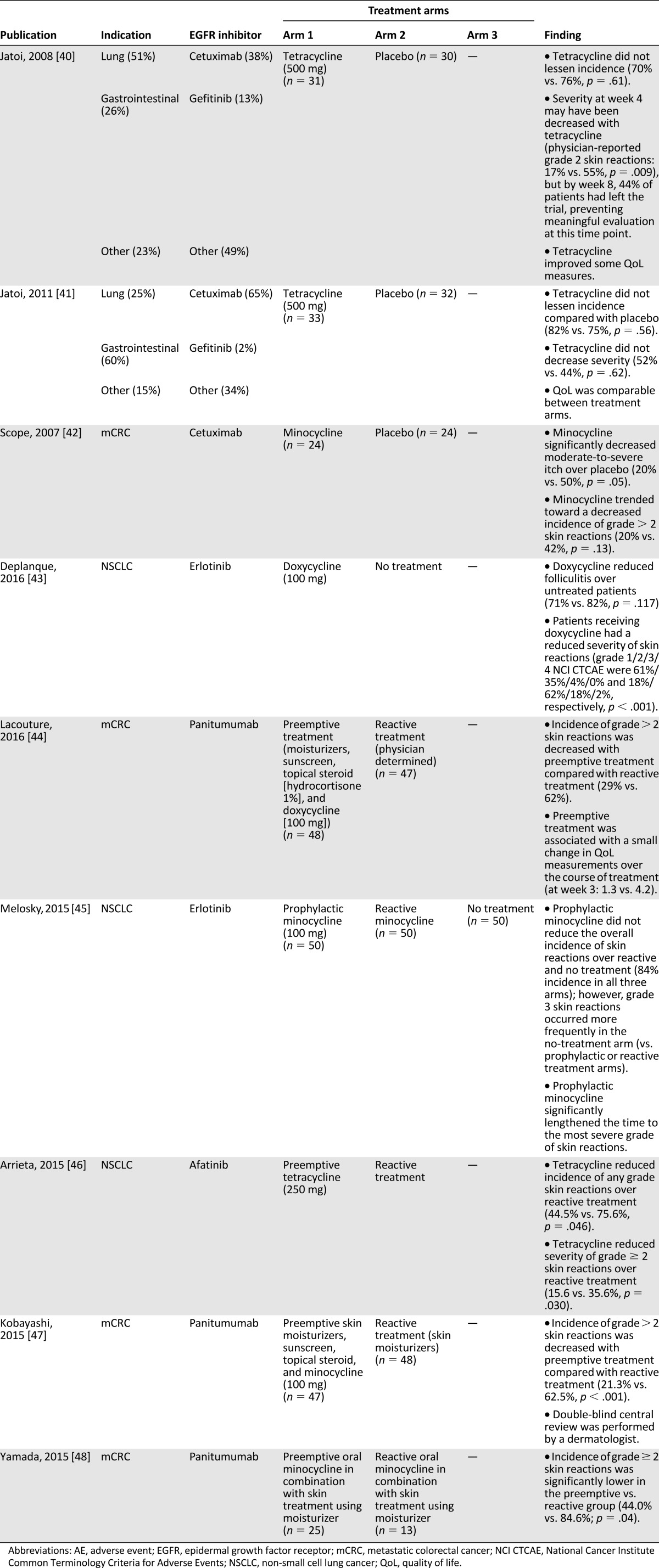
In light of the data cited above, which suggest that prophylactic care can potentially reduce the severity of skin reactions by twofold or greater, we provide the following evidence-based recommendations for the prophylactic management of skin reactions induced by EGFR inhibitors. Starting with the first EGFR-inhibitor dose, the application of prophylactic basic skin care can prevent and reduce the severity and extent of skin reactions. General recommendations for basic daily regimens involving gentle cleansing, skin care, and sunlight protection are summarized in Figure 1A, as is a list of activities that should be avoided when possible.
Figure 1.
Recommendations for the prophylactic management of skin reactions induced by EGFR inhibitors: general basic skin care recommendations for the early phase (A) and specific prophylaxis recommendations for acne-like rash (B), starting with the first cetuximab dose. Level of evidence and grades of recommendations reflect the following scoring system: Levels of evidence are as follows: IA, evidence from meta-analysis of randomized controlled trials; IB, evidence from at least one randomized controlled trial; IIA, evidence from at least one controlled study without randomization; IIB, evidence from at least one other type of quasi-experimental study; III, evidence from nonexperimental descriptive studies, such as comparative studies, correlation studies, and case-control studies; and IV, evidence from expert committee reports or opinions or clinical experience of respected authorities, or both. Grades of recommendation are as follows: A, directly based on level I evidence; B, directly based on level II evidence or extrapolated recommendations from level I evidence; C, directly based on level III evidence or extrapolated recommendations from level I or II evidence; and D, directly based on level IV evidence or extrapolated recommendations from level I, II, or III evidence.
Abbreviations: DAC, Deutscher Arzneimittel-Codex; LOE, level of evidence.
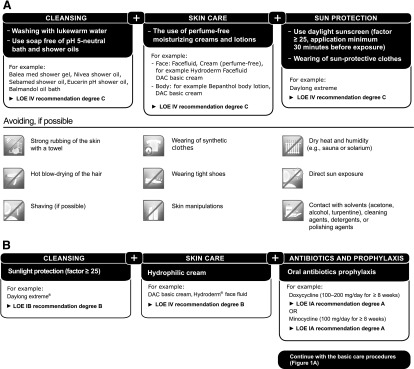
Specific evidence-based prophylactic recommendations for the management of EGFR inhibitor-induced acne-like rash are summarized in Figure 1B. These include sunlight protection, skin care with hydrophilic cream (initial positive results involving the prophylactic topical application of vitamin K cream have been reported [56–58]; confirmatory data are currently being sought in a German, randomized, multicenter phase II study), and oral antibiotics with doxycycline (100–200 mg/day for ≥8 weeks) or minocycline (100 mg/day for ≥8 weeks). Antibiotic administration should begin no later than the first day of EGFR-inhibitor treatment. The most common potential adverse effects of tetracyclines are sensitivity of skin to sunlight (rare with minocycline), cramps or burning of the stomach, and diarrhea; minocycline has also been reported to cause dizziness, light-headedness, or unsteadiness.
Additionally, we propose an opinion-based algorithm for managing patients who received prophylactic treatment, but who nevertheless experienced skin reactions in response to EGFR inhibitor-based therapy (Fig. 2). In brief, in accordance with the findings of the STEPP trial [44], patients whose skin reactions are either unaffected or worsen with the application of twice-daily topical antibiotics are recommended to receive twice-daily topical corticosteroids. In the event that the patients’ skin reactions still do not improve, options include dose reduction according to the summary of product characteristics, consultation with a dermatologist, or possible administration of isotretinoin (and concomitant discontinuation of oral antibiotics).
Figure 2.
Proposed algorithm for managing patients who experience skin reactions in response to EGFR inhibitor-based therapy.
Abbreviations: DAC, Deutscher Arzneimittel-Codex; EGFR, epidermal growth factor receptor.
Future Perspective
Additional trial data are soon anticipated. Enrollment is now completed for the EVITA (NCT01345526) and AIO-LQ-0110 (NCT01668498) trials, which are investigating the addition of vitamin K to doxycycline prophylaxis and the substitution of doxycycline by erythromycin cream, respectively. Another study (NCT01931150), which is investigating topical dapsone for cetuximab-induced skin reactions, is also ongoing. Furthermore, results supporting the prophylactic use of antibiotics in conjunction with dacomitinib were presented at MASCC 2015, and full publication is eagerly awaited. Also encouraging are findings emanating from the reactive deployment of epidermal growth factor ointment in patients experiencing erlotinib-related skin reactions [59], a reactive management strategy that could potentially also prove useful in the prophylactic setting.
Another important topic for future research is the current dearth of information regarding biomarkers that are possibly predictive of vulnerability to the development of skin reactions (either their incidence or severity). The elucidation of such biomarkers could aid in identifying patients for whom aggressive prophylactic treatment is most imperative.
Conclusion
Inhibition of EGFR is an established treatment that extends patient survival across a variety of tumor types. Skin reactions are the most common EGFR inhibitor-attributable AE and can be painful and debilitating, potentially having a negative impact on patients’ QoL and social functioning. Moreover, skin reactions can cause a negative impact on treatment duration; shortened treatment duration can, in turn, compromise efficacy. We espouse the viewpoint that prophylactic management of skin reactions should be recommended for all patients treated with EGFR inhibitors. Indeed, appropriate prophylactic management could effectively reduce the severity (although perhaps not incidence) of skin reactions in patients treated with EGFR inhibitors and therefore has the potential to directly benefit patients.
Acknowledgments
M.E.L. is supported in part through the NIH/National Cancer Institute Cancer Center Support Grant P30 CA008748. Medical writing assistance was provided by ClinicalThinking, Inc., Hamilton, NJ, and funded by Merck KGaA, Darmstadt, Germany.
Author Contributions
Conception/Design: Ralf-Dieter Hofheinz, Gaël Deplanque, Yoshito Komatsu, Yoshimitsu Kobayashi, Janja Ocvirk, Patrizia Racca, Silke Guenther, Jun Zhang, Mario E. Lacouture, Aminah Jatoi
Provision of study material or patients: Ralf-Dieter Hofheinz, Gaël Deplanque, Yoshito Komatsu, Yoshimitsu Kobayashi, Janja Ocvirk, Patrizia Racca, Jun Zhang, Mario E. Lacouture, Aminah Jatoi
Collection and/or assembly of data: Silke Guenther
Data analysis and interpretation: Ralf-Dieter Hofheinz, Gaël Deplanque, Yoshito Komatsu, Yoshimitsu Kobayashi, Janja Ocvirk, Patrizia Racca, Silke Guenther, Jun Zhang, Mario E. Lacouture, Aminah Jatoi
Manuscript writing: Ralf-Dieter Hofheinz, Gaël Deplanque, Yoshito Komatsu, Yoshimitsu Kobayashi, Janja Ocvirk, Patrizia Racca, Silke Guenther, Jun Zhang, Mario E. Lacouture, Aminah Jatoi
Final approval of manuscript: Ralf-Dieter Hofheinz, Gaël Deplanque, Yoshito Komatsu, Yoshimitsu Kobayashi, Janja Ocvirk, Patrizia Racca, Silke Guenther, Jun Zhang, Mario E. Lacouture, Aminah Jatoi
Disclosures
Ralf-Dieter Hofheinz: Merck Serono, Amgen (C/A, H); Yoshito Komatsu: Eli Lilly, Bristol-Myers Squibb, Merck Serono, Takeda, Merck Sharp & Dohme, Taiho, Yakult, Chugai, DaiichiSankyo, Abbott (RF, H); Janja Ocvirk: Merck (RF), Roche (H); Silke Guenther: Merck (E); Mario E. Lacouture: Memorial Sloan Kettering Cancer Center (E), Quintiles, Boehringer Ingelheim, AstraZeneca, Dignitana, Genentech, Foamix, Janssen R&D, RP Pharmaceuticals, Michael’s Mission, Oncology Training International (C/A), Berg, Roche, Bristol-Myers Squibb (RF); Aminah Jatoi: Amgen, EnteraHealth, Boston Biologics (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 2.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 3.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 4.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 5.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 6.Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 7.Vermorken JB, Stöhlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): An open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 8.Siu LL, Waladron JN, Chen BE, et al. Phase III randomized trial of standard fractionation radiotherapy (SFX) with concurrent cisplatin (CIS) versus accelerated fractionation radiotherapy (AFX) with panitumumab (PMab) in patients (pts) with locoregionally advanced squamous cell carcinoma of the head and neck (LA-SCCHN): NCIC clinical trials group HN.6 trial. J Clin Oncol. 2015;33(suppl):6000a. [Google Scholar]
- 9.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 11.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: A multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 13.Douillard JY, Ostoros G, Cobo M, et al. First-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: A phase-IV, open-label, single-arm study. Br J Cancer. 2014;110:55–62. doi: 10.1038/bjc.2013.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Soler R, Van Cutsem E. Clinical research of EGFR inhibitors and related dermatologic toxicities. Oncology (Williston Park) 2007;21(suppl 5):10–16. [PubMed] [Google Scholar]
- 15.Sipples R. Common side effects of anti-EGFR therapy: Acneform rash. Semin Oncol Nurs. 2006;22(suppl 1):28–34. doi: 10.1016/j.soncn.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16–26. doi: 10.3747/co.v16i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejwani A, Wu S, Jia Y, et al. Increased risk of high-grade dermatologic toxicities with radiation plus epidermal growth factor receptor inhibitor therapy. Cancer. 2009;115:1286–1299. doi: 10.1002/cncr.24120. [DOI] [PubMed] [Google Scholar]
- 18.Su X, Lacouture ME, Jia Y, et al. Risk of high-grade skin rash in cancer patients treated with cetuximab—an antibody against epidermal growth factor receptor: Systemic review and meta-analysis. Oncology. 2009;77:124–133. doi: 10.1159/000229752. [DOI] [PubMed] [Google Scholar]
- 19.Jia Y, Lacouture ME, Su X, et al. Risk of skin rash associated with erlotinib in cancer patients: A meta-analysis. J Support Oncol. 2009;7:211–217. [PubMed] [Google Scholar]
- 20.Balagula Y, Wu S, Su X, et al. The effect of cytotoxic chemotherapy on the risk of high-grade acneiform rash to cetuximab in cancer patients: A meta-analysis. Ann Oncol. 2011;22:2366–2374. doi: 10.1093/annonc/mdr016. [DOI] [PubMed] [Google Scholar]
- 21.Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): A randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569–579. doi: 10.1016/S1470-2045(14)70118-4. [DOI] [PubMed] [Google Scholar]
- 22.Price TJ, Peeters M, Kim TW, et al. Final results from ASPECCT: Randomized phase 3 non-inferiority study of panitumumab (pmab) vs cetuximab (cmab) in chemorefractory wild-type (WT) KRAS exon 2 metastatic colorectal cancer (mCRC) J Clin Oncol. 2015;33(suppl):3586a. doi: 10.1016/j.ejca.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 25.Lacouture ME. Insights into the pathophysiology and management of dermatologic toxicities to EGFR-targeted therapies in colorectal cancer. Cancer Nurs. 2007;30(suppl 1):S17–S26. doi: 10.1097/01.NCC.0000281758.85704.9b. [DOI] [PubMed] [Google Scholar]
- 26.Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 27.Rosen AC, Case EC, Dusza SW, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: A questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14:327–333. doi: 10.1007/s40257-013-0021-0. [DOI] [PubMed] [Google Scholar]
- 28.Venook AP, Niedzwiecki D, Lenz H-J, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) J Clin Oncol. 2014;31(suppl 5):LBA3a. [Google Scholar]
- 29.Láng I, Köhne CH, Folprecht G, et al. Quality of life analysis in patients with KRAS wild-type metastatic colorectal cancer treated first-line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer. 2013;49:439–448. doi: 10.1016/j.ejca.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi K, Ando M, Ooki A, et al. Quality of life analysis in patients with RAS wild-type metastatic colorectal cancer treated with first-line FOLFIRI + cetuximab in the CRYSTAL study. Eur J Cancer. 2015;51(suppl 3):2120a. doi: 10.1016/j.clcc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Siena S, Tabernero J, Bodoky G, et al. Quality of life (QoL) during first-line treatment with FOLFOX4 with or without panitumumab (pmab) in RAS wild-type (WT) metastatic colorectal carcinoma (mCRC) J Clin Oncol. 2015;33(suppl 3):693a. [Google Scholar]
- 32.Wagner LI, Berg SR, Gandhi M, et al. The development of a Functional Assessment of Cancer Therapy (FACT) questionnaire to assess dermatologic symptoms associated with epidermal growth factor receptor inhibitors (FACT-EGFRI-18) Support Care Cancer. 2013;21:1033–1041. doi: 10.1007/s00520-012-1623-4. [DOI] [PubMed] [Google Scholar]
- 33.Wagner LI, Lacouture ME. Dermatologic toxicities associated with EGFR inhibitors: the clinical psychologist’s perspective. Impact on health-related quality of life and implications for clinical management of psychological sequelae. Oncology (Williston Park) 2007;21(suppl 5):34–36. [PubMed] [Google Scholar]
- 34.Boers-Doets CB, Gelderblom H, Lacouture ME, et al. Experiences with the FACT-EGFRI-18 instrument in EGFRI-associated mucocutaneous adverse events. Support Care Cancer. 2013;21:1919–1926. doi: 10.1007/s00520-013-1752-4. [DOI] [PubMed] [Google Scholar]
- 35.Boers-Doets CB, Gelderblom H, Lacouture ME, et al. Translation and linguistic validation of the FACT-EGFRI-18 quality of life instrument from English into Dutch. Eur J Oncol Nurs. 2013;17:802–807. doi: 10.1016/j.ejon.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Chan A, Cameron MC, Garden B, et al. A systematic review of patient-reported outcome instruments of dermatologic adverse events associated with targeted cancer therapies. Support Care Cancer. 2015;23:2231–2244. doi: 10.1007/s00520-014-2564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clabbers JM, Boers-Doets CB, Gelderblom H, et al. Xerosis and pruritus as major EGFRI-associated adverse events. Support Care Cancer. 2016;24:513–521. doi: 10.1007/s00520-015-2781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osio A, Mateus C, Soria JC, et al. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol. 2009;161:515–521. doi: 10.1111/j.1365-2133.2009.09214.x. [DOI] [PubMed] [Google Scholar]
- 39.Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116:3916–3923. doi: 10.1002/cncr.25090. [DOI] [PubMed] [Google Scholar]
- 40.Jatoi A, Rowland K, Sloan JA, et al. Tetracycline to prevent epidermal growth factor receptor inhibitor-induced skin rashes: Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB) Cancer. 2008;113:847–853. doi: 10.1002/cncr.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jatoi A, Dakhil SR, Sloan JA, et al. Prophylactic tetracycline does not diminish the severity of epidermal growth factor receptor (EGFR) inhibitor-induced rash: Results from the North Central Cancer Treatment Group (Supplementary N03CB) Support Care Cancer. 2011;19:1601–1607. doi: 10.1007/s00520-010-0988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scope A, Agero AL, Dusza SW, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390–5396. doi: 10.1200/JCO.2007.12.6987. [DOI] [PubMed] [Google Scholar]
- 43.Deplanque G, Radj G, Vergnenegre A, et al. Doxycycline for prevention of erlotinib-induce rash in non-small-cell lung cancer patients after failure of first-line chemotherapy: A randomized, open-label trial. J Am Acad Dermatol. 2016;74:1077–1085. doi: 10.1016/j.jaad.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 44.Lacouture ME, Mitchell EP, Piperdi B, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:1351–1357. doi: 10.1200/JCO.2008.21.7828. [DOI] [PubMed] [Google Scholar]
- 45.Melosky B, Anderson H, Burkes RL, et al. Pan Canadian rash trial: A randomized phase III trial evaluating the impact of a prophylactic skin treatment regimen on epidermal growth factor receptor-tyrosine kinase inhibitor-induced skin toxicities in patients with metastatic lung cancer. J Clin Oncol. 2016;34:810–815. doi: 10.1200/JCO.2015.62.3918. [DOI] [PubMed] [Google Scholar]
- 46.Arrieta O, Vega-González MT, López-Macías D, et al. Randomized, open-label trial evaluating the preventive effect of tetracycline on afatinib induced-skin toxicities in non-small cell lung cancer patients. Lung Cancer. 2015;88:282–288. doi: 10.1016/j.lungcan.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi Y, Komatsu Y, Yuki S, et al. Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study; J-STEPP. Future Oncol. 2015;11:617–627. doi: 10.2217/fon.14.251. [DOI] [PubMed] [Google Scholar]
- 48.Yamada M, Iihara H, Fujii H, et al. Prophylactic effect of oral minocycline in combination with topical steroid and skin care against panitumumab-induced acneiform rash in metastatic colorectal cancer patients. Anticancer Res. 2015;35:6175–6181. [PubMed] [Google Scholar]
- 49.Burtness B, Anadkat M, Basti S, et al. NCCN task force report: Management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(suppl 1):S5–S21; quiz S22–S24. doi: 10.6004/jnccn.2009.0074. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair R. Anticipating and managing the cutaneous side effects of epidermal growth factor receptor inhibitors. Asia Pac J Clin Oncol. 2014;10(suppl 1):11–17. doi: 10.1111/ajco.12160. [DOI] [PubMed] [Google Scholar]
- 51.Curry JL, Torres-Cabala CA, Kim KB, et al. Dermatologic toxicities to targeted cancer therapy: Shared clinical and histologic adverse skin reactions. Int J Dermatol. 2014;53:376–384. doi: 10.1111/ijd.12205. [DOI] [PubMed] [Google Scholar]
- 52.Macdonald JB, Macdonald B, Golitz LE, et al. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J Am Acad Dermatol. 2015;72:203–218; quiz 219–220. doi: 10.1016/j.jaad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 53.Wollenberg A, Moosmann N, Klein E, et al. A tool for scoring of acneiform skin eruptions induced by EGF receptor inhibition. Exp Dermatol. 2008;17:790–792. doi: 10.1111/j.1600-0625.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- 54.Dascalu B, Kennecke HF, Lim HJ, et al. Prophylactic versus reactive treatment of acneiform skin rashes from epidermal growth factor receptor inhibitors in metastatic colorectal cancer. Support Care Cancer. 2016;24:799–805. doi: 10.1007/s00520-015-2846-y. [DOI] [PubMed] [Google Scholar]
- 55.Jatoi A, Thrower A, Sloan JA, et al. Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4) The Oncologist. 2010;15:1016–1022. doi: 10.1634/theoncologist.2010-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ocvirk J. The prophylactic use of K1 cream for reduction of skin toxicity during the cetuximab treatment. Support Care Cancer. 2012;20(suppl 1):446a. [Google Scholar]
- 57.Pinto C. Topical vitamin K1 in the management of skin rash during anti-EGFR monoclonal antibody treatment in patients with metastatic cancer: Italian Observational Study. J Clin Oncol. 2011;29(suppl):e14068a. [Google Scholar]
- 58.Pinto C. Skin toxicity and treatment compliance of first-line cetuximab with irinotecan, oxaliplatin, and fluoropyrimidines-based chemotherapy in metastatic colorectal cancer (mCRC): The preliminary analysis of observer study. J Clin Oncol. 2013;31(suppl 4):530a. [Google Scholar]
- 59.Hwang IG, Kang JH, Oh SY, et al. Phase II trial of epidermal growth factor ointment for patients with erlotinib-related skin effects. Support Care Cancer. 2016;24:301–309. doi: 10.1007/s00520-015-2783-9. [DOI] [PubMed] [Google Scholar]