Cancer patients often do not make informed decisions regarding clinical trial participation. By improving knowledge, helping patients clarify preferences for participation, and facilitating conversations on trials, decision aids could lead to decisions about participation that better match patients’ preferences, promoting patient-centered care and the ethical conduct of clinical research. Evidence regarding a decision tool to support patients’ decisions about trial participation is described.
Keywords: Decision aids, Clinical trials, Informed decision making
Abstract
Background.
Cancer patients often do not make informed decisions regarding clinical trial participation. This study evaluated whether a web-based decision aid (DA) could support trial decisions compared with our cancer center’s website.
Methods.
Adults diagnosed with cancer in the past 6 months who had not previously participated in a cancer clinical trial were eligible. Participants were randomized to view the DA or our cancer center’s website (enhanced usual care [UC]). Controlling for whether participants had heard of cancer clinical trials and educational attainment, multivariable linear regression examined group on knowledge, self-efficacy for finding trial information, decisional conflict (values clarity and uncertainty), intent to participate, decision readiness, and trial perceptions.
Results.
Two hundred patients (86%) consented between May 2014 and April 2015. One hundred were randomized to each group. Surveys were completed by 87 in the DA group and 90 in the UC group. DA group participants reported clearer values regarding trial participation than UC group participants reported (least squares [LS] mean = 15.8 vs. 32, p < .0001) and less uncertainty (LS mean = 24.3 vs. 36.4, p = .025). The DA group had higher objective knowledge than the UC group’s (LS mean = 69.8 vs. 55.8, p < .0001). There were no differences between groups in intent to participate.
Conclusions.
Improvements on key decision outcomes including knowledge, self-efficacy, certainty about choice, and values clarity among participants who viewed the DA suggest web-based DAs can support informed decisions about trial participation among cancer patients facing this preference-sensitive choice. Although better informing patients before trial participation could improve retention, more work is needed to examine DA impact on enrollment and retention.
Implications for Practice:
This paper describes evidence regarding a decision tool to support patients’ decisions about trial participation. By improving knowledge, helping patients clarify preferences for participation, and facilitating conversations about trials, decision aids could lead to decisions about participation that better match patients’ preferences, promoting patient-centered care and the ethical conduct of clinical research.
Background
Cancer clinical trials are essential to evaluate new therapies and advance evidence-based treatments. However, despite the widespread availability of cancer clinical trials in academic and nonacademic settings, patients often are not well informed about trials and do not make informed decisions about participation. Many patients harbor therapeutic misconceptions about the aim of clinical trials [1, 2] and overestimate benefits of standard treatment [3]. In a recent meta-analysis of 103 studies, more than 25% of clinical trial participants did not understand the nature of the study they were participating in or the voluntary nature of participation [4]. Only approximately 50% of participants understood central trial concepts such as the use of placebos or randomization [4]. Furthermore, members of the general public frequently have misconceptions about clinical trials, with nearly half of respondents believing that research participants do not always receive the best possible care [5].
Without an adequate understanding of trials and trial procedures, patients cannot make a fully informed decision about participation, a principal tenet of the ethical conduct of clinical research. Although a number of interventions have been developed to improve clinical trial informed consent, nearly all focus exclusively on improving patients’ knowledge [6, 7]. Even with the implementation of such interventions, a recent meta-analysis found that participant understanding of important concepts essential for informed consent has not increased during the 3 decades of studies examined [4]. To improve cancer clinical trial decision quality, interventions must go beyond knowledge provision to address aspects such as preference deliberation and decision preparedness, guiding patients through the trial decision-making process [6, 8].
Patient decision aids (DAs; Fig. 1) can be used to support high-quality cancer clinical trial decisions. In a variety of contexts, DAs have reduced patients’ uncertainty about health decisions, improved patients’ knowledge about decisions, improved patient-provider decision communication, and improved the match between patients’ values and their health choices [9]. DAs incorporate values clarification exercises that help individuals address fears, concerns, and preferences for options. These concerns and preferences might be particularly important for racial and ethnic minority populations typically excluded from trials, in part because of concerns and misinformation about clinical research [10, 11]. By improving knowledge about trials, clarifying patients’ preferences for participation, and facilitating conversations about trials, DAs could improve decisions about participation that better match patients’ preferences, promoting patient-centered care and the ethical conduct of clinical research. They could also improve trial retention rates, particularly among those who hesitate to participate based on misinformation about trials, if patients become more informed and aware of trials and details before participation. Given that there is often limited time for deliberation once a patient becomes trial eligible, implementing DAs about cancer clinical trials at multiple points in the cancer care continuum, even before patients are eligible for a trial, can prepare patients to make a choice about trial participation if or when one is offered to them.
Figure 1.
Web-based decision aid.
The purpose of this study was to compare a web-based DA to our cancer center’s usual care website in a randomized trial, examining whether and how the DA improves four elements of decision making about cancer clinical trial participation: knowledge, self-efficacy, preparedness to make a decision about trial participation, and intent to participate in a trial. Our hypothesis was that implementing the DA would improve each of these outcomes, increasing knowledge, self-efficacy, preparedness to make a decision about trial participation, and intent to participate in a trial among recently diagnosed cancer patients. A secondary hypothesis was that the DA would improve outcomes at a greater rate for racial minorities.
Methods
Decision Aid
The eight-section web-based DA was developed based on cancer survivor input in accordance with best practices in health literacy. It concentrates on knowledge, empowerment, and values clarification, aiming to correct common misconceptions about trials while promoting preference deliberation (Fig. 1). It is intended to prepare patients to have an informed conversation about cancer clinical trial participation should one be offered to them.
In previous work, semistructured interviews were conducted among 45 Hispanic and black cancer survivors representative of the prior population of interest. Feedback about attitudes, barriers, and facilitators toward cancer clinical trial participation elicited in those interviews was incorporated into the initial DA. The DA was then refined after usability testing with 10 additional minority survivors. It was found to improve knowledge, self-efficacy, and preparedness to make a decision about cancer clinical trial participation in pilot testing with 64 Hispanic and black cancer survivors. In a previous phase of the current study, a survey of 30 key stakeholders (e.g., principal investigators, institutional review board members) at an institution outside its initial development revealed high acceptability of the DA among those who develop and deliver informed consent (M.D. Kuzemchak, M.M. Byrne, K.A. Kaphingst et al., manuscript submitted for publication). Feedback was incorporated prior to the current phase.
The final DA includes eight sections: Introduction, About Studies, Common Questions, Talking to Your Doctor, What’s Important to You, Research Terms, and Additional Resources (Fig. 1). It includes an interactive education component explaining common trial concepts, hypothetical patient stories illustrating choices a patient could make (to participate or to not participate, both in the context of existing effective standard treatment and in the context of no existing effective standard treatment), and an interactive values clarification component in which users slide a toolbar to indicate their perceived importance of various trial attributes. Generic in nature, the DA is intended to supplement existing trial-specific informed consent documents.
In the current phase, we evaluated the effectiveness of the web-based DA in comparison with an enhanced usual care control in a diverse population of newly diagnosed cancer patients. The trial was registered with clinicaltrials.gov (protocol no. NCT01964222) before data collection [12].
Participants
Participants were recruited in various cancer clinics within a major urban academic medical center. Adults 18 years or older who had been diagnosed with cancer in the past 6 months and who had not previously participated in a cancer clinical trial were eligible to participate. Consistent with the intent of the tool and the fact that trial eligibility can change across the care continuum, patients were included whether or not they were eligible for a trial at the time of study enrollment. Eligible participants were identified through clinician referrals and/or through review of medical records by a member of the research team.
Data Collection
Eligible patients were approached during clinic visits. At the direction of clinic staff, trained research assistants invited eligible patients to participate in the waiting room and/or in the exam room. Eligible patients had had no prior contact about the study. After a brief explanation of the study, interested individuals completed a brief screening survey to confirm eligibility. Written consent was obtained from those who were interested and eligible before viewing any additional study materials. Participants were then allocated by a computer-generated random number sequence to one of two study conditions: DA or enhanced usual care (UC). Those randomized to the DA group were instructed to view the web-based DA (Fig. 1). Those randomized to the UC group were instructed to view the institution’s website on cancer clinical trials (Fig. 2). Immediately after viewing one of the conditions, participants completed a survey assessing cancer clinical trial knowledge, self-efficacy for finding cancer clinical trial information, decisional conflict, intent to participate in a cancer clinical trial, and cancer clinical trial perceptions. They also completed information about sociodemographic characteristics and cancer type and stage. Participants could choose to complete the survey online or on paper. They could complete it in the clinic at the time of consent on an electronic tablet device or at home with a link to the website. For participants who opted to complete the intervention and survey at the clinic, research assistants remained available to participants if they encountered technical challenges; however, to reduce response bias, they did not stay with the participant as he/she completed the survey. Participants were allowed as much time as they needed to navigate the website and to complete the survey. They were provided a $20 gift card for their time.
Figure 2.
Usual care website.
Measures
Knowledge
Eleven objective knowledge items evaluated how well participants understood cancer clinical trials after viewing their assigned website (supplemental online Appendix). An overall knowledge score was calculated for each participant with the percentage of items correctly answered, consistent with the validated measure’s scoring procedures [13–15]. We examined whether there were differences between the two groups in the average percentage of correct answers. Participants were also asked to rank their self-perceived knowledge on a single-item 5-point Likert scale, with values ranging from 1 (not at all knowledgeable) to 5 (completely knowledgeable).
Self-Efficacy for Finding Information About Cancer Clinical Trials
Self-efficacy for finding information about cancer clinical trials was measured on a single-item 5-point scale with higher scores indicating greater self-efficacy for finding information.
Decisional Conflict
Evaluation of decisional conflict consisted of two subscales: the Values Clarity Subscale and the Uncertainty Subscale of the low literacy version of the Decisional Conflict Scale [16]. Each contains two items with three response categories. Following standard scoring guidelines for the validated measure, each item was scored, and then the sum was divided by 2 and multiplied by 25 to produce overall “Values Clarity” and “Uncertainty” scores, respectively. Scores ranged from 1 to 100, with higher values representing less values clarity and less certainty, respectively.
Clarity of Opinions
Clarity of opinions about cancer clinical trials was measured on a single-item 5-point scale, with higher scores indicating clearer opinions.
Intent to Participate
Intent to participate in a cancer clinical trial was measured on a single-item 5-point scale, with higher scores indicating greater intent to participate.
Intent to Encourage Others to Participate
Intent to encourage others to participate in a cancer clinical trial was measured on a single-item 5-point scale, with higher scores indicating greater intent to encourage others to participate.
Decision Readiness
Readiness to make a decision about whether to participate in a cancer clinical trial was measured on a single-item 5-point scale, with higher scores indicating greater readiness to make a decision.
Perceptions of Cancer Clinical Trials
Participants’ perceptions of the pleasantness, safeness, easiness, helpfulness, value, convenience, and goodness of cancer clinical trials were measured with individual items on a 7-point Likert scale, with values ranging from 1 (very unpleasant, very unsafe, etc.) to 7 (very pleasant, very safe, etc.).
Implementation Outcomes
To evaluate the potential for implementation of the web-based DA, three implementation outcomes were examined: time spent on the website, number of visits to the website, and number of participants who visited each page of the website in the DA group.
Sociodemographic Information
Sociodemographic information was collected including age, gender, cancer type, education, income, ethnicity, race, language, and health literacy level [17].
Data Analysis
All analyses were conducted using Stata version 13 (StataCorp, College Station, TX, http://www.stata.com). Descriptive statistics were calculated for continuous and categorical variables. Bivariate analyses explored the relationship between randomized group (DA or UC) and outcomes. Primary outcomes were knowledge and decisional conflict; secondary outcomes were self-efficacy, intent to participate, decision readiness, and trial perceptions. Because the outcome variables for secondary outcomes may not be interval because they are measured using a Likert scale, we assessed the robustness of significant differences in outcome by group assignment using nonparametric χ2 tests. As the nonparametric analyses showed the same results as the parametric analyses in terms of significant differences by group assignment for these outcome variables, we present only results from the parametric analyses. Controlling for whether participants had ever heard of cancer clinical trials and educational attainment, multivariable linear regression models examined group (DA or UC) on knowledge, self-efficacy for finding information about trials, decisional conflict (values clarity and uncertainty about choice), and intent to participate, decision readiness, and perceptions of cancer clinical trials. Sample size was set at 180 (90 per group) to achieve 80% power at α = 0.05, using knowledge as the primary outcome and controlling for up to three covariates. Statistical significance was set at .05 using two-sided analyses.
Results
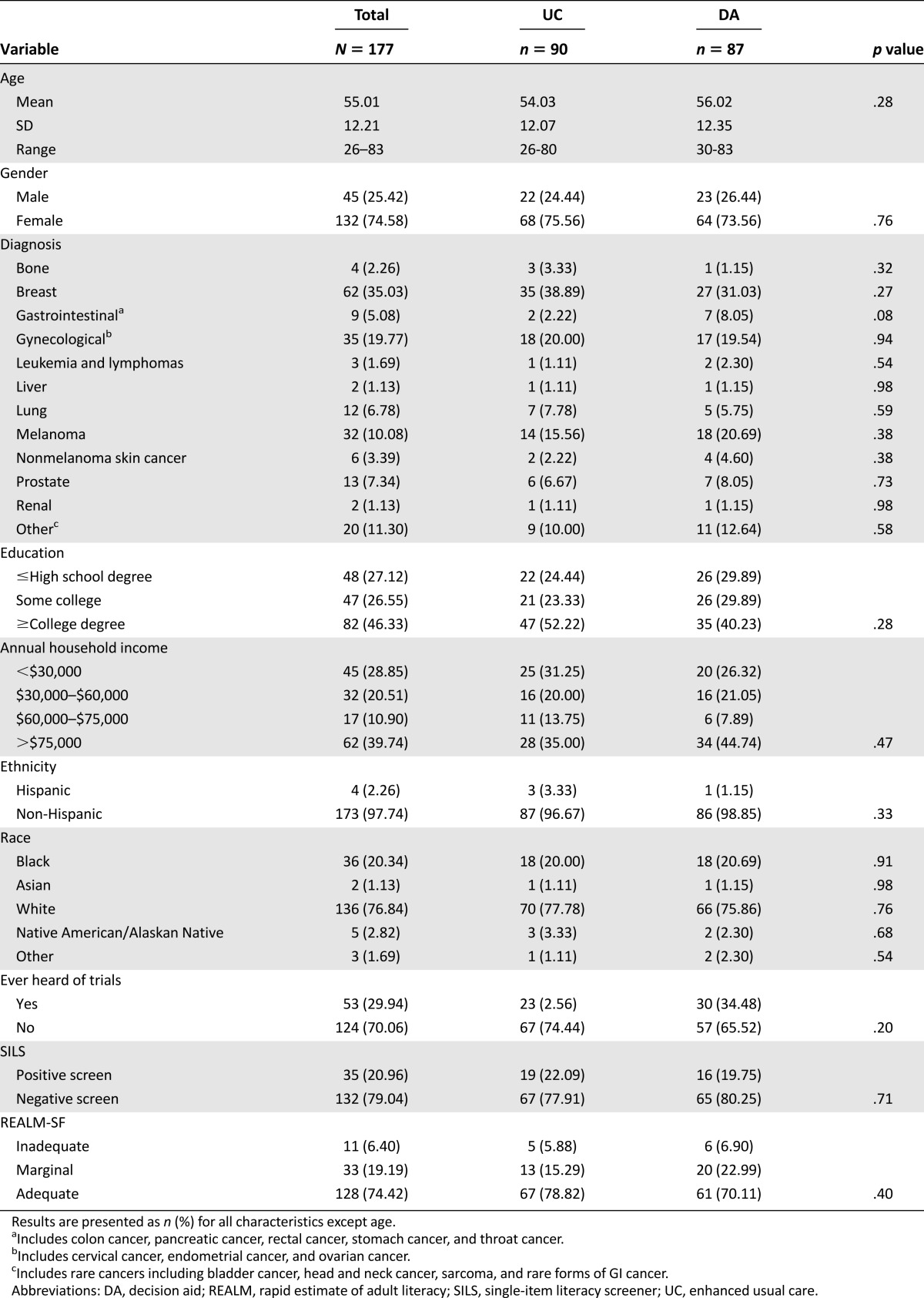
Participant Characteristics
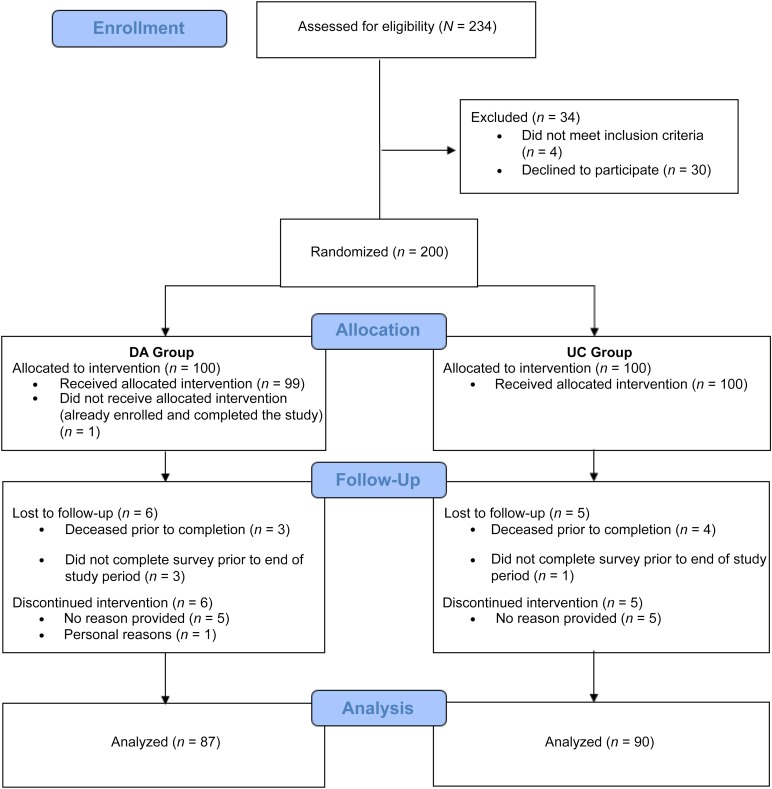
Figure 3 details our recruitment and enrollment in a CONSORT diagram. A total of 200 patients were consented to participate out of 234 approached (86%) between May 2014 and April 2015. Patient-provided reasons for declining participation included being uninterested at that time (20), having limited time (3), not feeling well (1), having difficulty reading (1), and none (9). Of the consented patients, 100 were randomized to view the web-based DA, whereas 100 were randomized to view the UC website. After viewing their assigned website, surveys were completed by 87 participants in the DA group (87%), and 90 participants in the UC group (90%). Reasons for incompletion included death (3 DA, 4 UC), failure to complete in the study period (3 DA, 1 UC), voluntary withdrawal (6 DA, 5 UC), and ineligibility due to screening failure (1 in DA). Individuals who did not complete the survey were significantly older than those who did complete the survey (mean age 62.1 years [SD, 2.2] vs. 55.0 [0.9], respectively; p = .009), but did not significantly differ in any other measured demographic characteristics. Table 1 displays demographic characteristics of the final sample. There were no significant differences in demographic characteristics between individuals randomized to the DA versus the UC. Most participants (162/177; 91.5%) chose to view the website at home rather than at their clinic visit, with 107 (60.5%) completing the survey by e-mail, 44 (24.9%) by postal mail, and 11 (6.2%) by phone.
Figure 3.
CONSORT flow diagram.
Abbreviations: DA, decision aid; UC, enhanced usual care.
Table 1.
Participant demographics (N = 177)
Bivariate Outcomes by Group
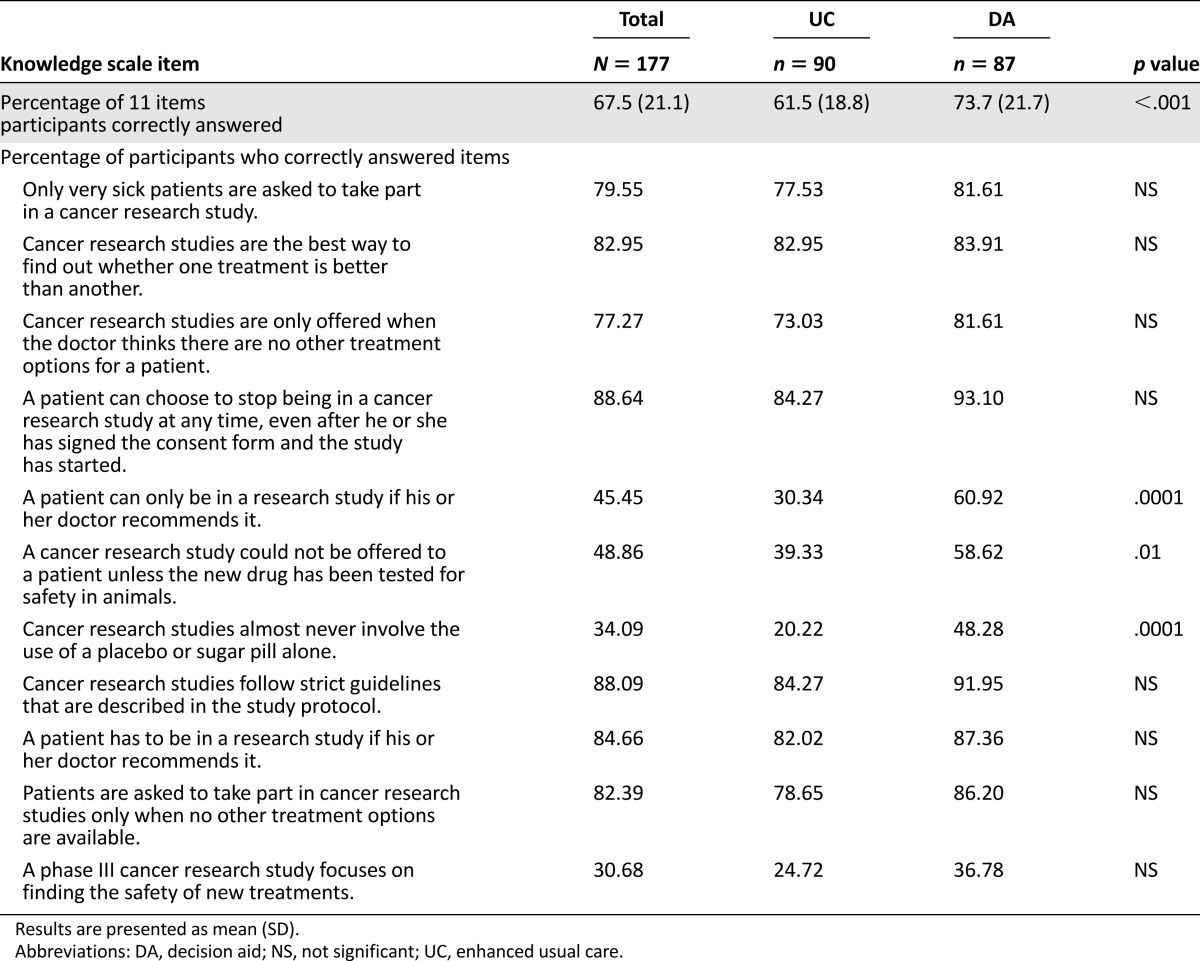
Participants in the DA group performed better on overall knowledge on the 11-item knowledge scale, answering an average of 73.7% of the items (8.1/11) correctly, compared with 61.5% (6.8/11) in the UC group (p = .0001; Table 2). The percentage of participants answering correctly was greater in the DA group than in the UC group for all items, with statistically significant differences between groups on four of the 11 items (Table 2). However, certain items were frequently answered incorrectly by participants in both groups. In particular, items regarding placebo use and study phases were answered correctly by fewer than half the participants in both the DA and UC conditions (Table 2).
Table 2.
Knowledge by study condition (N = 177)
Table 3 shows mean scores on self-reported outcomes by group. Compared with those in the UC group, those in the DA group had higher self-perceived knowledge about cancer clinical trials (M = 3.1 and 3.6, respectively; p = .003), self-efficacy for finding information about cancer clinical trials (M = 3.5 and 4.1, respectively; p = .0006), and clarity of opinions about cancer clinical trials (M = 3.5 and 3.9, respectively; p = .0114). Those in the UC group also reported less clear values and less certainty about choice compared with those in the DA group (M = 31.5 and 16.7, respectively; p = .0034 for values clarity; M = 36.5 and 24.1, respectively; p = .02 for uncertainty). There were no significant differences in either the intent to participate in a cancer clinical trial or in the intent to encourage others to participate in a cancer clinical trial between groups. There were no significant differences in participants’ perceptions of pleasantness, safeness, easiness, helpfulness, value, convenience, or goodness of cancer clinical trials across groups (data not shown in table).
Table 3.
Self-reported bivariate outcomes by study condition (N = 177)
Multivariable Analyses
Table 4 summarizes outcomes of our multivariable models with intent to participate, uncertainty, clarity of values, and knowledge of cancer clinical trials as the variables. Controlling for whether participants had ever heard of cancer clinical trials and educational attainment, there were no differences in intent to participate in cancer clinical trial. However, participants in the DA group reported clearer values regarding trial participation than the UC group (least squares [LS] mean = 15.8 and 32, respectively; p < .0001) and less uncertainty about participation (LS mean = 24.3 and 36.4, respectively; p = .025). Those in the DA group also had higher objective knowledge scores than those in the UC group (LS mean = 69.8 and 55.8, respectively; p < .0001). There were no significant interactions of race/ethnicity and group assignment in the multivariable analyses.
Table 4.
Multivariable models examining group on primary outcomes, controlling for whether patients had heard of cancer clinical trials and educational attainment (N = 177)
Because our outcome variables do not strictly meet the assumptions for OLS regressions, we ran alternatively specified models using ordered logit regression (intent, clarity of values, and uncertainty) and generalized linear models (knowledge) as a sensitivity analyses. There were no changes in the significance of these outcome variables after controlling for whether participants had heard of cancer clinical trials and educational attainment.
In exploratory analyses, we assessed whether race/ethnicity was associated with outcome variables. In multivariable analyses controlling for whether participants had ever heard of cancer clinical trials and educational attainment, we found no significant difference when comparing white non-Hispanics to all others in intent to participate, clarity of opinions, uncertainty, and knowledge.
Association of Objective Knowledge With Intent to Participate and Subjective Outcome Variables
We explored the association of the proportion of correct knowledge questions with individuals’ intent to participate and subjective views on knowledge and decision readiness using pairwise correlations. We found no significant association with knowledge and intent to participate or intent to encourage others to participate. However, there were significant associations between knowledge and variables for self-perceived knowledge, clarity of opinions, uncertainty, and clarity of values, with higher levels of objective knowledge being associated with higher perceived knowledge and decision readiness (Table 5). These significant associations were maintained after controlling for group assignment, ever heard of cancer clinical trials, and educational attainment in multivariate regressions (data not shown in tables).
Table 5.
Correlation of intentions and decision outcomes with objective knowledge

Decision Aid Implementation Outcomes
Of the 86 participants in the DA group, data on implementation outcomes was available for the 64 participants who opted to view the tool online rather than on paper. There were 94 unique visits to the website, with an average of 1.4 visits per participant. Participants spent an average of 20.1 minutes on the website.
Discussion
Overall, participants who viewed the DA website performed better on key decision outcomes than those who viewed the usual care website. Although there was no difference in participants’ intent to participate in a cancer clinical trial if one were offered, there were statistically significant differences in decision-support factors such as their overall knowledge of cancer clinical trials, self-perceived knowledge of cancer clinical trials, self-efficacy for finding information about cancer clinical trials, certainty about choice, and values clarity. This suggests that DAs can support informed decisions about trial participation among cancer patients faced with this preference-sensitive choice.
Moreover, the DA was received positively by patients even among older adults who were not comfortable using computers or the Internet. Our response rate (86%) and completion rate of those enrolled (90% in the DA group) was high for a study on newly diagnosed cancer patients. By offering multiple methods to complete the DA in both web- and paper-based formats, inside and outside of the clinic environment, we were able to reach a range of participants. In our study, 61% of participants completed the study electronically outside of the clinic environment. National studies of Internet use among older adults [18] suggest that approximately 58% of older adults use the Internet in their home, and this number is growing. Electronic DAs such as this one can be an important avenue for delivering cancer-related information, but steps should be taken to ensure that patients regardless of computer literacy can benefit from the information while there is still a sizable minority of the older adult population who might not be comfortable using computers.
The DA discussed information that stakeholders identified as essential for patients to understand about trial participation. Many of these points, such as the voluntary nature of trials and their potential risks and benefits, correspond to current principles of informed consent and shared decision making. Moreover, DAs that clearly explain what clinical trials aim to do could help alleviate therapeutic misconceptions, an important barrier to informed consent [1, 2]. By improving patients’ knowledge of trial features and by helping them deliberate what they believe about those features, DAs such as this one can facilitate informed, preference-sensitive choices among patients considering a cancer clinical trial.
There are several reasons we might not have seen differences in intent to participate in trials. Intent to participate is often related to cancer stage, and our patients had a mix of cancer types and stages. It is possible that cancer type or stage contributed to this outcome, but given the diversity of patients in the study, we did not have the power to explore specific disease characteristics on outcomes. In addition, DAs are not designed to persuade individuals to participate or not in a particular trial; thus, intent might not be expected to change as a result of this intervention. It is possible that retention rates could increase with the use of DAs as patients match their decision about participation to their preferences, but this study was not designed to measure changes in retention through time. Finally, intent was high in both groups. We did find qualitative information that supported increased intentions to participate after viewing the DA. For example, as one patient explained after viewing the DA website, “This really has changed my mind about clinical trials. When I was first diagnosed, my husband said no trials, we are doing what they know works. Now I would have no objections [to participating].” Future studies of specific cancer types or stages could explore the role of DAs on recruitment or retention rates.
In our study, we found that the DA website performed better than the usual care website among both white and nonwhite patients. This finding suggests that DAs could help mitigate some of the racial disparities in trial knowledge and self-efficacy for finding information about trials. Future studies could look specifically at minority groups’ responses to the DA and/or could develop targeted institutional based strategies to help address the disparities in trial enrollment rates.
It is important to interpret these findings in the context of the study. The study was conducted at a single academic medical center and results might not be generalizable to other centers. The study included a mix of cancer types and stages, and it is possible that focusing on a specific cancer type, stage, or type of clinical trial (phase I, II, or III) could lead to different results. The study was cross-sectional and we did not track enrollment rates through time if patients were offered trials. Future studies should explore these findings in diverse settings longitudinally to confirm our results. Studies could also explore the role of DAs regarding specific cancer clinical trials rather than trials in general.
Conclusion
Since the mid-1990s, government and academic organizations have worked together to support cancer clinical trial participation, particularly among vulnerable subgroups of the population such as racial and ethnic minorities [19, 20]. Our findings suggest that DAs, such as the one studied, have the potential to improve informed consent and decision quality with regard to cancer clinical trials. They can help improve patients’ knowledge of cancer clinical trials and can help them feel confident talking to clinicians about trials should one be offered to them. More research is needed on ways to implement DAs about cancer clinical trials participation and how to systematically measure their impact on trial decisions [21].
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This research was supported by an investigator-initiated grant funded from the Merck Health Literacy/Diversity/Adherence Investigator Studies Program to Dr. Politi. The authors would like to thank Norah Rast and Denise Monti for their contributions to this work.
Author Contributions
Conception/Design: Mary C. Politi, Kimberly A. Kaphingst, Margaret M. Byrne
Collection and/or assembly of data: Mary C. Politi, Marie D. Kuzemchak, Hannah Perkins
Data analysis and interpretation: Mary C. Politi, Hannah Perkins, Jingxia Liu, Margaret M. Byrne
Manuscript writing: Mary C. Politi, Marie D. Kuzemchak, Kimberly A. Kaphingst, Jingxia Liu, Margaret M. Byrne
Final approval of manuscript: Mary C. Politi, Marie D. Kuzemchak, Hannah Perkins, Kimberly A. Kaphingst, Jingxia Liu, Margaret M. Byrne
Disclosures
Mary C. Politi: Merck Sharpe & Dohme (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Daugherty CK. Impact of therapeutic research on informed consent and the ethics of clinical trials: A medical oncology perspective. J Clin Oncol. 1999;17:1601–1617. doi: 10.1200/JCO.1999.17.5.1601. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum PS, Roth LH, Lidz CW, et al. False hopes and best data: Consent to research and the therapeutic misconception. Hastings Cent Rep. 1987;17:20–24. [PubMed] [Google Scholar]
- 3.Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367:1616–1625. doi: 10.1056/NEJMoa1204410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tam NT, Huy NT, Thoa TB, et al. Participants’ understanding of informed consent in clinical trials over three decades: Systematic review and meta-analysis. Bull World Health Organ. 2015;93:186–98H. doi: 10.2471/BLT.14.141390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns KE, Magyarody NM, Duffett M, et al. Attitudes of the general public toward alternative consent models. Am J Crit Care. 2011;20:75–83. doi: 10.4037/ajcc2010645. [DOI] [PubMed] [Google Scholar]
- 6.Gilles K, Cotton SC, Brehaut JC, et al. Decision aids for people considering taking part in clinical trials. Cochrane Database Syst Rev. 2015;11:CD009736. doi: 10.1002/14651858.CD009736.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: A systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 8.Gillies K, Skea ZC, Campbell MK. Decision aids for randomised controlled trials: A qualitative exploration of stakeholders’ views. BMJ Open. 2014;4:e005734. doi: 10.1136/bmjopen-2014-005734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Syst Rev. 2014;(1):CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 10.Entwistle V. Supporting participation in clinical research: Decision aids for trial recruitment? Health Expect. 2008;11:205–207. doi: 10.1111/j.1369-7625.2008.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson GM, Ward AJ. Recruiting minorities into clinical trials: Toward a participant-friendly system. J Natl Cancer Inst. 1995;87:1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- 12.Washington University School of Medicine. Available at https://clinicaltrials.gov/ct2/show/NCT01964222. 2014. Accessed July 18, 2016.
- 13.Wells KJ, Quinn GP, Meade CD, et al. Development of a cancer clinical trials multi-media intervention: Clinical trials: are they right for you? Patient Educ Couns. 2012;88:232–240. doi: 10.1016/j.pec.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30:2516–2521. doi: 10.1200/JCO.2011.39.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KJ, Jacobsen PB, Quinn GP et al. Development and validation of measures of patients’ perceptions regarding cancer clinical trials. Paper presented at: American Public Health Association 138th Annual Meeting & Expo; 2010; Denver, Colorado; 2010. [Google Scholar]
- 16.Linder SK, Swank PR, Vernon SW, et al. Validity of a low literacy version of the Decisional Conflict Scale. Patient Educ Couns. 2011;85:521–524. doi: 10.1016/j.pec.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45:1026–1033. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 18.Perrin A, Duggan M. Americans’ Internet Access: 2000-2015. Available at http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015. Accessed July 18, 2016.
- 19.National Institutes of Health . NIH Guidelines on the inclusion of women and minorities as subjects in clinical research. Bethesda, MD: National Institutes of Health; 1994. p. 23. [Google Scholar]
- 20.McCaskill-Stevens W, McKinney MM, Whitman CG, et al. Increasing minority participation in cancer clinical trials: The minority-based community clinical oncology program experience. J Clin Oncol. 2005;23:5247–5254. doi: 10.1200/JCO.2005.22.236. [DOI] [PubMed] [Google Scholar]
- 21.Gillies K, Cotton SC, Brehaut JC, et al. Decision aids for people considering taking part in clinical trials. Cochrane Database Syst Rev. 2015;(11):CD009736. doi: 10.1002/14651858.CD009736.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.