Abstract
An in vitro transport assay, established with a modified Shiga toxin B subunit (STxB) as a marker, has proved to be useful for the study of transport from the early/recycling endosome (EE/RE) to the trans-Golgi network (TGN). Here, we modified this assay to test antibodies to all known soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) that have been shown to localize in the Golgi and found that syntaxin 5, GS28, Ykt6, and GS15 antibodies specifically inhibited STxB transport. Because syntaxin 5, GS28, Ykt6, and GS15 exist as a unique SNARE complex, our observation indicates that these four SNAREs function as a complex in EE/RE-TGN transport. The importance of GS15 in EE/RE-TGN transport was further demonstrated by a block in recombinant STxB transport in HeLa cells when GS15 expression was knocked down by its small interfering iRNA. Morphological analyses showed that some GS15 and Ykt6 were redistributed from the Golgi to the endosomes when the recycling endosome was perturbed by SNX3-overexpression, suggesting that GS15 and Ykt6 might cycle between the endosomes and the Golgi apparatus. Further studies indicated that syntaxin 5 and syntaxin 16 exerted their role in EE/RE-TGN transport in an additive manner. The kinetics of inhibition exhibited by syntaxin 16 and syntaxin 5 antibodies is similar.
INTRODUCTION
Mammalian cells endocytose a variety of molecules. Some of them escape from the lysosomal degradative pathway and are instead delivered to the Golgi apparatus. Examples of these are some protein toxins such as cholera toxin, Shiga toxin, and ricin (Sandvig and van Deurs, 2002) as well as some endogenous proteins such as TGN38 (Ghosh et al., 1998; Mallet and Maxfield, 1999), mannose 6-phosphate receptor (Goda and Pfeffer, 1988), furin (Mallet and Maxfield, 1999), GLUT4 (Shewan et al., 2003), and some glycosylphosphatidylinositol (GPI)-anchored proteins (Nichols et al., 2001; Nichols, 2002). To date, several retrograde transport pathways in the endocytic route to the trans-Golgi network (TGN) have been identified. These include a well-studied pathway from the late endosome to the TGN taken by mannose 6-phosphate receptors (Goda and Pfeffer, 1988) and furin (Mallet and Maxfield, 1999), and a newly discovered direct pathway from the early/recycling endosome to the TGN taken by TGN38 (Ghosh et al., 1998; Mallet and Maxfield, 1999), GLUT4 (Shewan et al., 2003), and exogenously added Shiga toxin B subunit (Mallard et al., 1998). Similar transport from the late endosome (prevacuolar compartment) and the early/recycling endosome (post-Golgi compartment) to the TGN (late Golgi) has been described in the yeast Saccharomyces cerevisiae (Bensen et al., 2001; Siniossoglou et al., 2001). Recently, GPI-anchored green fluorescent protein (GFP), CD59, and a fraction of cholera toxin B subunit have been found to accumulate in a discrete population of endosomes en route to the Golgi apparatus (Nichols, 2002). These endosomes are devoid of markers for classical early and recycling endosomes, but they do contain caveolin-1. However, another study suggests that GPI-anchored proteins are endocytosed to the recycling endosomal compartment via nonclathrin, noncaveolae-mediated pathway (Sabharanjak et al., 2002).
Shiga toxin is a bacterial toxin that is highly toxic to a large number of eukaryotic cells. Its toxicity is dependent on intracellular membrane transport for reaching its targets that reside in the cytoplasm (Sandvig and van Deurs, 2000). Shiga toxin is composed of two subunits (Sandvig and van Deurs, 2000). The A-subunit is an enzyme with RNA N-glycosidase activity. It inhibits protein synthesis by inactivating 28S RNA of the 60S ribosomal subunit (Endo et al., 1988). The B-subunit is a pentamer and is responsible for binding to the cell surface receptor glycosphingolipid Gb3 and directing the holotoxin from plasma membrane, via endosomes and the Golgi apparatus, to the endoplasmic reticulum (Sandvig et al., 1992). Shiga toxin B subunit is therefore a useful marker for studying retrograde transport to the TGN. Johannes et al. (1997) have generated a recombinant Shiga toxin B fragment (STxB) that contains two tyrosine sulfation sites. The addition of these tyrosine sulfation sites facilitates biochemical and quantitative monitoring the arrival of STxB at the Golgi apparatus because tyrosine sulfation is a trans-Golgi/TGN-specific event (Niehrs and Huttner, 1990). Morphological and biochemical studies by Mallard et al. (1998) have shown that, at low temperature, internalized STxB partitions away from markers destined for the late endocytic pathway and colocalizes extensively with cointernalized transferrin. On subsequent incubation at 37°C, STxB is rapidly transported to the Golgi apparatus. Transport to the Golgi is insensitive to actin-depolymerizing and pH-neutralizing drugs that affect vesicular transport at the late endocytic pathway. Based on these results it has been proposed that STxB follows a direct pathway from the early/recycling endosomes to the Golgi apparatus, bypassing the late endosome. Mechanistically, STxB transport from the endosome to the Golgi has been shown to involve Rab11 (Wilcke et al., 2000), a Rab6 isoform, Rab6a′ (Mallard et al., 2002), and a SNARE complex consisting of syntaxin 16, syntaxin 6, Vti1a, and VAPM3/VAMP4 (Mallard et al., 2002).
Soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) are a group of membrane proteins that play important roles in the final stage of docking and fusion of vesicular transport (Söllner et al., 1993; Weber et al., 1998; McNew et al., 2000; Parlati et al., 2000). SNAREs on transport vesicles (v-SNARE) interact with SNAREs on the target membrane (t-SNARE) to mediate membrane docking and fusion. In mammalian cells, at least 12 SNAREs have been described to specifically or partially associate with the Golgi apparatus. These include syntaxin 5 (Bennett et al., 1993; Hay et al., 1998), syntaxin 6 (Bock et al., 1996, 1997, syntaxin 16 (Tang et al., 1998a), GS27/membrin (Hay et al., 1997; Lowe et al., 1997), Sec22b/ERS24 (Zhang et al., 1999), Bet1 (Zhang et al., 1997), GS28/GOS28 (Subramaniam et al., 1995, 1996), Ykt6 (Zhang and Hong, 2001), VAMP4 (Steegmaier et al., 1999; Zeng et al., 2003), GS15 (Xu et al., 1997, 2002), Vti1a/Vti1-rp2 (Xu et al., 1998), GS32/SNAP-29 (Wong et al., 1999), and syntaxin 10 (Tang et al., 1998b). Syntaxins 6, 10, and 16 are localized to the TGN (Bock et al., 1997; Tang et al., 1998a, b). Detailed morphological studies show that Sec22b and Bet1 are preferentially associated with the pre-Golgi intermediate compartments (ICs) and with the cis-Golgi (Zhang et al., 1997, 1999; Hay et al., 1998). GS27 is mainly localized in the cis-Golgi (Hay et al., 1998). Syntaxin 5 and GS28, originally thought to be enriched in the early Golgi, are now shown to be present in every Golgi cisternae (Hay et al., 1998; Orci et al., 2000; Volchuk et al., 2004). GS15 is recently shown to have increasing concentrations across the cisternae toward the trans-Golgi (Volchuk et al., 2004). The syntaxin 5/GS27/Sec22/Bet1 complex is involved in early stage of ER-Golgi transport and perhaps early intra-Golgi transport (Hay et al., 1998), the syntaxin 5/GS28/Ykt6/Bet1 complex is involved in the late stage of ER-to-Golgi transport (Zhang et al., 2001). The syntaxin 5/GS28/Ykt6/GS15 complex is likely to act within the Golgi apparatus (Xu et al., 2002), whereas the syntaxin 16/syntaxin 6/Vit1a/VAMP4 (or VAMP3) complex is shown to participate in early/recycling endosome (EE/RE)-TGN transport (Mallard et al., 2002).
An assay that reconstitutes the EE/RE-TGN transport of STxB has been recently established and was used to demonstrate the functional importance of Rab6a′ and the syntaxin 16/syntaxin 6/Vit1a/VAMP4 (or VAMP3) complex in this transport event (Mallard et al., 2002). This assay, however, is performed on tissue culture plates and is therefore, inconvenient for testing a large number of samples. The reaction volumes are rather large and consume a lot of protein or antibody reagents. To overcome these difficulties, we modified the assay using suspension of semi-intact cells. Examining all known Golgi SNAREs by using this modified assay, we found that syntaxin 5, GS28, Ykt6, and GS15 are involved in STxB transport. Because syntaxin 16-containing SNARE complex has been shown to participate in this transport, our results suggest that there are two SNARE complexes involved in EE/RE-TGN transport of STxB.
MATERIALS AND METHODS
Antibodies, Antigens, and Chemicals
Polyclonal antibodies against syntaxin 5 (Subramaniam et al., 1997), syntaxin 16 (Tang et al., 1998a), syntaxin 7 (Wong et al., 1998), Vti1a (Xu et al., 1998), GS15 (Xu et al., 1997), Ykt6 (Zhang and Hong, 2001), Bet1 (Zhang et al., 1997), Sec22b (Zhang et al., 1999), GS32 (Wong et al., 1999), Sec31 (Tang et al., 2000), GT (β1,4-galactosyltransferase) (Subramaniam et al., 1992), and monoclonal antibodies against GS27 (Lowe et al., 1997) and GS28 (HFD9) (Subramaniam et al., 1995) as well as the antigens for syntaxin 5, GS15, and Ykt6 antibodies GST-syntaxin 5 (Subramaniam et al., 1997), GST-GS15 (Xu et al., 1997), and GST-Ykt6 (Zhang and Hong, 2001) have been described previously. Monoclonal antibodies against GM130 and GS15 were purchased from BD Biosciences (San Diego, CA). Polyclonal antibody against Giantin was from Covance (Princeton, NJ). Polyclonal antibody against tyrosylprotein sulfotransferase (TPST) was a generous gift by Dr. Chinnaswamy Kasinathan (University of Medicine and Dentistry of New Jersey, Newark, NJ). β-Tubulin monoclonal antibody (mAb) was from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal antibody against the myc epitope tag was from Upstate biotechnology (Charlottesville, VA). Antibodies against myc tag (9E10, hybridoma from American Type Culture Collection, Manassas, VA), and transferrin receptor (OKT9, hybridoma from American Type Culture Collection) were purified from mice ascitic fluid. All antibodies used for transport assays were either affinity-purified (for polyclonal antibodies) or purified using protein G-Sepharose (Pierce Chemical, Rockford, IL) (for monoclonal antibodies). Purified antibodies were dialyzed in 25/125 buffer (25 mM HEPES, pH 7.2, and 125 mM KOAc) and checked for their immunolabeling qualities by immunofluorescence microscopy and/or Western blot analyses. Fab fragments were prepared from syntaxin 5 and GS15 antibodies according to the manufacturer's instructions (Pierce). Streptolysin O was obtained from Dr. S. Bhakdi (Johannes-Gutenberg-Universität, Mainz, Germany). All other chemicals were from Sigma-Aldrich or Merck (Whitehouse Station, NJ) and are of analytical grade or better.
Preparation of Recombinant STxB
A plasmid expressing the modified Shiga toxin B subunit, STxB-Sulf2, was described previously and periplasmic extraction and protein purification were performed essentially as described previously (Johannes et al., 1997).
In Vitro Transport Assays
HeLa cells grown on a 10-cm tissue culture dish were starved for 1 h in minimum essential medium without sulfate (Sigma-Aldrich) supplemented with 5% dialyzed fetal bovine serum, 1 mM Ca2+, and 10 mM HEPES, pH 7.3. The cells were then incubated in fresh medium containing 1 μM STxB for 1 h at 18°C followed by 30-min chase at 18°C in fresh medium without STxB, allowing its accumulation in the early/recycling endosome. Cells were then placed on ice and incubated for 10 min with 20 μg/ml Streptolysin O in permeabilization buffer [25 mM HEPES, pH 7.3, 125 mM KOAc, 2.5 mM Mg(OAc)2, and 1 mM dithiothreitol (DTT)]. After removing unbound Streptolysin O, cells were perforated at 18°C for 30 min in permeabilization buffer. The cells were scrapped, pelleted at 1000 × g and resuspended in 150 μl of membrane buffer (25 mM HEPES, pH 7.3, 250 mM sucrose, and 1 mM EDTA). Typically, 200 μl of 1.5-4 mg/ml semi-intact cells would be obtained from one 10-cm tissue culture dish and was sufficient for 20 transport assays. The cells were either used immediately or stored at -80°C until needed.
The EE/RE-TGN transport was reconstituted in a 1.5-ml Eppendorf tube. A standard transport assay contains 10 μl of semi-intact cells, 5 μl of rat liver cytosol at 17.5 mg/ml in buffer 25/125, 6 μl of ATP-mix (10 mM ATP, pH 7.0, 150 mM creatine phosphate, 210 U/ml creatine phosphokinase, and 20 mM MgCl2), 4 μl of reaction buffer [250 mM HEPES, pH 7.3, 250 mM KCl, 15 mM Mg(OAc)2], 20 μl of buffer 25/125 (used as control) or buffer 25/125 containing the reagents to be tested, 1 mM DTT (final concentration), 0.2 mM GTP (final concentration), 1 mCi/ml [35S]sulfate (final concentration) (Amersham Biosciences, Little Chalfont, Buckinghamshire, United Kingdom), and H2O to a final volume of 75 μl. The tubes were left on ice for 1 h before being transferred to a 37°C water bath and incubated for a further 90 min. Membrane pellets were recovered after centrifugation at 16,000 × g for 30 min in an Eppendorf microcentrifuge and were solubilized in 20 μl of Laemmli sample buffer. Proteins were separated on 15% modified Laemmli peptide separation gels and blotted onto nitrocellulose filters. Radioactive bands were recorded with a Molecular Imager FX (Bio-Rad, Hercules, CA), and band intensities were quantified with its software. Cytosol-dependent transport was calculated by subtracting the basal transport obtained in the absence of exogenous cytosol and was used to calculate relative transport or inhibition levels in all experiments except for those shown in Figures 1, A and C, 4B, 5C, 6B, and 7B in which the original values were used for the calculations.
Figure 1.
Reconstitution of the EE/RE-TGN transport by using a modified STxB. (A) Transport was carried out in the absence or presence of 0.6, 1.2, 1.8, 2.4, and 3.0 mg/ml rat liver cytosol as indicated. The relative level of transport was shown, with the highest transport level being defined arbitrarily as 100%. (B-D) Standard transport mixtures containing perforated cells, rat liver cytosol, and ATP regeneration system were preincubated for 1 h on ice with BFA (B), nocodazol (B), GTPγS (C), or various antibodies (D) as indicated. They were then shifted to 37°C and incubated for a further 90 min. Controls were the standard transport setups without any additional reagents or antibodies, and the levels of transport were arbitrarily defined as 100%. The respective concentrations (micrograms per milliliter) of the added reagents were shown in brackets (B and D). H, heat inactivated.
Figure 4.
STxB transport to the TGN was inhibited in HeLa cells when GS15 expression is knocked down by its siRNA. HeLa cells grown on 24-well plates were transfected with GS15 or control siRNA for 48 h followed by Western blot analysis with antibodies against GS15, Bet1, GS27, or β-tubulin (A) or analysis of the levels of 35S-labeled STxB (assessed by in vivo transport) and β-tubulin (assessed by Western blot) after the in vivo STxB transport assay (B).
Figure 5.
Two SNARE complexes are involved in the EE/RE-TGN transport. (A) Inhibition by syntaxin 5 and syntaxin 16 antibodies was additive. (B) Inhibition by syntaxin 5 and GS15 antibodies was not additive, whereas the inhibition by syntaxin 16 and GS15 antibodies was. (A and B) Transport was carried out in the presence of buffers (controls), antibodies, or combination of two different antibodies as indicated. (C) Inhibition by GS15 siRNA in vivo and syntaxin 16 antibody in vitro was additive. HeLa cells transfected with GS15 or control siRNA were processed to make semi-intact cells and used for in vitro transport in the presence or absence of syntaxin 16 antibodies as indicated. (A-C) Extent of transport relative to each control (set as 100%) was calculated from two to three separate experiments. Error bars represented standard deviations. The respective concentrations (micrograms per milliliter) of the added reagents were shown in brackets.
Figure 6.
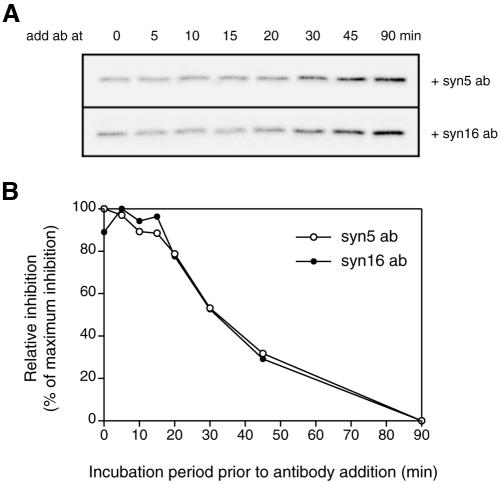
Antibodies against syntaxin 5 and syntaxin 16 exhibited similar kinetics of inhibition. Standard transport reactions at 37°C were allowed to proceed for 0, 5, 10, 15, 20, 30, 45, and 90 min as indicated and paused on ice. Either 67 μg/ml syntaxin 5 or 20 μg/ml syntaxin 16 antibodies were added at these time points, and the mixture was left on ice for 50 min. Transport reactions were resumed at 37°C and allowed to proceed until 90 min in total. (A) Representative phosphorimaging data. (B) Graph plotted from duplicates. Maximum inhibition values were set as 100%. This result suggests that on its way from endosomes to the TGN, STxB passes syntaxin 5- and syntaxin 16-sensitive stages simultaneously.
Figure 7.
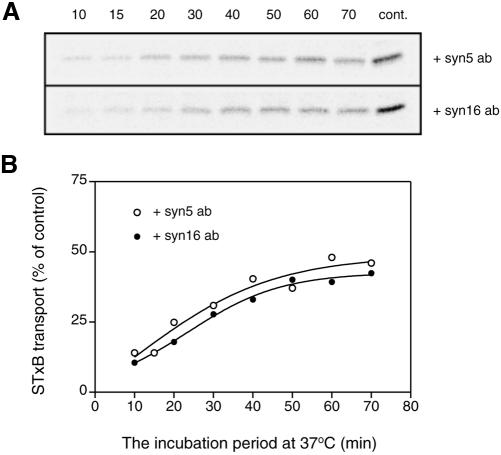
Syntaxin 16-mediated (syntaxin 5-independent) and syntaxin 5-mediated (syntaxin 16-independent) routes have similar time courses of transport. Transport reactions with either 67 μg/ml syntaxin 5 or 20 μg/ml syntaxin 16 antibodies were allowed to proceed for 10, 15, 20, 30, 40, 50, 60, and 70 min at 37°C and stopped on ice. Controls (cont.) were transport reactions that proceeded for 70 min in the absence of any antibody. Transport relative to control (set as 100%) at each time point was calculated. (A) Representative phosphorimaging data. (B) Graph plotted from duplicates. Curves fitting each set of data are presented.
Mammalian Expression Constructs
The mouse SNX3 coding sequence was amplified by polymerase chain reaction (PCR) (primers 5′-CAG TAC GAA TTC GGC GGA GAC GGT AGC GGA CAC-3′ and 5′-GTC GTC TCT AGA GGC ATG TCT TAT TTT AGA TGG AG-3′) by using Pfu DNA polymerase. The PCR product was digested by EcoRI and XbaI and subsequently cloned into EcoRI/XbaI-digested pDMycneo vector (pCI-neo vector from Promega, Madison, WI, with two myc epitopes inserted in between NheI and XhoI sites) to express the N-terminal double myc-tagged SNX3. cDNA fragment encoding rat α2,6-sialyltransferase (ST) (amino acids 1-64) was cloned by PCR and inserted into NheI/BamHI sites of pEGFP-N3 (ST-EGFP).
Transfection and Indirect Immunofluorescence Microscopy
A431 cells were maintained in DMEM supplemented with 10% fetal bovine serum and transfected with pDMyc-neo/SNX3 vector using the Effectene transfection reagent (QIAGEN, Hilden, Germany) following the manufacturer's protocol. Indirect immunofluorescence microscopy was performed as described previously (Lu et al., 2001).
GS15 Knockdown by Small Interfering RNA (siRNA) Followed by In Vivo and In Vitro STxB Transport Assays
siRNA targeting human GS15 (5′-AAG CAU GAC CAG CCU GCU UAC-3′), referred to as GS15 siRNA, was purchased from Dharmacon Research (Lafayette, CO). The control siRNA was described previously (Lu and Hong, 2003). HeLa cells grown on a 24-well plate (for the in vivo assays) and a six-well plate (for the in vitro assays) were transfected with GS15 or control siRNA by using the Oligofectamine transfection reagent (Invitrogen, Carlsbad, CA) according to the protocol provided by Dharmacon Research. After 48 h of incubation, cells were washed and used for in vivo or in vitro transport assays. The in vitro transport assays were performed as described above. The in vivo transport assays were performed as follows. The cells were first starved for 1 h in minimum essential medium without sulfate followed by incubation in fresh medium containing 1 μM STxB and 0.5 mCi/ml [35S]sulfate for 90 min at 37°C. Subsequently, the cells were washed with cold phosphate-buffered saline and solubilized in Laemmli sample buffer. Proteins were separated and radioactive bands were recorded and quantified. To check the efficiency of siRNA treatment, another set of cells was treated with siRNA in an identical way, and the expression of GS15 and other proteins was examined by Western blot with GS15 mAb and other respective antibodies.
RESULTS
A Modified In Vitro Assay Reconstituting EE/RE-TGN Transport
An in vitro EE/RE-TGN transport assay was established using Streptolysin O-permeabilized HeLa cells and a marker protein STxB-Sulf2, a recombinant Shiga toxin B subunit containing two sulfation sites (STxB) (Mallard et al., 2002). This transport assay, involving the permeabilization of cells adherent on tissue culture plates, is reagent consuming and not particularly convenient for high throughput analyses. To investigate the function of several reagents in an economical way, we have modified this assay. HeLa cells are first allowed to accumulate STxB in the EE/RE at 18°C. These cells are then perforated with Streptolysin O at 18°C, instead of 37°C to minimize the basal level of transport. The permeabilized cells are scraped off and resuspended in buffer for immediate use or snap-frozen in liquid nitrogen and maintained at -80°C. Finally, transport assays are assembled in microcentrifuge tubes with equal volumes of aliquoted semi-intact cell suspensions, effectively achieving normalization of cell quantity, a necessity difficult to accomplish with adherent cells. Above all, because the reaction volume is significantly reduced, the modified assay requires much less protein or antibody reagents relative to the assay in the tissue culture plate format.
The efficiency of the modified assay is shown in Figure 1. STxB transport is cytosol dependent with basal levels of only 5-10% relative to the maximal levels that are achieved with 1.2 mg/ml or more of rat liver cytosol (Figure 1A). Consequently, 1.2 mg/ml rat liver cytosol was used for all assays reported in this study.
The validity of the modified assay was first established with reagents whose functions are well documented. The drug brefeldin A (BFA) is a fungal metabolite that is known to inhibit STxB retrograde transport (Mallard et al., 1998, 2002). A microtubule-destabilizing drug nocodazol, on the other hand, has no effect on STxB transport from endosomes to Golgi (Mallard et al., 1998, 2002). In good agreement with these previous observations, Figure 1B shows that 5 and 10 μg/ml BFA inhibited ∼80% of this in vitro STxB transport, whereas nocodazol (Noc) did not. Guanosine 5′-O-(3-thio)triphosphate (GTPγS), a nonhydrolyzable GTP analogue, has been used in many cases to establish the importance of GTPases in a particular process. In agreement with previous findings and other transport assays (Goda and Pfeffer, 1988; Mallard et al., 2002), 1 mM GTPγS (net concentration 0.8 mM) inhibited >90% of STxB transport (Figure 1C).
The specificity of the modified assay was further demonstrated by antibody inhibition studies (Figure 1D). Antibodies to SNARE proteins syntaxin 16, syntaxin 7, and Vti1a were tested. Syntaxin 16 (syn16 ab) and Vti1a (vti1a ab) antibodies inhibited potently the transport in our modified suspension assay, whereas syntaxin 7 antibodies (syn7 ab) did not, in agreement with previous findings (Mallard et al., 2002). These data together establish that our modified assay has indeed faithfully reconstituted the EE/RE-TGN transport in vitro.
The SNARE Proteins Syntaxin 5, Ykt6, GS28, and GS15 Are Involved in EE/RE-TGN Transport
We next screened a panel of Golgi-associated SNAREs for involvement in the EE/RE-TGN transport by using our modified in vitro assay. Syntaxin 5 has previously been shown to localize at the cis-Golgi and participate in ER-to-Golgi transport (Bennett et al., 1993; Dascher et al., 1994; Rowe et al., 1998). Surprisingly, syntaxin 5 antibody (syn5 ab) dramatically reduced STxB transport to the TGN (Figure 2A). The typical level of inhibition ranged between 50 and 70%. The inhibition exhibited by the antibodies was abolished by heat-denaturing the antibody (syn5 ab-H), suggesting that interaction with the endogenous syntaxin 5 is the basis for its inhibition of EE/RE-TGN transport. Preincubation of the antibody with its antigen glutathione S-transferase (GST)-syntaxin 5 (GST-syn5) neutralized its inhibition of the STxB transport, yielding levels of ∼70% relative to the control, similar to levels obtained with GST-syn5 alone. In contrast, the inhibition remained unchanged after preincubation of the antibody with GST. GST alone had no effect on STxB transport. To eliminate the possibility that the antibody inhibition was caused by divalent cross-linking, we prepared Fab fragments from syntaxin 5 antibodies (syn5 Fab). Similar to intact antibody molecules, the Fab fragments inhibited STxB transport (by ∼50%) and the inhibition could be reversed by pretreatment of Fab fragments with GST-syn5 or by heat inactivation (syn5 Fab-H) (Figure 3A). Together, these data confirmed that the syntaxin 5 antibody specifically inhibits STxB transport and implied that syntaxin 5 is involved in EE/RE-TGN transport of STxB.
Figure 2.
Syntaxin 5, GS28, Ykt6, and GS15 are involved in the EE/RE-TGN transport. (A-C) Syntaxin 5, GS15, and Ykt6 polyclonal antibodies inhibited the EE/RE-TGN transport. (D) GS28, but not GS27 mAb inhibited the in vitro transport. As indicated, transport reactions were carried out in the presence of buffer (controls), antigens, GST, antibodies, or heat-inactivated antibodies. Where both antibodies and antigens (or GST) were present, antibodies were incubated with antigens (or GST) on ice for 1 h before being added to the standard reactions. Extent of transport relative to each control (defined as 100%) was an average from two to four separate experiments. Error bars represented standard deviations. The respective concentrations (micrograms per milliliter) of the added reagents were shown in brackets. H, heat inactivated.
Figure 3.
Fab fragments derived from syntaxin 5 or GS15 antibodies inhibited the EE/RE-TGN transport. Fab fragments were prepared from syntaxin 5 or GS15 antibodies. Transport was carried out in the presence of buffer (controls), Fab fragments, antigens, or heat-inactivated Fab fragments. Where both Fab fragments and antigens were present, Fab fragments were incubated with antigens on ice for 1 h before being added to the transport reactions. The extent of transport relative to each control (defined as 100%) was calculated from two to three separate experiments. (A) Transport in the presence of the Fab fragments and/or antigens of syntaxin 5 antibodies. (B) Transport in the presence of the Fab fragments and/or antigens of GS15 antibodies. Error bars represented standard deviations. The respective concentrations (micrograms per milliliter) of the added reagents were shown in brackets. H, heat inactivated.
We also observed that antibodies to three other Golgi SNARE proteins, namely, GS15, Ykt6, and GS28 also inhibited STxB transport to varying extents. Figure 2, B and C, show that GS15 and Ykt6 polyclonal antibodies inhibited STxB transport by 40-60% and 30-50%, respectively. The inhibition could be lifted by pretreatment of antibodies with their antigens, GST-GS15 or GST-Ykt6, but not with the GST moiety alone. The Fab fragment of anti-GS15 (GS15 Fab) also inhibited the in vitro transport (Figure 3B).
Figure 2D shows that GS28 but not GS 27 mAb inhibited STxB transport. The moderate level of inhibition (∼30%) by GS28 antibody is clearly significant, because it was lost when GS28 mAb was heat inactivated before the transport reaction (GS28 ab-H). GM130 mAb was not inhibitory (unpublished data). Antibodies against other Golgi-associated SNAREs, such as Bet1, Sec22b, and GS32 or antibody against COPII coat protein Sec31 also did not inhibit STxB transport under similar conditions (unpublished data), although antibodies to Bet1, Sec22b, and Sec31 inhibited in vitro ER-to-Golgi transport, as described previously (Zhang et al., 1997, 1999; Tang et al., 2000).
To rule out the possibility that the Golgi apparatus was disrupted by the addition of SNARE antibodies, which results in mislocalization of tyrosylprotein sulfotransferase, we checked the integrity of Golgi apparatus after Streptolysin O permeabilization and antibody treatment. When HeLa cells transfected with ST-EGFP, a trans-Golgi/TGN marker, were permeabilized and treated with GS15 polyclonal and GS28 monoclonal antibodies, respectively, ST-EGFP was found to remain concentrated in the perinuclear region and colocalized with the bound GS15 as well as GS28 antibodies in the Golgi apparatus (Supplementary Figure 1). This suggested that the Golgi apparatus remained intact after permeabilization and the treatment with GS15 and GS28 antibodies. Also in cells treated with GS28 mAb, TPST remained enriched in the Golgi apparatus with some peripheral labeling, which is similar to the labeling obtained from cells that were fixed directly for immunofluorescence microscopy (Supplementary Figure 2), indicating that TPST distribution is not altered by the permeabilization and antibody treatment.
Together, these data demonstrate that antibodies against syntaxin 5, GS28, Ykt6, and GS15 specifically inhibited STxB transport to the TGN, suggesting that endogenous syntaxin 5, GS28, Ykt6, and GS15 are involved in the EE/RE-TGN transport. Because these four SNAREs exist as a unique SNARE complex (Xu et al., 2002; Shorter et al., 2002) and a similar SNARE complex was shown to be present in yeast (Parlati et al., 2002), it is likely that they function together as a novel SNARE complex regulating the EE/RE-TGN transport.
STxB Transport Is Reduced When GS15 Is Knocked Down by Its siRNA
To further establish the importance of the SNARE complex in STxB transport, we used a knockdown of protein expression approach with siRNA. Syntaxin 5 was not selected for this because we reasoned that its knockdown would lead to massive impairment of cell health arising from its absolute requirement for ER-Golgi transport and its presence in several distinct SNARE complexes (Bennett et al., 1993; Dascher et al., 1994; Rowe et al., 1998). GS15 was chosen instead because it does not act in the ER-Golgi transport, and its antibody exhibited similar efficacy of inhibition compared with syntaxin 5 antibody. HeLa cells were transfected with GS15 or control siRNA. The effectiveness of siRNA treatment in “knocking down” specific protein expression was verified by Western blot analysis. Figure 4A shows that GS15 expression was dramatically reduced in cells transfected with GS15 siRNA compared with expression levels in the cells transfected with the control siRNA. The expression of other proteins such as Bet1, GS27, and β-tubulin was not affected by GS15 siRNA. Significantly, STxB transport in cells transfected with GS15 siRNA was reduced to 36% in the in vivo transport assay (Figure 4B) and 42% in the in vitro transport assay by using the resulting semi-intact cells (Figure 5C), respectively, compared with the cells transfected with control siRNA. This indicates that GS15 is necessary for proper EE/RE-TGN transport of STxB both in vivo and in vitro. The possibility that the reduction of STxB transport was caused by impaired endocytosis was ruled out by the observation that endocytosis of STxB into endosomes was not altered in transfected cells (unpublished data). The inhibition was also unlikely due to any gross disturbance of Golgi apparatus, because a medial Golgi marker, Giantin, and a trans-Golgi marker, GT (β1,4-galactosyltransferase), did not show any morphological changes in GS15 siRNA-transfected cells (unpublished data).
Two SNARE Complexes Are Involved in EE/RE-TGN Transport
In yeast, the SNAREs Sed5p, Gos1p, Ykt6p, and Sft1p have been shown to form a complex both in vivo and in vitro (Parlati et al., 2002). Sed5p, Gos1p, and Sft1p are the yeast homologues of mammalian syntaxin 5, GS28, and GS15, respectively. In mammalian cells, syntaxin 5, GS28, Ykt6, and GS15 are known to form a complex such that antibodies against any one of the four SNAREs coimmunoprecipitate the other three (Shorter et al., 2002; Xu et al., 2002). To investigate whether these SNARE proteins work as a complex in STxB transport, we compared the degree of inhibition in the presence of individual antibody with that in the presence of two antibodies in combination. Theoretically, if two SNAREs exist in different complexes their antibodies would show additive inhibitory effects. Figure 5A shows that the extent of transport when both syntaxin 5 and syntaxin 16 antibodies were present was reduced to 17% of control, much lower than transport levels in the presence of either syntaxin 5 antibody (41%) or syntaxin 16 antibody (33%) alone. This suggests that syntaxin 5 and syntaxin 16 exist in different complexes, which is in good agreement with previous studies. Figure 5B shows that the STxB transport in the presence of both syntaxin 5 and GS15 antibodies was 30%, similar to that obtained in the presence of syntaxin 5 antibody alone (33%). In contrast, GS15 and syntaxin 16 antibodies together inhibited transport to a much lower level (Figure 5B). Similar to the GS15-syntaxin 16 antibody combination, when semi-intact cells derived from GS15 siRNA-treated cells were used for the in vitro assay, syntaxin 16 antibody also showed an additional inhibition. Whereas GS15 siRNA (assessed for in vitro transport by using the resulting semi-intact cells) or syntaxin 16 antibody (added in the semi-intact cells derived from control siRNA-treated cells) alone reduced transport level to 42 or 31%, respectively, GS15 siRNA and syntaxin 16 antibody together reduced transport level to 12% (Figure 5C). These results, together, suggest that GS15 and syntaxin 5 function within the same complex but independently of the syntaxin 16 complex in mediating EE/RE-TGN transport. As with GS15 antibody, the combination of syntaxin 5 antibody with either Ykt6 or GS28 antibodies did not show additive effects (unpublished data). Along with earlier demonstrations that these four SNAREs can form a unique SNARE complex, our results suggest that at least two distinct SNARE complexes are involved in EE/RE-TGN transport of STxB.
Two SNARE Complexes Act Simultaneously with Similar Kinetics
We attempted to explore whether it is possible to temporally distinguish the involvement of the two SNARE complexes in STxB transport through a time course study. Standard STxB transport reactions at 37°C were allowed to proceed for various time intervals and stopped by incubation on ice. At these time points, either syntaxin 5 or syntaxin 16 antibodies were added to the assay mixture. Transport reactions were then resumed at 37°C. It is well established that SNARE proteins function in vesicle docking and fusion steps, a final stage in vesicular transport. Thus, antibodies added before the docking and fusion step will inhibit transport, whereas antibodies added after the docking and fusion step will not affect transport. Figure 6 shows that the time course profiles of inhibition by syntaxin 5 and syntaxin 16 antibodies are very similar. In both cases, antibodies added earlier than 15 min exerted maximum inhibitions. At 20 min, the inhibition exhibited by the antibodies began to decrease, indicating that some STxB molecules (∼20%) had already passed the antibody sensitive stage. At 30 and 45 min, ∼50 and 70% of the molecules had passed this antibody-sensitive stage, respectively. The nearly identical kinetics of antibody susceptibility for syntaxin 5 and syntaxin 16 between 20 and 90 min indicate that the STxB molecules pass the syntaxin 5- and syntaxin 16-sensitive stages simultaneously. In other words, syntaxin 5 and syntaxin 16 were required for docking and fusion function at roughly the same time for EE/RE-TGN transport of STxB. Given that syntaxin 5 and syntaxin 16 exist in two distinct functional complexes, these results suggest that STxB-containing vesicles traveled along two routes to reach TGN, one depending on syntaxin 16 complex and the other on syntaxin 5 complex as part of their fusion machineries.
Although both routes started from endosomes and ended up at the TGN (as the transport assay monitors STxB sulfation, a trans-Golgi/TGN-specific event), their itineraries between the endosomes and the TGN might be different. The syntaxin 5 complex was previously believed to reside and therefore function in the cis-Golgi (Bennett et al., 1993; Dascher et al., 1994; Rowe et al., 1998). If this is the case, vesicles requiring syntaxin 5 for transport should first arrive at the cis-Golgi and subsequently move across the Golgi stack and finally arrive at the TGN. Thus, vesicles traveling via the syntaxin 5-dependent route would take longer to arrive at the TGN relative to those taking the syntaxin 16-dependent route for direct fusion with the TGN. To test this possibility, we measured the kinetics of syntaxin 5-dependent (in the presence of saturating amounts of anti-syntaxin 16 antibodies) and syntaxin 16-dependent (in the presence of saturating amounts of anti-syntaxin 5 antibodies) transport events. Two sets of assays were set up. One set contained syntaxin 5 antibody to inhibit syntaxin 5-dependent transport and the other contained syntaxin 16 antibody to inhibit syntaxin 16-dependent transport. Both sets underwent standard transport reactions for various time intervals and transport was then terminated by incubation on ice. In this way, the net transport event observed in the former reactions was syntaxin 5-independent transport that is mediated mainly by the syntaxin 16 complex; and in the later reaction, syntaxin 16-independent transport mediated mainly by the syntaxin 5 complex was measured. Figure 7 shows that syntaxin 16-mediated (top) and syntaxin 5-mediated (bottom) transport followed similar time courses. The temporal profiles for the curves fitting each set of data are essentially identical (Figure 7B). This result suggests that both routes may have similar itineraries, i.e., the syntaxin 5 complex also might function at the TGN as does the syntaxin 16 complex. The recent demonstration that syntaxin 5 and GS28 are distributed widely across the Golgi stack (Hay et al., 1998; Orci et al., 2000; Volchuk et al., 2004) is consistent with the possibility that a syntaxin 5 containing complex acts at the TGN.
Some GS15 and Ykt6 Are Redistributed to the Endosomes in SNX3-overexpressing Cells
v-SNAREs on the vesicles and t-SNAREs on target membrane pair up to mediate vesicle docking and fusion. Some v-SNAREs must be present on the donor membrane, and some t-SNAREs must be present on the target membrane. In STxB transport, the donor membrane is the endosomes and the target membrane is the TGN. Therefore, a functional SNARE complex must have a component(s) in the endosomes to serve as the v-SNARE. However, all members of the syntaxin 5 complex that we show here to be necessary for STxB transport (syntaxin 5, GS28, Ykt6, and GS15) have been reported to localize primarily to the Golgi apparatus at steady state, an observation difficult to reconcile with their function in EE/RE-TGN transport. This apparent contradiction is lightened somewhat by our observation that some GS15 and Ykt6 were redistributed to the endosomes when the recycling endosome was perturbed by the overexpression of SNX3 (Figure 8). When A431 cells were transfected with SNX3, a sorting nexin that can perturb endosomal recycling upon overexpression (Xu et al., 2001), a clear redistribution of some GS15 and Ykt6 to the SNX3-marked endosomes was observed. The SNX3-marked structures represent a mixture of early, recycling, and late endosomes (Xu et al., 2001). This redistribution to endosomes by SNX3 overexpression is specific for GS15 and Ykt6 and does not affect other Golgi SNAREs such as syntaxin 5, GS27 (Figure 8), or GS28 (unpublished data). These results suggest that GS15 and Ykt6, although predominantly Golgi localized at steady state, might cycle between the Golgi and endosomes with low levels in the endosomes that are difficult to detect using conventional methods. When endosomal recycling is disturbed by SNX3 overexpression, GS15 and Ykt6 might then be partially arrested in the endosomes and become effectively enriched. Thus, the SNX3 overexpression might have highlighted a phenomenon that is not obvious under normal circumstances. Consistent with this possibility, transport of cholera toxin B fragment was significantly arrested in some peripheral structures in cells overexpressing SNX3 (Supplementary Figure 3), suggesting that SNX3 overexpression did arrest cycling between the endosomes and the Golgi apparatus. These observations suggest that GS15 and/or Ykt6 could potentially serve as endosomal v-SNAREs in the EE/RE-TGN transport. For Ykt6 at least, the notion of a post-Golgi function is in agreement with recently described observations in yeast (Kweon et al., 2003).
Figure 8.
Some GS15 and Ykt6 were redistributed to endosomes in SNX3-overexpressing cells. A431 cells were transfected with myc-SNX3 and double labeled with antibodies against GS15 (B), Ykt6 (E), syntaxin 5 (G), or GS27 (J) and anti-Myc antibody in SNX3-overexpressing cells (A, D, F, and I). Bar, 10 μM.
DISCUSSION
In an attempt to systematically examine the participation of various proteins in the EE/RE-TGN transport and to identify/isolate new factors involved in this transport event, we have developed a simpler, more consistent and quantitative EE/RE-TGN transport assay based on a previous system relying on adherent cells (Mallard et al., 2002). An examination of all possible parameters and requirements suggests that this cell suspension-based assay is fundamentally and mechanistically similar to the earlier version. The role of the syntaxin 16 SNARE complex was faithfully reflected in both assays. We had originally intended to use the syntaxin 5 antibody as a negative control, because syntaxin 5 had been shown to localize in the cis-Golgi and participate in ER-Golgi transport (Bennett et al., 1993; Dascher et al., 1994; Rowe et al., 1998). Surprisingly, we observed that syntaxin 5 antibody potently and specifically inhibited the EE/RE-TGN transport in both assay systems, and we further characterized its involvement by using the modified assay. The finding that syntaxin 5 is involved in the EE/RE-TGN transport led us to check antibodies to other Golgi-associated SNAREs. We found that antibodies against GS28, Ykt6, and GS15 also inhibited the EE/RE-TGN transport to varying degrees. The inhibitions were specific because antibodies to other Golgi-associated SNAREs such as Bet1, Sec22b, GS27, and GS32, did not inhibit the transport under the same conditions, although they had profound effects in the ER-Golgi transport (for example, Bet1 and Sec22) (Zhang et al., 1997, 1999). In addition, GST-syntaxin 5 not only exhibited some consistent inhibition (albeit at low levels) (Figure 2A) but also could neutralize the more potent inhibition exhibited by its antibody. The inhibition by syntaxin 5 antibody was abolished by heat inactivation. These results suggest that binding with endogenous syntaxin 5 is the basis for anti-syntaxin 5 antibody to inhibit the EE/RE-TGN transport. To rule out the possibility that the inhibition is a secondary effect of antibody cross-linking, we have prepared Fab fragments of anti-syntaxin 5 and anti-GS15 antibodies and have demonstrated that the resulting Fab fragments similarly inhibited the EE/RE-TGN transport. These results therefore provide strong evidence for a role of syntaxin 5, GS15, GS28, and Ykt6 in the EE/RE-TGN transport. This conclusion was corroborated by our demonstration that knockdown of GS15 by its siRNA potently affected STxB transport to the TGN both in vivo and in vitro.
To rule out the possibility that the inhibition by antibodies and GS15 siRNA was caused by Golgi perturbation due to antibody or siRNA treatment, we examined the distribution of several trans-Golgi and TGN localized enzymes: GT, TPST, and ST. The distribution of these markers was largely unchanged in siRNA- and antibody-treated cells.
Intriguingly, these four SNAREs have recently been shown by two independent studies to form a unique SNARE complex (Shorter et al., 2002; Xu et al., 2002). A similar complex was independently demonstrated for yeast cells (Parlati et al., 2002). To investigate whether syntaxin 5, GS28, Ykt6, and GS15 work together as a complex in EE/RE-TGN transport, we compared the inhibitory effects obtained by the individual antibodies with those obtained by a combination of two antibodies. Whereas the syntaxin 5 antibody did not show any additive inhibitory effects with antibodies against the other three components of the same SNARE complex, the syntaxin 16 antibody showed clear additive inhibitory effects with syntaxin 5 or GS15 antibody. Similar additive inhibitory effects also were observed when cells were first treated with GS15 siRNA followed by in vitro transport by using the resulting semi-intact cells supplemented with syntaxin 16 antibody. These results suggest that syntaxin 5, GS28, Ykt6, and GS15 work together as a complex distinct from the syntaxin 16 complex identified previously (Mallard et al., 2002).
The notion of a syntaxin 5-based SNARE complex functioning in the EE/RE-TGN transport poses a problem regarding the identity of the v-SNARE in this complex. Both Ykt6 and GS15 were redistributed to endosomes in SNX3-overexpressing cells, suggesting that Ykt6 and/or GS15 might cycle between endosome and Golgi. The finding that Ykt6 and GS15 localize in compartments other than the Golgi in mammalian cells is unprecedented. In yeast, however, a significant portion of Ykt6p has been found to associate with the vacuole (equivalent to the mammalian late endosome and lysosome) and participate in yeast vacuole homotypic fusion. This indicates that Ykt6p is localized in multiple compartments and participates in multiple transport events (Ungermann et al., 1999; Kweon et al., 2003). That Golgi proteins cycle through the endosome is a known phenomenon. The cis-Golgi proteins GPP130 and GP73 have been observed to cycle to the cell surface and back along the late endosome-independent TGN38/46 pathway (Puri et al., 2002). This pathway also is shared by STxB (Mallard et al., 1998). It is possible that some Ykt6 and GS15 may cycle between endosomes and the Golgi in such a way that the residency time at the endosomes is very short, resulting in a predominant Golgi localization at the steady state. However, the fast cycling from endosomes to the Golgi is arrested at the endosomes when SNX3 is overexpressed to perturb the endocytic pathway. This observation indicates that GS15 and/or Ykt6 might function as v-SNARE(s) in the EE/RE-TGN transport process.
The finding that two SNARE complexes are involved in the EE/RE-TGN transport and that the complexes exert their effects simultaneously suggests that STxB could travel to TGN via two routes. One is syntaxin 16 dependent and the other is syntaxin 5 dependent. According to previous studies, the syntaxin 16 route is direct from the endosome to the TGN. At present, little is known about the syntaxin 5 route. Although the possibility still exists that STxB transport via the syntaxin 5-dependent route first progresses to the cis-Golgi, to be accepted by the syntaxin 5 complex and subsequently takes the anterograde pathway to the TGN, several lines of evidence indicate that the syntaxin 5 complex also may mediate direct transport from the endosome to the TGN. First, antibodies against syntaxin 5 and syntaxin 16 inhibited EE/RE-TGN transport with similar kinetics, suggesting that they act at a similar stage of transport in these two routes. Second, we have investigated more directly the transport process mediated by syntaxin 5 (in the presence of anti-syntaxin 16 antibodies) in comparison with that mediated by syntaxin 16 (in the presence of anti-syntaxin 5 antibodies) and have found that these two routes have similar kinetics of transport. Third, the redistribution of GS15 and Ykt6 to endosomes upon SNX3 overexpression indicates that endosomes are likely the donor compartment for the syntaxin 5 route as well as the syntaxin 16 route. Finally, the recent establishment that both syntaxin 5 and GS28 are present throughout the entire Golgi complex, whereas GS15 has increasing concentrations across the cisternae toward the trans-Golgi is consistent with the possibility for them to act directly at the TGN (Hay et al., 1998; Orci et al., 2000; Volchuk et al., 2004).
In yeast, components of the Sed5p (syntaxin 5) complex also have been found to participate in protein recycling from the endosomes to the Golgi. Thus, Gos1p, Ykt6p, and a dominant activator of Sed5p (syntaxin 5), Sly1p (SLY1-20), function as multicopy suppressors of ric1Δ and ypt6Δ cells that exhibit defects in protein retrieval from the endosomes to the TGN (Bensen et al., 2001). Snc1p that normally recycles to the Golgi via early endosomes accumulates in transport vesicles in a Gos1p deletion mutant (Siniossoglou and Pelham, 2001).
Why does the EE/RE-TGN transport of STxB occur in two routes mediated by two distinct SNARE complexes? One possibility is that the STxB carrying intermediates may not originate from and/or heading for the same domain of a given compartment. Both endosomes and TGN are highly mosaic with different domains or subdomains. It is possible that STxB is incorporated into two different populations of transport intermediates that travel along two routes toward the same or different domains of the TGN.
Although other possibilities also could also potentially explain the requirements of two SNARE complexes in the EE/RE-TGN transport, the discovery and clear demonstration of the role of the two SNARE complexes in the EE/RE-TGN transport are significant and will open new avenues to better understand this important transport event.
Supplementary Material
Acknowledgments
We thank Dr. Singh Paramjeet for critical reading of this manuscript and Dr. Chinnaswamy Kasinathan for providing TPST antibody. This work was supported by a grant from Agency for Science, Technology, and Research, Singapore (to W.H.). B.L.T. is an adjunct member of the Institute of Molecular and Cell Biology. W.H. is also a faculty member of the Department of Biochemistry, National University of Singapore.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-12-0876. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-12-0876.
Abbreviations used: EE/RE-TGN, early/recycling endosomes to trans-Golgi network; STxB, recombinant Shiga Toxin B subunit.
Online version of this article contains supporting material. Online version is available at www.molbiolcell.org.
References
- Bennett, M., Garcia-Arraras, J., Elferink, L., Peterson, K., Fleming, A., Hazuka, C., and Scheller, R. (1993). The syntaxin family of vesicular transport receptors. Cell 74, 863-873. [DOI] [PubMed] [Google Scholar]
- Bensen, E.S., Yeung, B.G., and Payne, G.S. (2001). Ric1p and the Ypt6p GTPase function in a common pathway required for localization of trans-Golgi network membrane proteins. Mol. Biol. Cell 12, 13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, J.B., Lin, R.C., and Scheller, R.H. (1996). A new syntaxin family member implicated in targeting of intracellular transport vesicles. J. Biol. Chem. 271, 17961-17965. [DOI] [PubMed] [Google Scholar]
- Bock, J.B., Klumperman, J., Davanger, S., and Scheller, R.H. (1997). Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol. Biol. Cell 8, 1261-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher, C., Matteson, J., and Balch, W. (1994). Syntaxin 5 regulates endoplasmic reticulum to Golgi transport. J. Biol. Chem. 269, 29363-29366. [PubMed] [Google Scholar]
- Endo, Y., Tsurugi, K., Yutsudo, T., Takeda, Y., Ogasawara, T., and Igarashi, K. (1988). Site of action of a vero toxin (VT2) from Escherichia coli O 157, H7 and of Shiga toxin on eukaryotic ribosomes. Eur. J. Biochem. 171, 45-50. [DOI] [PubMed] [Google Scholar]
- Ghosh, R.N., Mallet, W.G., Soe, T.T., McGraw, T.E., and Maxfield, F.R. (1998). An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 142, 923-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda, Y., and Pfeffer, S.R. (1988). Selective recycling of the mannose 6-phosphate/IGF-II receptor to the trans Golgi network in vitro. Cell 55, 309-320. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., Chao, D.S., Kuo, C.S., and Scheller, R.H. (1997). Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell 89, 149-158. [DOI] [PubMed] [Google Scholar]
- Hay, J.C., Klumperman, J., Oorschot, V., Steegmaier, M., Kuo, C.S., and Scheller, R.H. (1998). Localization, Dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs. J. Cell Biol. 141, 1489-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes, L., Tenza, D., Antony, C., and Goud, B. (1997). Retrograde transport of KGEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 272, 19554-219561. [DOI] [PubMed] [Google Scholar]
- Kweon, Y., Rothe, A., Conibear, E., and Stevens, T.H. (2003). Ykt6p is a multifunctional yeast R-SNARE that is required for multiple membrane transport pathways to the vacuole. Mol. Biol. Cell 14, 1868-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, S., L., Peter, F., Subramaniam, V.N., Wong, S.H., and Hong, W. (1997). A SNARE involved in protein transport through the Golgi apparatus. Nature 389, 881-884. [DOI] [PubMed] [Google Scholar]
- Lu, L., and Hong, W. (2003). Interaction of Arl1-GTP with GRIP domains recruits autoantigens Golgin-97 and Golgin-245/230 onto the Golgi. Mol. Biol. Cell 14, 3767-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, L., Horstmann, H., Ng, C., and Hong, W. (2001). Regulation of Golgi structure and function by ARF-like protein 1 (Arl1). J. Cell Sci. 114, 4543-4555. [DOI] [PubMed] [Google Scholar]
- McNew, J.A., Parlati, F., Fukuda, R., Johnston, R.J., Paz, K., Paumet, F., Söllner, T.H., and Rothman, J.E. (2000). Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature 407, 153-159. [DOI] [PubMed] [Google Scholar]
- Mallard, F., Antony, C., Tenza, D., Salamero, J., Goud, B., and Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B-fragment transport. J. Cell Biol. 143, 973-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F., Tang, B.L., Galli, T., Tenza, D., Saint-Pol, A., Xu, Y., Antony, C., Hong, W., Goud, B., and Johannes, L. (2002). Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet, W.G., and Maxfield, F.R. (1999). Chimeric forms of furin and TGN38 are transported from the plasma membrane to the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146, 345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B.J., Kenworthy, A.K., Polishchuk, R.S., Lodge, R., Roberts, T., Hirschberg, K., Phair, R.D., and Lippincott-Schwartz, J. (2001). Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B.J. (2002). A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 4, 374-378. [DOI] [PubMed] [Google Scholar]
- Niehrs, C., and Huttner, W.B. (1990). Purification and characterization of tyrosylprotein sulfatransferase. EMBO J. 9, 35-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci, L., Ravazzola, M., Volchuk, A., Engel, T., Gmachl, M., Amherdt, M., Perrelet, A., Sollner, T.H., and Rothman, J.E. (2000). Anterograde flow of cargo across the Golgi stack potentially mediated via bi-directional “percolating” COPI vesicles. Proc. Natl. Acad. Sci. USA 97, 10400-10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati, F., McNew, J.A., Fukuda, R., Miller, R., Söllner, T.H., and Rothman, J.E. (2000). Topological restriction of SNARE-dependent membrane fusion. Nature 407, 194-198. [DOI] [PubMed] [Google Scholar]
- Parlati, F., Varlamov, O., Paz, K., McNew, J.A., Hurtado, D., Söllner, T.H., and Rothman, J.E. (2002). Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA 99, 5424-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri, S., Bachert, C., Fimmel, C.J., and Linstedt, A.D. (2002). Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic 3, 641-653. [DOI] [PubMed] [Google Scholar]
- Rowe, T., Dascher, C., Bannykh, S., Plutner, H., and Balch, W.E. (1998). Role of vesicle-associated syntaxin 5 in the assembly of pre-Golgi intermediates. Science 279, 696-700. [DOI] [PubMed] [Google Scholar]
- Sabharanjak, S., Sharma, P., Parton, R.G., and Mayor, S. (2002). GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2, 411-423. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., Garred, Ø., Prydz, K., Kozlov, J., Hansen, S., and van Deurs, B. (1992). Retrograde transport of endocytosed Shiga toxin to the endoplasmic reticulum. Nature 358, 510-512. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2000). Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 19, 5943-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2002). Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 529, 49-53. [DOI] [PubMed] [Google Scholar]
- Shewan, A.M., van Dam, E.M., Martin, S., Tang, B.L., Hong, W., Bryant, N.J., and James, D.E. (2003). GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in syntaxins 6 and 16 but not TGN 38, involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter, J., Beard, M.B., Seemann, J., Dirac-Svejstrup, B., and Warren, G. (2002). Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell. Biol. 157, 45-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou, S., and Pelham, H.R.B. (2001). An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 20, 5991-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner, T., Whiteheart, S.W., Brunner, M., Erdjument-Bromage, H., Geromanos, S., Tempst, P., and Rothman, J.E. (1993). SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318-324. [DOI] [PubMed] [Google Scholar]
- Steegmaier, M., Klumperman, J., Foletti, D.L., Yoo, J.-S., and Scheller, R.H. (1999). Vesicle-associated membrane protein 4 is implicated in trans-Golgi network vesicle trafficking. Mol. Biol. Cell 10, 1957-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam, V.N., Krijnse-Locker, J., Tang, B.L., Ericsson, M., Yusoff, A., Griffiths, G., and Hong, W. (1995). Monoclonal antibody HFD9 identifies a novel 28kDa integral membrane protein on the cis-Golgi. J. Cell Sci. 108, 2405-2414. [DOI] [PubMed] [Google Scholar]
- Subramaniam, V.N., Peter, F., Philip, R., Wong, S.H., and Hong, W. (1996). GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science 272, 1161-1163. [DOI] [PubMed] [Google Scholar]
- Subramaniam, V.N., bin Mohd Yusof, A.R.,Wong, S.H., Lim, G.B., Chew, M., and Hong, W. (1992). Biochemical fractionation and characterization of proteins from Golgi-enriched membranes. J. Biol. Chem. 267, 12016-12021. [PubMed] [Google Scholar]
- Subramaniam, V.N., Loh, E., and Hong, W. (1997). N-Ethylmaleimide-sensitive factor (NSF) and α-soluble NSF attachment proteins (SNAP) mediate dissociation of GS28-syntaxin 5 Golgi SNAP receptors (SNARE) complex. J. Biol. Chem. 272, 25441-25444. [DOI] [PubMed] [Google Scholar]
- Tang, B.L., Low, D.Y., Lee, S. S. Tan, A.E., and Hong, W. (1998a). Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem. Biophys. Res. Commun. 242, 673-679. [DOI] [PubMed] [Google Scholar]
- Tang, B.L., Low, D.Y., Tan, A.E., and Hong, W. (1998b). Syntaxin 10, a member of the syntaxin family localized to the trans-Golgi network. Biochem. Biophys. Res. Commun. 242, 345-350. [DOI] [PubMed] [Google Scholar]
- Tang, B.L., Zhang, T., Low, D.Y.H., Wong, E.T., Horstmann, H., and Hong, W. (2000). Mammalian Homologues of Yeast Sec31p. J. Biol. Chem. 275, 13597-13604. [DOI] [PubMed] [Google Scholar]
- Ungermann, C., von Mollard, G.F., Jensen, O.N., Margolis, N., Stevens, T.H., and Wickner, W. (1999). Three v-SNAREs and two t-SNAREs, present in a pentameric cis-SNARE complex on isolated vacuoles, are essential for homotypic fusion. J. Cell Biol. 145, 1435-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volchuk, A., et al. (2004). Countercurrent distribution of two distinct SNARE complexes mediating transport within the Golgi stack. Mol. Biol. Cell 15, 1506-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T.H., and Rothman, J.E. (1998). SNAREpins: minimal machinery for membrane fusion. Cell 92, 759-772. [DOI] [PubMed] [Google Scholar]
- Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B., and Salamero, J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151, 1207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, S.H., Xu, Y., Zhang, T., Griffiths, G., Lowe, S.L., Subramaniam, V.N., Seow, K.T., and Hong, W. (1999). GS32, a novel Golgi SNARE of 32 kDa, interacts preferentially with syntaxin 6. Mol. Biol. Cell 10, 119-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, S.H., Xu, Y., Zhang, T., and Hong, W. (1998). Syntaxin 7, a novel syntaxin member associated with the early endosomal compartment. J. Biol. Chem. 273, 375-380. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Hortsman, H., Seet, L., Wong, S.H., and Hong, W. (2001). SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIn(3)P. Nat. Cell Biol. 3, 658-666. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Martin, S., James, D., E. and Hong, W. (2002). GS15 forms a SNARE complex with syntaxin 5, GS28 and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol. Biol. Cell 13, 3493-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., Wong, S.H., Tang, B.L., Subramaniam, V.N., Zhang, T., and Hong, W. (1998). A 29-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (Vti1-rp2) implicated in protein trafficking in the secretary pathway. J. Biol. Chem. 273, 21783-21789. [DOI] [PubMed] [Google Scholar]
- Xu, Y., Wong, S.H., Zhang, T., Subramaniam, V.N., and Hong, W. (1997). GS15, a 15-kilodalton Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) homologous to rBet1. J. Biol. Chem. 272, 20162-20166. [DOI] [PubMed] [Google Scholar]
- Zeng, Q., Tran, T.T., Tan, H.X., and Hong, W. (2003). The cytoplasmic domain of VAMP4 and VAMP5 is responsible for their correct subcellular targeting: the N-terminal extension of VAMP4 contains a dominant autonomous targeting signal for the trans-Golgi network. J. Biol. Chem. 278, 23046-23054. [DOI] [PubMed] [Google Scholar]
- Zhang, T., and Hong, W. (2001). Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participate in a late stage in endoplasmic reticulum-Golgi transport. J. Biol. Chem. 276, 27480-27487. [DOI] [PubMed] [Google Scholar]
- Zhang, T., Wong, S.H., Tang, B.L., Xu, Y., and Hong, W. (1999). Morphological and functional association of Sec22b/ERS24 with the pre-Golgi intermediate compartment. Mol. Biol. Cell 10, 435-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T., Wong, S.H., Tang, B.L., Xu, Y., Peter, F., Subramaniam, V.N., and Hong, W. (1997). The mammalian protein (rbet1) homologous to yeast Bet1p is primarily associated with the pre-Golgi intermediate compartment and is involved in vesicular transport from the endoplasmic reticulum to the Golgi apparatus. J. Cell Biol. 139, 1157-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.