Abstract
Background
Recently, as an independent fracture factor from Bone mineral density (BMD), muscle weakness due to the fatty degeneration of thigh muscles have been attracting attentions as causes of hip fracture. The purpose of this study is to investigate the correlation between the body composition and BMD and fatty degeneration of thigh muscles of the female patients over 65 years old with osteoporotic hip fracture.
Methods
This study was conducted with 178 female osteoporotic hip fracture patients. Total hip BMD was measured using dual energy X-ray absorptiometry. Cross-sectional area (CSA), cross-sectional muscle area (CSmA), muscle attenuation coefficient (MAC), and intramuscular adipose tissue (IMAT) of gluteus maximus, hip abductors, quadriceps and hamstring muscle were measured with computed tomography. Normalized IMAT (nIMAT) was calculated by dividing the fat area in the muscle into the size of each muscle. The correlation between each measurement is examined then the differences between the intertrochanteric fracture group and the femoral neck fracture group were analyzed.
Results
CSmA and MAC of quadriceps were the largest and nIMAT was the lowest. CSA and CSmA of the four muscles showed a statistically significant positive correlation with weight, height, body mass index (BMI), and BMD. MAC of 2 gluteal muscles was positively correlated with weight, BMI and BMD. nIMAT of all four muscles was positively correlation with weight and BMI but nIMAT of 2 mid-thigh muscles was positively correlation with BMD.
Conclusions
Muscle size and fatty degeneration in the thigh muscles were most positively correlated with the body weight. BMD was positively correlation with CSA and CSmA of all thigh muscles, and MAC of 2 gluteal muscles and fatty degeneration of 2 mid-thigh muscles. There was no statistically significant difference in the size of the femoral muscle and the degree of fatty degeneration between the two fracture groups.
Keywords: Bone density, Fatty degeneration, Hip fractures, Muscle attenuation, Osteoporosis
INTRODUCTION
Progressive reduction of bone mass and muscle mass are inevitable processes which occur with aging.[1,2] Bone mineral density (BMD) has a positive correlation between muscle mass and muscle strength, and therefore, sarcopenia is related to osteoporosis.[3,4] In the process of aging of these muscles, the degree of intramuscular fatty degeneration increased, which is also associated with a decrease in mobility function.[5] Osteoporotic hip fracture is an important geriatric disease that threatens the life of the patient and impairs quality of life and is rapidly increasing with aging in elderly people over 65 years old.[6,7] The decrease of BMD and morphological characteristics of the proximal femur have been identified as factors affecting the osteoporotic hip fracture.[8,9] In recent years, muscle size change around the thigh area and the weakening of muscle strength by fatty degeneration are getting attention as factors affecting the osteoporotic hip fracture.[10,11] Visser et al.[12,13] reported that the muscle amount and fatty degeneration were related to lower extremity performance and mobility limitation in well-functioning older persons. This weakening of muscle strength is related to the risk of falling.[14] Lang et al.[15] reported that the fatty degeneration of thigh muscles increases the relative risk of hip fracture by 42%, which is independent of BMD and a predictive factor of hip fracture. The purpose of this study is to investigate the correlation between body composition and measured BMD, muscle size, muscle weakness index and the degree of fatty degeneration of thigh muscles in women over 65 years old with osteoporotic hip fracture. Hip fracture patients were divided into femoral intertrochanteric fracture group and femoral neck fracture group, then BMD, thigh area muscle size, and fatty degeneration were investigated to find out the difference between the two groups.
METHODS
1. Subjects
This study was conducted with 178 patients, out of 316 women osteoporotic hip fracture patients who were hospitalized at orthopedic surgery department of Ajou University from March 2013 to May 2016, who conducted both total hip BMD measurement and computed tomography (CT) of thigh muscles. Among them, the number of femoral neck fracture patient was 73 and the number of femoral intertrochanteric fracture patient was 105. We excluded patients with high-energy damage such as car accidents or falling, patients with diseases that may affect bone metabolism, patients who cannot walk well after being injured, and patients whose BMD of hip region cannot be measured owing to the hip fracture at both sides.
2. Measurement
1) Physical characteristics
The height and weight of the patient were measured and body mass index (BMI) was calculated.
2) BMD
Total hip BMD was measured in the subject patient using dual energy X-ray absorptiometry (DXA; Lunar Prodigy Advance, GE Lunar, Medison, WI, USA). Patient measured BMD of the proximal femur without fracture in the supine position within 7 days of injury.
3) Muscle measurements of the femoral region
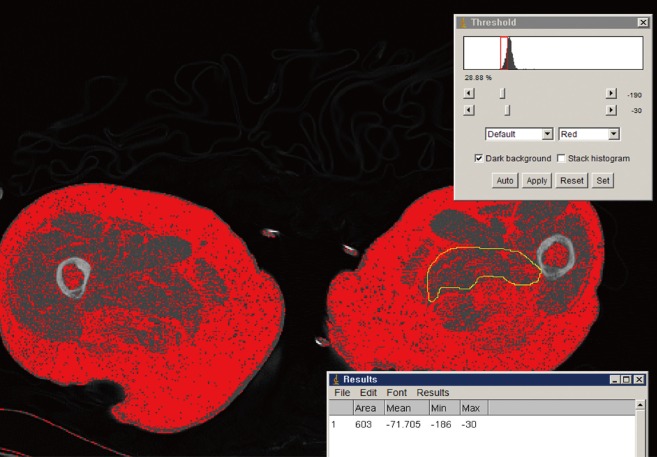
Four muscles were selected from the gluteal region (gluteus maximus and hip abductors) and mid-thigh region (quadriceps and Hamstring muscle) for the measurement. In order to measure these muscles, two axial images were taken using CT before surgery and within 7 days after being injured. To measure gluteal muscles, axial cut was made on the basis of the third sacrum, and to measure mid-thigh region muscles, another axial cut was made on the basis of the mid-femur (50% of femur length; Siemens Healthcare, Erlangen, Germany) The length of the femur was measured from the tip of the femur to the intertrochanter notch. In the axial images, cross-sectional area (CSA) of each muscle, cross-sectional muscle area (CSmA), muscle attenuation coefficient (MAC) and intramuscular adipose tissue (IMAT) were measured using National Institutes of Health (NIH) Image J ver. 1.49 K (NIH, Bethesda, MD, USA) software program which automatically distinguish muscle and fat based on the Hounsfield unit (HU) within the muscle area, between 30 to 80 HU is recognized as muscle, and between -190 and -30 HU is recognized as fat (Fig. 1).[15,16] The IMAT value was divided by the size of each muscle to compensate for the difference in the body size of the patient, which was expressed as the normalized IMAT (nIMAT) value.[17]
Fig. 1. Measurement of intramuscular fat area and average Hounsfield unit (HU) within muscle by software program (between -190 to -30 HU).
| Normalized IMAT (%) = IMAT×100/CSA |
3. Statistical analysis
Variables such as age, height, weight, BMI, BMD, and measurements of four muscles around thigh area of patient were expressed in the form of mean and standard deviation. The correlation between the variables was obtained with Pearson correlation and was statistically analyzed using SSPC statistical program (version 22; SPSS Inc., Chicago, IL, USA). Differences in the variables between the femoral neck fracture group and the intertrochanteric fracture group were determined using independent sample t-test, with a statistical significance of less than 0.05.
RESULTS
1. Physical characteristics and muscle measurements
The physical characteristics of the subject patients are shown in Table 1. The mean age of patients was 78.3 years old, ranging from 65 to 91 years old. The mean BMI was 22.6, ranging from 14.4 to 35.2 and 46 patients were in the obese group (25.8% of the total patient). The mean BMD was 0.62 g/cm2, ranging from 0.35 to 0.98. The mean T-score was -2.79 and 119 patients (66.9% of the total patient) were under -2.5 score which is in the range of osteoporosis under the World Health Organization (WHO) criteria. The measured CSmA, MAC and nIMAT of the four muscles are shown in Table 2. The MAC value of quadriceps muscle was 53.64 HU, which was the highest among the four muscles, with the lowest nIMAT of 12.1%. The nIMAT of the gluteus muscle was the highest at 35.6%.
Table 1. Demographic characteristics.
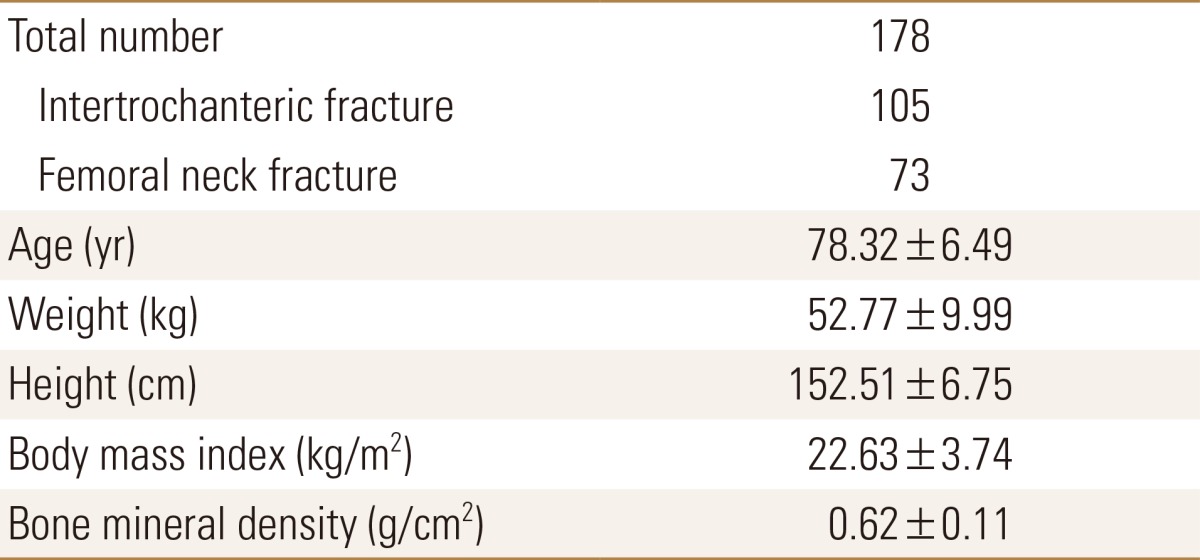
Table 2. Cross sectional muscle area, muscle attenuation coefficient and normalized intramuscular adipose tissue of muscles.
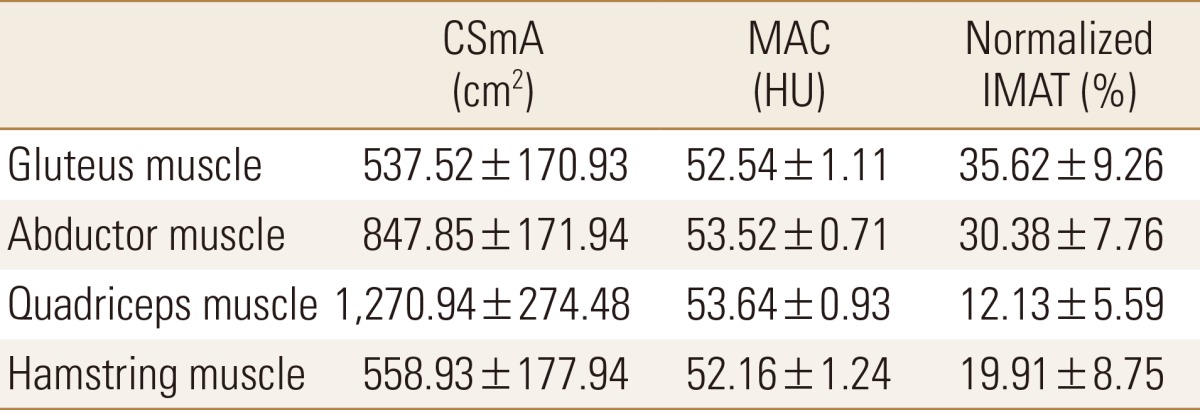
SmA, cross sectional muscle area; MAC, muscle attenuation coefficient; HU, Hounsfield units; IMAT, intramuscular adipose tissue.
2. Correlation between the measurements
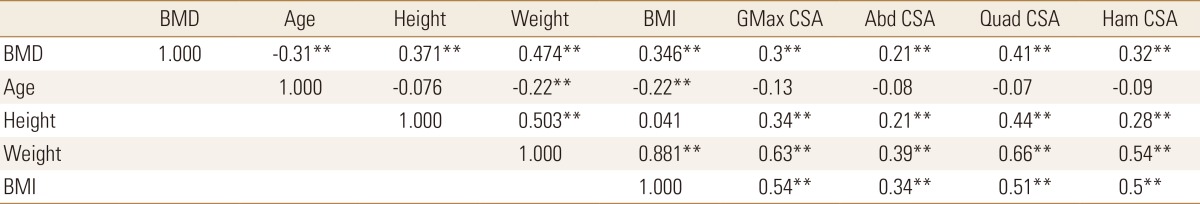
The correlation between the measurements and the statistical significance are shown in Tables 3, 4, 5. BMD showed statistically significant decrease with increasing age and showed positive correlations with height, weight, and BMI. As the weight and BMI were increased, BMD increased. CSA of the four muscles showed statistically significant positive correlations with BMD, weight, height, and BMI, and a negative correlation with age. In other words, muscle size decreased with age.
Table 3. Correlation between bone mineral density, age, height, weight, body mass index, cross sectional area.
*P<0.05. **P<0.01.
BMD, bone mineral density; BMI, body mass index; GMax, gluteus maximus; CSA, cross sectional area; Abd, abductor; Quad, quadriceps; Ham, hamstring.
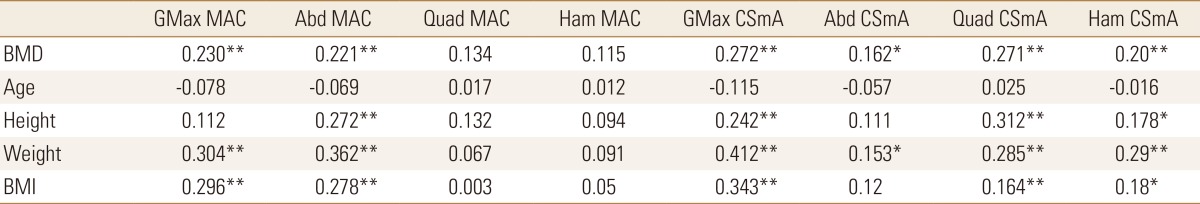
Table 4. Correlation between bone mineral density, age, height, weight, body mass index and muscle attenuation coefficient, cross sectional muscle area.
*P<0.05. **P<0.01.
GMax, gluteus maximus; MAC, muscle attenuation coefficient; Abd, abductor; Quad, quadriceps; Ham, hamstring; CSmA, cross sectional muscle area; BMD, bone mineral density; BMI, body mass index.
Table 5. Correlation between bone mineral density, age, height, weight, body mass index and normalized intramuscular adipose tissue.
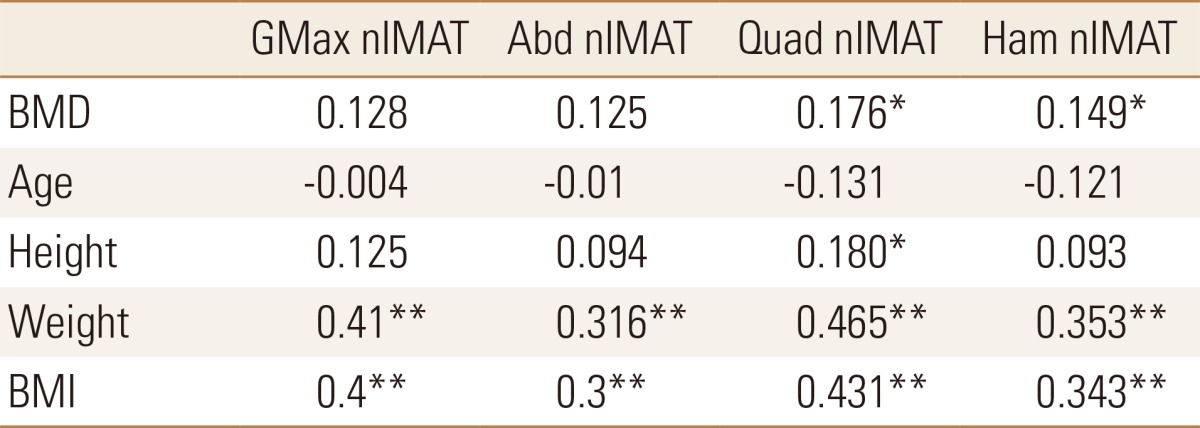
*P<0.05. **P<0.01.
GMax, gluteus maximus; nIMAT, normalized intramuscular adipose tissue; Abd, abductor; Quad, quadriceps; Ham, hamstring; BMD, bone mineral density; BMI, body mass index.
Regarding the MAC, which represents the muscle density of each muscle, gluteus maximus and abductor muscles in the gluteal area showed significantly positive correlations with BMD, weight, and BMI. However, quadriceps and hamstring muscles in the mid-thigh area showed positive correlations with BMD, weight, and BMI but no statistical significance.
CSmA in four muscles statistically increased with increasing BMD and showed a negative correlation with age except quadriceps. In other words, CSmA showed a decrease with increasing age but no statistical significance. CSmA of gluteus maximus, quadriceps and hamstring muscle showed statistically significant positive correlation with BMD, height, weight and BMI, on the contrary, CSmA of abductor muscle showed a positive correlation with BMD and weight.
The nIMAT, which indicates the degree of fatty degeneration of each muscle, of all four muscles showed a statistically significant positive correlation with weight and BMI, on the other hand, showed negative correlations with age but no statistical significance. In quadriceps and hamstring muscles of mid-thigh area, nIMAT showed a statistically significant positive correlation with BMD.
3. The difference between intertrochanteric fracture group and femoral neck fracture group
The measurement values of the two groups are shown in Table 6. The age and BMD of the patients were statistically different between the two groups. Age was higher in the intertrochanteric fracture group and BMD was higher in femoral neck fracture group. The muscle size and a degree of fatty degeneration of the muscles were not significantly different between the two groups.
Table 6. Comparison between intertrochanteric and femoral neck fracture groups on the same demographics and muscle quantity and quality.
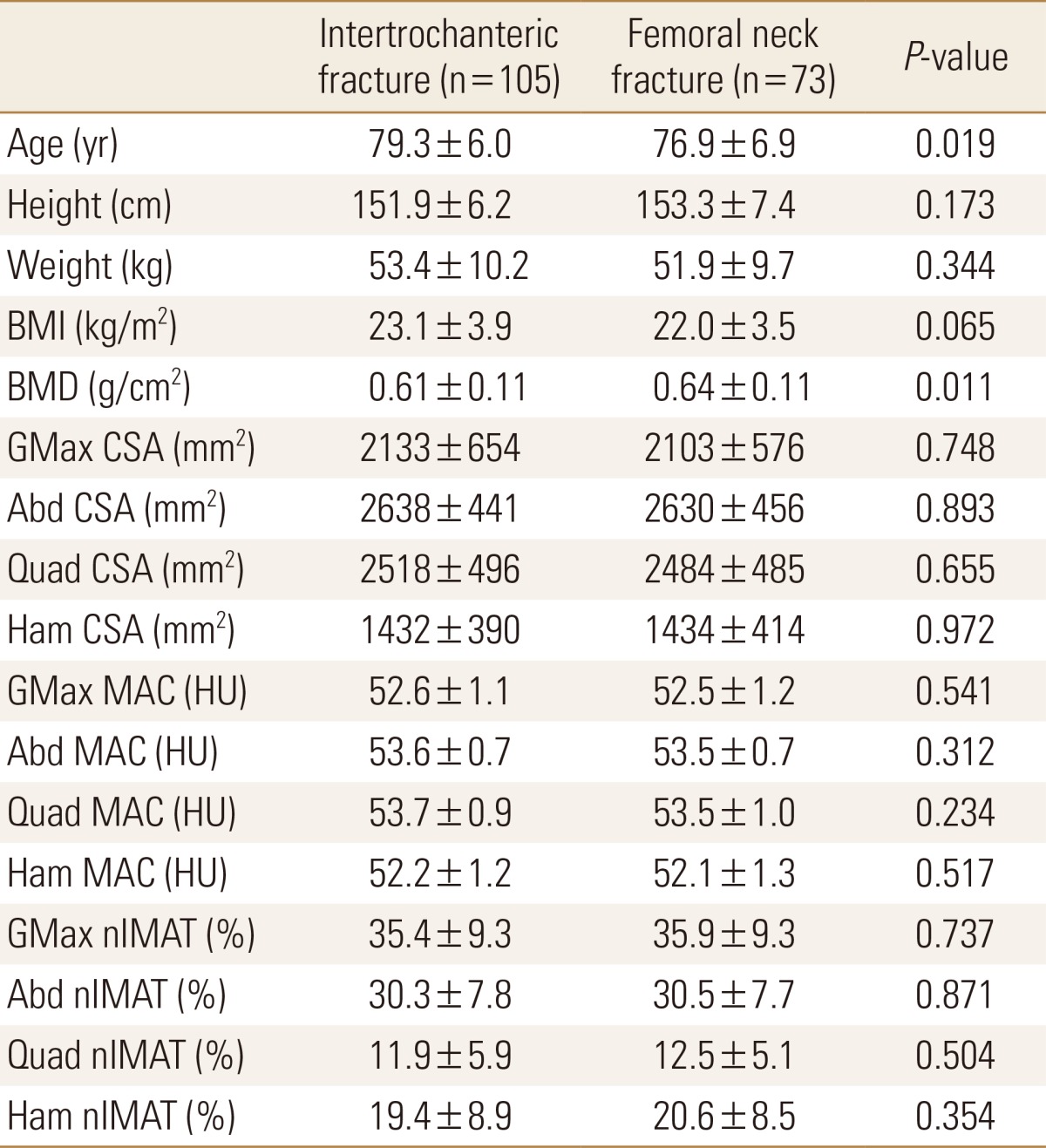
Data is expressed as mean±standard deviation. Comparisons significant for P<0.05.
BMI, body mass index; BMD, bone mineral density; GMax, gluteus maximus; CSA, cross sectional muscle area; Abd, abductor; Quad, quadriceps; Ham, hamstring; MAC, muscle attenuation coefficient; HU, Hounsfield units; nIMAT, normalized intramuscular adipose tissue.
DISCUSSION
With age, loss of muscle mass is associated with loss of bone mass. The precise mechanism responsible for synchronizing bone and muscle mass remain unclear. Muscle decline with age appears to occur before bone decline with age. With age, greater adiposity is observed in both bone marrow and muscle and fat infiltrated are also observed in nerves and capillaries.[18]
Goodpaster et al.[19,20] argued that muscle strength decline is much more rapid than the concomitant loss of muscle mass which suggest a decline in muscle quality. Moreover, the degree of muscle weakness is much more related with the muscle strength reduction than muscle mass does. Marcus et al.[21] reported that intramuscular fat deposition is associated with metabolic deficits and lack of exercise in the elderly and increases with age and the level of inactivity. To measure the deposition of fat tissue in muscle, invasive methods such as biopsy of muscle tissue, and noninvasive methods such as DXA, CT and h magnetic resonance (H-MR) spectroscopy are being used.[11,16,22] Among these, the measurement of fatty degeneration in muscles using a CT showed that the fat tissue was dark and the muscle tissue was bright (depending on the proton content per unit mass, the adipose tissue appears in the negative area, ranging from -190 to -30 HU, and the muscle appears in the 0 to 100 HU area), so that the two tissues could be easily distinguished. Therefore, despite the radiation exposure and price disadvantages, this method is suitable for measuring the deposition of fat tissue in muscle.[23] Recently, Malkov et al.[11] reported that value of the CSA, MAC, and subcutaneous fat thickness of the muscle around the femur which was measured using DXA, showed a statistically significant correlation with the value of those measured using a CT. Although it is convenient for the clinical use, it does not have enough resolution that can surpass the tissue distinguishing ability by CT.
In this study, the MAC value of quadriceps muscle was 53.64 HU, the highest among the four muscles, with the lowest nIMAT of 12.13%. On the other hand, the nIMAT of gluteus maximus muscle was the highest at 35.6%. This suggests that the level of fatty degeneration of the extensor of knee joint is low even in the elderly. Inacio et al.[24] measured the MAC and IMAT values of the thigh muscles in the elderly over 65 years of age. MAC was the highest in the quadriceps and the lowest in the gluteus maximus and medius muscles and IMAT was the lowest measure in quadriceps and showed the same result as that of this study. This is explained by the fact that muscles in the gluteal lesion are more responsive to muscle changes with age. Thelen et al.[25] reported that the responsive muscular activation patterns of elderly subjects were similar to those of young subjects in the forward fall experiment. However, there was a difference in muscle activation time due to the delayed quadriceps activity before the swing phase and during the swing phase. The importance of quadriceps muscles in the case of falling was emphasized.
As expected, analysis of the correlation between the measurements demonstrated that BMD, body weight, and BMI showed statistically significant decrease with age. BMD showed a statistically significant positive correlation with height, body weight, and BMI. CSA and CSmA decreased with age and the degree of fatty degeneration also tends to decrease but no statistical significance. In previous studies, it has been reported that the degree of fatty degeneration of muscle increases with age, however in this study, the degree of fatty degeneration of muscle decrease trend with age but no statistical significance. It seems because ages of the subject patients are high and body weight is more sensitive than age. CSA, CSmA and IMAT showed a statistically significant positive correlation with body weight and BMI in four muscles. So the heavier the body weight, the larger CSA, CSmA and the grater the degree of fatty degeneration. The changes of BMD showed a statistically significant positive correlation with CSA and CSmA. MAC of the quadriceps and hamstring muscles in the mid-thigh area did not show a significant correlation with age, height, weight, BMI and BMD (Table 4). This suggests that 2 muscles in the mid-thigh area is less responsive to body weight changes than in gluteal area. nIMAT of 2 mid-thigh muscles showed positive correlation with weight, BMI and BMD. On the contrary, nIMAT of 2 gluteal muscles showed no correlation with BMD. That means, the higher BMD, the greater MAC of the gluteal muscles and the more fatty degeneration of the mid-thigh muscles.
Recently, other risk factors of the osteoporotic hip fracture which independent from BMD were being announced. Lang et al.[15,26] reported that combining body composition with skeletal measures may improve fracture prediction compared to bone measure alone. Moreover they reported that the CSA and muscle weakness index of the thigh muscle were independent fracture risk factors. Malkov et al.[11] reported that the thickness of subcutaneous fat in the thigh, cross sectional muscle area and muscle density were the risk factor for hip fracture in both sexes.
In this study, hip fracture patient group was divided into femur intertrochanteric fracture group and femoral neck fracture group. Age and BMD were statistically different between the two groups. Age was higher in femoral intertrochanteric fracture patients group and BMD was higher in femoral neck fracture patients group. The size and the degree of fatty degeneration of the muscle around the femur was not significantly different between the two groups. Although there are many studies on the morphologic difference of the proximal femur and the BMD in the two fracture groups, this study may be the first to compare the size and CSA of the surrounding muscles.
The limitation of this study is that the study was retrospective and the fractured patient was included only so couldn't compare with the non-fracture group. The number of fracture patients was not large enough to obtain statistical significance in comparison between the intertrochanteric fracture group and the neck fracture group. In addition, measurements of vitamin D levels, lipid profile, and blood glucose, which directly affect muscle mass and intramuscular fat deposition, are missing. Park et al.[27] reported that the risk of reduced muscle mass in elderly women with type 2 diabetes was twice as high as in those without type 2 diabetes. Lang et al.[15,26] reported that patients with diabetes had more intramuscular fatty degeneration than those without diabetes and 42% had a higher risk of fracture in the diabetic patient group. In addition, there was no evaluation of the exercise capacity of the patients before the injury. We suggest that a prospective study supplemented with these findings is needed.
In conclusion, muscle size and degree of fatty degeneration of thigh muscles showed a statistically significant positive correlation with the body weight. BMD was positively correlation with CSA and CSmA of all thigh muscles, and MAC of 2 gluteal muscles and fatty degeneration of 2 mid-thigh muscles. There was no statistically significant difference in the size of the femoral muscle and the degree of fatty degeneration in the femur intertrochanteric fracture and neck fracture patients group.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Edwards MH, Dennison EM, Aihie Sayer A, et al. Osteoporosis and sarcopenia in older age. Bone. 2015;80:126–130. doi: 10.1016/j.bone.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarantino U, Baldi J, Celi M, et al. Osteoporosis and sarcopenia: the connections. Aging Clin Exp Res. 2013;25(Suppl 1):S93–S95. doi: 10.1007/s40520-013-0097-7. [DOI] [PubMed] [Google Scholar]
- 3.He H, Liu Y, Tian Q, et al. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27:473–482. doi: 10.1007/s00198-015-3241-8. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Won CW, Kim BS, et al. The association between the low muscle mass and osteoporosis in elderly Korean people. J Korean Med Sci. 2014;29:995–1000. doi: 10.3346/jkms.2014.29.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcus RL, Addison O, Dibble LE, et al. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J Aging Res. 2012;2012:629637. doi: 10.1155/2012/629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon HK, Park C, Jang S, et al. Incidence and mortality following hip fracture in Korea. J Korean Med Sci. 2011;26:1087–1092. doi: 10.3346/jkms.2011.26.8.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon BH, Lee YK, Kim SC, et al. Epidemiology of proximal femoral fractures in South Korea. Arch Osteoporos. 2013;8:157. doi: 10.1007/s11657-013-0157-9. [DOI] [PubMed] [Google Scholar]
- 8.Svejme O, Ahlborg HG, Nilsson JA, et al. Low BMD is an independent predictor of fracture and early menopause of mortality in post-menopausal women--a 34-year prospective study. Maturitas. 2013;74:341–345. doi: 10.1016/j.maturitas.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Broy SB, Cauley JA, Lewiecki ME, et al. Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD official positions part 1: hip geometry. J Clin Densitom. 2015;18:287–308. doi: 10.1016/j.jocd.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Schafer AL, Vittinghoff E, Lang TF, et al. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. J Clin Endocrinol Metab. 2010;95:E368–E372. doi: 10.1210/jc.2010-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malkov S, Cawthon PM, Peters KW, et al. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30:1414–1421. doi: 10.1002/jbmr.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 13.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- 14.Moreland JD, Richardson JA, Goldsmith CH, et al. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 15.Lang T, Cauley JA, Tylavsky F, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 17.Bouche KG, Vanovermeire O, Stevens VK, et al. Computed tomographic analysis of the quality of trunk muscles in asymptomatic and symptomatic lumbar discectomy patients. BMC Musculoskelet Disord. 2011;12:65. doi: 10.1186/1471-2474-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonewald LF, Kiel DP, Clemens TL, et al. Forum on bone and skeletal muscle interactions: summary of the proceedings of an ASBMR workshop. J Bone Miner Res. 2013;28:1857–1865. doi: 10.1002/jbmr.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 21.Marcus RL, Addison O, Kidde JP, et al. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14:362–366. doi: 10.1007/s12603-010-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa Y, Hattori M, Harada K, et al. Age-related changes in intramyocellular lipid in humans by in vivo H-MR spectroscopy. Gerontology. 2007;53:218–223. doi: 10.1159/000100869. [DOI] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Kelley DE, Thaete FL, et al. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 24.Inacio M, Ryan AS, Bair WN, et al. Gluteal muscle composition differentiates fallers from non-fallers in community dwelling older adults. BMC Geriatr. 2014;14:37. doi: 10.1186/1471-2318-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thelen DG, Muriuki M, James J, et al. Muscle activities used by young and old adults when stepping to regain balance during a forward fall. J Electromyogr Kinesiol. 2000;10:93–101. doi: 10.1016/s1050-6411(99)00028-0. [DOI] [PubMed] [Google Scholar]
- 26.Lang T, Koyama A, Li C, et al. Pelvic body composition measurements by quantitative computed tomography: association with recent hip fracture. Bone. 2008;42:798–805. doi: 10.1016/j.bone.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32:1993–1997. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]