Abstract
Cell surface CD147 shows remarkable variations in size (31-65 kDa) because of heterogeneous N-glycosylation, with the most highly glycosylated forms functioning to induce matrix metalloproteinase (MMP) production. Here we show that all three CD147 N-glycosylation sites make similar contributions to both high and low glycoforms (HG- and LG-CD147). l-Phytohemagglutinin lectin binding and swainsonine inhibition experiments indicated that HG-CD147 contains N-acetylglucosaminyltransferase V-catalyzed, β1,6-branched, polylactosamine-type sugars, which account for its excess size. Therefore, CD147, which is itself elevated on invasive tumor cells, may make a major contribution to the abundance of β1,6-branched polylactosamine sugars that appear on invasive tumor cells. It was shown previously that caveolin-1 associates with CD147, thus inhibiting CD147 self-aggregation and MMP induction; now we show that caveolin-1 associates with LG-CD147 and restricts the biosynthetic conversion of LG-CD147 to HG-CD147. In addition, HG-CD147 (but not LG-CD147) was preferentially captured as a multimer after treatment of cells with a homobifunctional cross-linking agent and was exclusively recognized by monoclonal antibody AAA6, a reagent that selectively recognizes self-associated CD147 and inhibits CD147-mediated MMP induction. In conclusion, we have 1) determined the biochemical basis for the unusual size variation in CD147, 2) established that CD147 is a major carrier of β1,6-branched polylactosamine sugars on tumor cells, and 3) determined that caveolin-1 can inhibit the conversion of LG-CD147 to HG-CD147. Because it is HG-CD147 that self-aggregates and stimulates MMP induction, we now have a mechanism to explain how caveolin-1 inhibits these processes. These results help explain the previously established tumor suppressor functions of caveolin-1.
INTRODUCTION
CD147/basigin/EMMPRIN, a cell surface transmembrane glycoprotein widely expressed on many cell types, appears at especially high levels on human tumor cells (Ellis et al., 1989; Muraoka et al., 1993; Polette et al., 1997). Elevated CD147 expression is correlated with tumor progression of gliomas (Sameshima et al., 2000a), hepatomas (Jiang et al., 2001), squamous cell carcinomas (Bordador et al., 2000), and melanomas (van den Oord et al., 1997; Kanekura et al., 2002). CD147/EMMPRIN on tumor cells stimulates production of multiple matrix metalloproteinases (MMPs) by tumor stromal cells, which is the basis of the name EMMPRIN (extracellular matrix metalloproteinase inducer) (Ellis et al., 1989; Kataoka et al., 1993). Tumor cell CD147 can stimulate stromal fibroblast production of interstitial collagenase (MMP-1), gelatinase A (MMP-2), and stromelysin (MMP-3) (Ellis et al., 1989; Kataoka et al., 1993; Sameshima et al., 2000b; Li et al., 2001; Suzuki et al., 2004). Similarly, recombinant purified or soluble secreted EMMPRIN glycoprotein stimulates fibroblasts to produce MMP-1, MMP-2, and MMP-3 (Guo et al., 1997; Li et al., 2001; Sun and Hemler, 2001; Taylor et al., 2002), and tumor-derived CD147/EMMPRIN promoted production of MMP-1, MMP-2, and MMP-3 from human umbilical vein endothelial cells (HUVEC) (Caudroy et al., 2002). Also, CD147 plays a role in squamous cell carcinoma invasion and MMP-2 production (Bordador et al., 2000), and CD147 in breast cancer cells promoted MMP-2 and MMP-3 production in vitro and in vivo in nude mice, accompanied by markedly increased tumor growth and metastasis (Zucker et al., 2001; Caudroy et al., 2002). These studies support a model in which tumor cell CD147 stimulates MMP-1, MMP-2, and MMP-3 production, thereby leading to extracellular matrix degradation and increased tumor growth and metastasis. CD147 also can promote hyaluronan production and mammary carcinoma cell growth (Marieb et al., 2004), affect the activation and development of T cells (Igakura et al., 1996; Koch et al., 1999; Renno et al., 2002; Staffler et al., 2003), target monocarboxylate transporters (MCTs) to the plasma membrane (Kirk et al., 2000), and act as a receptor for cyclophilin A (Pushkarsky et al., 2001; Yurchenko et al., 2002). Deletion of the CD147/basigin gene in mice led to defects in spermatogenesis, female fertilization, and retinal development (Igakura et al., 1998; Kuno et al., 1998; Hori et al., 2000).
CD147 contains two extracellular Ig domains, a single transmembrane domain, and a 39-amino acid cytoplasmic domain (Biswas et al., 1995; Toole, 2003). The first Ig domain of CD147 is required for homophilic counterreceptor binding activity, as noted in domain deletion and antibody blocking studies (Yoshida et al., 2000; Sun and Hemler, 2001). The first Ig domain also is involved in MMP induction, as this activity is inhibited by anti-domain 1 antibodies (Biswas et al., 1995; Koch et al., 1999; Sun and Hemler, 2001). The second Ig domain of CD147 is required for association with caveolin-1, which leads to decreased CD147 self-association on the cell surface (Tang and Hemler, 2004). Conversely, loss of caveolin-1 promotes, in parallel, CD147-dependent MMP production and self-association (Tang and Hemler, 2004). Therefore, caveolin-1 is a negative regulator of CD147 self-association and MMP-inducing activity. Self-association can be assayed with low-affinity anti-CD147 antibodies such as M6/13 and AAA6 (Koch et al., 1999; Tang and Hemler, 2004). Because such antibodies tend to bind bivalently to cell surface CD147, they bind preferentially to homo-clustered CD147 (Koch et al., 1999).
On nearly all cell and tissue types analyzed, the CD147/EMMPRIN/basigin/5A11/HT7 protein with 2 Ig domains, from human (Berditchevski et al., 1997; Guo et al., 1997; Sun and Hemler, 2001), mouse (Kanekura et al., 1991; Li et al., 2001), and avian (Fadool and Linser, 1993) species, appears as multiple, discrete, glycosylated forms, typically differing in size by 15-25 kDa. This large size variation is atypical for cell surface glycoproteins in general and for Ig superfamily proteins in particular. It is highly glycosylated (HG) forms of CD147 that are active in the induction of MMPs (Guo et al., 1997; Sun and Hemler, 2001). Here we demonstrate that elevated glycosylation of CD147, yielding HG-CD147, is attributable to high polylactosamine content. Furthermore, we show that caveolin-1 selectively associates with less glycosylated (LG)-CD147, leading to a downward shift in the HG/LG ratio, in parallel with diminished self-aggregation on the cell surface. Our results support a model in which caveolin-1 associates with LG-CD147 during biosynthesis in the Golgi complex and escorts LG-CD147 to the cell surface. Caveolin-1 thus prevents polylactosamine addition and conversion to HG-CD147, impairing cell surface clustering and MMP induction. Consequently, the often-noted positive link between β1,6-branched N-polylactosamines and tumor progression (Dennis et al., 1999; Chakraborty and Pawelek, 2003) could be at least partly attributable to elevated HG-CD147 activity on tumor cells. In addition, the well-established tumor suppressor properties of caveolin-1 (Wiechen et al., 2001; Aldred et al., 2003; Hino et al., 2003; Williams et al., 2003) could be at least partly attributable to its negative effects on CD147 glycosylation, clustering, and MMP induction.
MATERIALS AND METHODS
Cell Lines, Antibodies, and Reagents
Human cell lines from embryonic kidney (HEK293), epidermoid carcinoma (A431), breast carcinoma (MCF7), and fibrosarcoma (HT1080) were grown in DMEM supplemented with antibiotics (penicillin and streptomycin) and 10% fetal calf serum. The primary fibroblast cell line NHDF-AD was purchased from Cambrex (East Rutherford, NJ). HUVEC were obtained from Children's Hospital (Boston, MA). CD147-transfected cell lines were maintained in DMEM with 0.5-1 mg/ml G418. CD147-green fluorescent protein (GFP) fusion protein (Tang and Hemler, 2004) was stably transfected into HEK293 and HT1080 cells, with Fugene 6 (Roche, Indianapolis, IN), and transfectants were selected with 1 mg/ml G418. Antibodies to CD147 were rabbit polyclonal antiserum B10 and mAb 8G6 (Berditchevski et al., 1997; Sun and Hemler, 2001) and mAb AAA6 (Kasinrerk et al., 1992; Koch et al., 1999). We also used anti-caveolin-1 rabbit polyclonal antibody (BD Transduction Laboratories, San Diego, CA), anti-integrin β1 (Chemicon, Temecula, CA), and anti-GFP mAb 3E6 (Obiogene, Carlsbad, CA), as well as tunicamycin (Sigma, St. Louis, MO), endoglycosidase H (endo H) (New England Biolabs, Beverly MA), l-PHA (Vector Laboratories, Burlingame, CA), and swainsonine (Sigma).
Immunoprecipitation and Immunoblotting
Cells (3-5 × 106) were lysed for 2-4 h in 1.5 ml of immunoprecipitation buffer (1% Brij 96, 25 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM MgCI2, 2 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin) at 4°C. After centrifugation (12,000 rpm, 10 min), lysates were incubated overnight with protein A-conjugated beads, to remove nonspecific binding material. After incubation of lysates with antibody for 1 h, protein A-conjugated beads were added, and lysates were incubated overnight at 4°C, followed by four washes with lysis buffer. Immune complexes were eluted from the beads with Laemmli sample buffer, resolved with SDS-PAGE under nonreduced conditions, and transferred to nitrocellulose membranes (BA85, 0.45 μm; Schleicher & Schuell, Keene, NH). After blocking for 1 h with phosphate-buffered saline containing 0.1% Tween 20 and 5% powdered skim milk, blots were incubated for 2 h or overnight with primary antibody (in the same buffer as described above), washed three times with phosphate-buffered saline with 0.1% Tween 20, and then incubated with secondary antibody. All bands were detected with an electrochemiluminescence detection kit (RPN 2106; Amersham Biosciences, Piscataway, NJ).
RESULTS
Characterization of CD147 LG and HG Forms
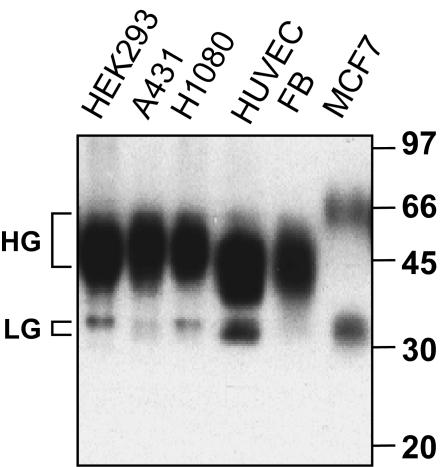
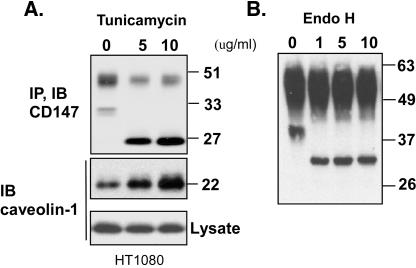
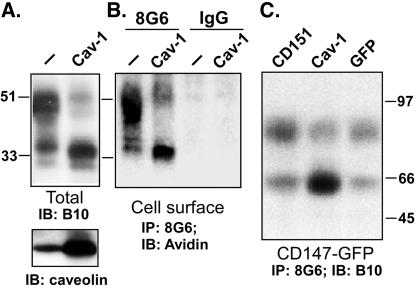
Variability in CD147 N-glycosylation is illustrated in Figure 1. There is a substantial amount of the LG form (∼32 kDa), relative to the HG form (∼45-65 kDa), in HUVEC and MCF7 cells. However, the LG form is minor in HEK293 and HT1080 cells, and the LG form is barely detectable in A431 cells and primary fibroblasts. Treatment of HT1080 cells with tunicamycin, an inhibitor of N-linked glycosylation of newly synthesized proteins, affected both LG and HG forms. The LG form (32 kDa) completely disappeared, and the level of the HG form (∼50 kDa) was greatly diminished. The 27-kDa band that appeared is consistent with the size of the core protein. The fraction of HG-CD147 remaining at ∼50 kDa was likely synthesized before tunicamycin treatment. In contrast to tunicamycin, endoglycosidase H (endo H) caused a reduction in size for the LG form but not the HG form of CD147 (Figure 2B). Therefore, both forms of CD147 are N-glycosylated, with the HG form containing complex-type carbohydrate (resistant to endo H) and the LG form containing high-mannose-type carbohydrate (sensitive to endo H).
Figure 1.
Variability among CD147 glycoforms. The indicated cell lines were lysed with 1% Brij 96 and then blotted with anti-CD147 polyclonal antibody B10 for endogenous CD147. FB, fibroblasts.
Figure 2.
Characterization of CD147 glycosylation. (A) HT1080 cells were treated with 0, 5, or 10 μg/ml tunicamycin for 12 h. CD147 immunoprecipitates were then blotted for CD147 (top) or associated caveolin-1 (middle). Caveolin-1 in total cell lysates was also blotted (bottom). (B) CD147 was immunoprecipitated from HT1080 cells (with mAb 8G6), and samples were then treated with 1, 5, or 10 μl of endo H (1000 U/μl), in 1.0 ml of buffer, at 37°C for 30 min before SDS-PAGE. CD147 was then detected by blotting with polyclonal antibody B10. IP, immunoprecipitated; IB, immunoblotted.
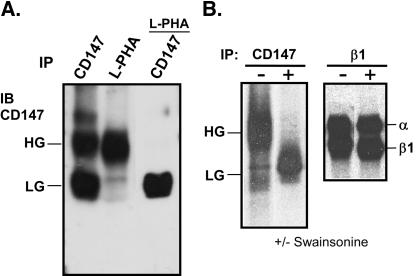
The addition of multiple N-acetyllactosamine units can result in a considerable increase in the size of a glycoprotein (Fukuda, 1991). Polylactosamine synthesis is initiated by β1,6-N-acetylglucosaminyltransferase V (GnT-V), which catalyzes the formation of β1,6 branches on the trimannosyl terminus of N-linked oligosaccharides. Binding by the lectin l-PHA is indicative of β1,6 branching and polylactosamines (Cummings and Kornfeld, 1982). l-PHA blotted almost exclusively the HG form, but not the LG form, of CD147 (Figure 3A, lane 2). Furthermore, depletion of l-PHA-reactive material removed nearly all HG-CD147, leaving only the LG form remaining to be blotted with anti-CD147 antibody (Figure 3A, lane 3). The two forms of CD147 were initially present at comparable levels, as indicated by CD147 blotting (Figure 3A, lane 1).
Figure 3.
Evidence for β1,6-branched polylactosamines. (A) HT1080 cells stably transfected with CD147-GFP were lysed in 1% Brij 96 detergent and then immunoprecipitated with anti-CD147 mAb 8G6 (lane 1) or the lectin l-PHA (lane 2). After depletion of l-PHA-binding material, CD147 was again immunoprecipitated (lane 3). (B) HEK293 cells were treated with 100 μM swainsonine for 2 h, incubated in cysteine- and methionine-free medium for 1 h, and then pulsed for 4 h in medium containing 0.5 mCi/ml [35S]methionine/cysteine and 5.0% dialyzed fetal calf serum. Labeled cells were lysed in RIPA buffer (25 mM Tris, 2 mM EDTA, 150 mM NaCl, 0.1% NaN3, pH 7.2, plus 1% Triton X-100, 1% deoxycholate, and 0.1% sodium dodecyl sulfate), endogenous CD147 and integrin β1 were immunoprecipitated, and labeled proteins were detected with a PhosphorImager (Storm 860; Molecular Dynamics, Sunnyvale, CA).
Swainsonine is an alkaloid that inhibits α-mannosidase II enzyme activity, thus eliminating substrate for GnT-V and preventing β1,6 branching (Dennis et al., 1999). After treatment of HEK293 cells with 100 μM swainsonine for 4 h, the appearance of HG-CD147 was almost completely inhibited (Figure 3B). These l-PHA and swainsonine results indicate that HG-CD147 contains polylactosamine extensions added to N-glycans during protein maturation. In a control experiment, β1-integrin and associated integrin α subunits did not show sensitivity to swainsonine (Figure 3B), thus emphasizing the specialized properties of CD147, compared with other proteins on the surface of these cells.
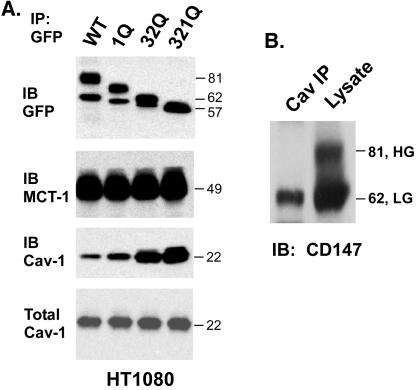
A mutation (N44Q, i.e., 1Q) removing the sole N-linked glycosylation site in Ig domain 1 caused the HG form of CD147 to decrease by ∼5-6 kDa (Figure 4A). Mutation of both N-linked glycosylation sites (N152Q and N186Q, i.e., 32Q) in Ig domain 2 caused the HG form to lose ∼12 kDa (down to ∼32 kDa), and mutation of all three sites (321Q) yielded the core protein (∼27 kDa). In the same experiment, LG-CD147 decreased by 1-2 kDa in the 1Q mutant, by ∼3 kDa in the 32Q mutant, and by 4-5 kDa in the 321Q mutant (Figure 4A). Therefore, all three sites are making comparable contributions to the glycosylation of HG-CD147 and LG-CD147.
Figure 4.
Effects of N-glycosylation on CD147 size and association with caveolin-1. (A) HT1080 cells stably expressing mutant and wild-type GFP-CD147 were lysed in 1% Brij 96. CD147 was then immunoprecipitated with anti-GFP mAb, and immunoprecipitates were immunoblotted for CD147 (anti-GFP) (top), MCT-1 (second), or caveolin-1 (third). Caveolin-1 present in total lysates was also detected by blotting (bottom). CD147 mutants were obtained with recombinant PCRs and subcloned into pEGFP-N1 vector (Clontech, Palo Alto, CA), yielding a C-terminal GFP fusion protein. All constructions were confirmed by sequencing (1Q, N44Q; 32Q, N152Q and N186Q; 321Q, N44Q and N152Q and N186Q). (B) HT1080 cells expressing wild-type CD147-GFP were lysed in 1% Brij 96, and then endogenous caveolin-1 was immunoprecipitated with polyclonal antibody. CD147 was detected in the caveolin-1 immunoprecipitate (lane 1) or in the total lysate (lane 2) by blotting with anti-GFP mAb. It should be noted that increasing exposure time by five to tenfold longer still did not reveal the HG form of CD147 in lane 1. Similar results were observed with HEK293 cells overexpressing caveolin-1 (not shown). Cav, caveolin-1.
Glycosylation Impairs Caveolin-1 Association
In parallel with decreased glycosylation resulting from tunicamycin inhibition, the extent of caveolin-1 association was increased (Figure 2A, middle), whereas total caveolin-1 levels did not change (Figure 2A, bottom). Similarly, the decreased extent of glycosylation resulting from asparagine (Asn) mutagenesis was correlated with an increase in the recovery of associated caveolin-1 (Figure 4A), whereas levels of another CD147-associated protein, MCT-1, did not change and total caveolin-1 levels did not change. These results indicate that N-glycosylation is not required for caveolin-1 association but instead interferes with caveolin-1 association. Consistent with this latter point, we observed preferential caveolin-1 association with the LG form of CD147 and not the HG form. Immunoprecipitation of caveolin-1 yielded only LG-CD147 (Figure 4B), although both the LG and HG forms were amply present in the HT1080 cell lysate. Similar results were obtained in a separate experiment; anticaveolin-1 antibody selectively immunoprecipitated only the LG form of CD147 from A431 cells, whereas anti-CD147 antibody yielded both the HG and LG forms (W. Tang, S.B. Chang, and M.E. Helmer; unpublished observations). Furthermore, immunoprecipitation of HG-CD147 with l-PHA (Figure 3A) did not yield any associated caveolin (unpublished data). Therefore, caveolin-1 association with CD147 is markedly impaired by the presence of N-linked glycosylation, especially in the case of HG-CD147.
Caveolin-1 Association Impairs Glycosylation
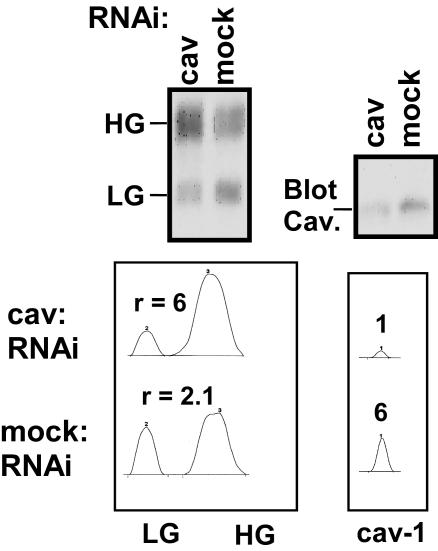
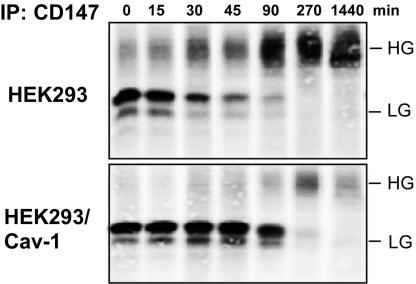
N-linked glycosylation impaired caveolin-1 association. Conversely, the presence of caveolin-1 impaired CD147 glycosylation. As shown in Figure 5, caveolin-1 levels in the human rhabdomyosarcoma cell line RD were decreased by ∼83% with an RNA interference strategy. In parallel, the HG-CD147/LG-CD147 ratio shifted from ∼2.1 in mock-treated cells to ∼6 in caveolin-1 RNA interference-treated cells. Overexpression of caveolin-1 had the opposite effect on the HG/LG ratio (Figure 6). Expression of caveolin-1 in HEK293 cells caused a marked decrease in the HG/LG ratio, as seen for total endogenous CD147 (Figure 6A), endogenous cell surface-labeled CD147 (Figure 6B), and transfected CD147-GFP protein (Figure 6C). Similar results were obtained when caveolin-1 was overexpressed in A431 cells (unpublished observations). In control experiments, overexpression of GFP alone or the CD151 molecule did not decrease the HG/LG ratio (Figure 6C), and no CD147 was observed in control Ig immunoprecipitations (Figure 6B). Blotting of caveolin-1 confirmed that caveolin-1 levels were up-regulated (five- to tenfold, compared with endogenous levels) in caveolin-1 transfectants (Figure 6A, bottom). Caveolin-1 overexpression had no detectable effect on the size of other glycoproteins in HEK293 cells, including β1-integrin, CD44, and CD9 (unpublished observations). An 35S-pulse-labeling experiment showed that the LG form was at least partially converted to the HG form during biosynthesis, such that, after 1.5 h, nearly all of the LG form was either converted or degraded (Figure 7). In contrast, with caveolin-1 overexpression, minimal conversion to the HG form occurred in 90 min, and levels of the HG form remained low even after 4.5 or 24 h.
Figure 5.
Effects of caveolin-1 removal on the CD147 HG/LG ratio. A small interfering RNA oligonucleotide (gagaagcaagtgtacgacg), previously established to reduce caveolin-1 expression (Nichols, 2002), was inserted into the vector pSilencer3.1-H1hygro (Ambion, Austin, TX) and was stably expressed in RD cells with the Oligofectamine reagent (Invitrogen, Carlsbad, CA), followed by selection with 200 μg/ml hygromycin (Sigma) for ∼2 wk. Left, results of CD147 blotting from RD cell lysates (top) and quantitative scans of CD147 blots (bottom) (scanned with GeneTools version 3.0; Syngene Laboratories, Frederick, MD). The r values refer to HG/LG ratios. Right, caveolin-1 blotting (top) and scans of caveolin-1 blots (bottom). The numbers 1 and 6 indicate the relative areas of caveolin-1 peaks. Similar results were obtained with many different film exposures and for multiple clones of RD transfectants. RNAi, RNA interference.
Figure 6.
Caveolin-1 effects on the CD147 HG/LG ratio. (A) HEK293 cells stably expressing the control vector or caveolin-GFP were lysed, and then endogenous CD147 (top) or caveolin-1 (bottom) was detected by blotting of total lysates. (B) HEK293 cells overexpressing caveolin-1 or the control vector were surface-labeled with biotin and lysed in 1% Brij 96. Immunoprecipitates of CD147 (mAb 8G6) or control IgG were then blotted for surface-labeled material with avidin-horseradish peroxidase. (C) HEK293 cells were stably cotransfected with CD147-GFP and CD151, caveolin-1-GFP, or GFP alone. CD147 was then immunoprecipitated with mAb 8G6 and analyzed by blotting. Caveolin-1-GFP was prepared as described (Tang and Hemler, 2004).
Figure 7.
Caveolin-1 effects on CD147 biosynthesis. In control HEK293 cells or cells stably expressing caveolin-1, endogenous CD147 was labeled with [35S]methionine/cysteine for 1 h, immunoprecipitated, and detected as in Figure 3B. After the 1-h pulse, the 35S-label was chased for the additional times indicated, in medium containing 5.0% dialyzed fetal calf serum and 25× excess unlabeled l-methionine.
HG-CD147 Is Preferentially Cross-linked/Clustered
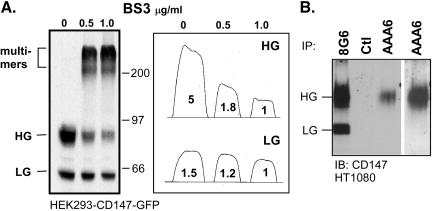
Caveolin-1 decreases the HG/LG ratio (Figure 6) at the same time that it decreases CD147 self-association (Tang and Hemler, 2004), strongly suggesting that HG-CD147 but not LG-CD147 becomes self-associated. Here we provide two additional results in support of this conclusion. First, after addition of a covalent cross-linking agent to intact cells, the appearance of CD147 multimers was accompanied by a marked dimunition in levels of HG-CD147 but only a slight decrease in the level of LG-CD147 (Figure 8A). This preferential cross-linking of HG-CD147 is consistent with its preferential self-association. This conclusion is supported by earlier demonstrations that cross-linking of CD147 yields homo-oligomers (Fadool and Linser, 1996; Yoshida et al., 2000). Second, anti-CD147 mAb AAA6 immunoprecipitated exclusively the HG form of CD147 (Figure 8A, lanes 3 and 4), whereas mAb 8G6 recognized both forms (Figure 8A, lane 1). Therefore, the excess N-glycosylation present in HG-CD147 must contribute to the AAA6 epitope, which was previously mapped to Ig domain 1 (Koch et al., 1999). Notably, low-affinity anti-CD147 mAbs, such as mAb AAA6, preferentially recognize self-associated CD147 on the surface of cells, because self-associated CD147 is able to support bivalent mAb binding (Koch et al., 1999). Therefore, when mAb AAA6 is recognizing self-associated CD147 (Koch et al., 1999), it is recognizing HG-CD147.
Figure 8.
Selective multimerization of HG-CD147. (A) Intact HEK293 cells were treated for 2 h with the homobifunctional cross-linking agent BS3 (bis[sulfosuccinimido]suberate), at 0.5 or 1.0 μg/ml, and then CD147-GFP was immunoprecipitated from cell lysates with mAb 8G6 and blotted with anti-GFP polyclonal antibody. Because of the presence of the GFP tag, sizes are increased (∼75 and 57 kDa), compared with Figures 1 and 2 (50 and 32 kDa). Band intensities corresponding to the HG and LG forms were quantitated with GeneTools version 3.0 (Syngene Laboratories), confirming the selective decrease in the HG form after cross-linking. The slight decrease in the amount of the LG form is consistent with decreased antibody recognition, rather than decreased interchain cross-linking. (B) Endogenous CD147 was immunoprecipitated from HT1080 cells with mAb 8G6, negative control antibody, or mAb AAA6 and was then detected with CD147 immunoblotting. Even with longer exposure of the AAA6 sample (lane 4), no LG form can be observed. Ctl, control.
DISCUSSION
CD147 Glycosylation
HG forms of CD147 have been frequently observed (see Introduction) and are functionally relevant with respect to MMP induction (Guo et al., 1997; Sun and Hemler, 2001). Compared with other cell surface transmembrane glycoproteins, CD147 is quite unusual. It often contains 15-25 kDa of glycosylation content, which is well beyond that (∼5 kDa) typically observed for a glycoprotein containing three N-linked glycosylation sites. Here we provide evidence that excess CD147 glycosylation is attributable to GnT-V-generated, β1,6-branched, polylactosamine content. Tunicamycin, endo H, and asparagine mutagenesis experiments established that all CD147 glycosylation is N-linked, with HG-CD147 containing complex-type carbohydrate and LG-CD147 containing the high-mannose form. Selective binding of l-PHA lectin to HG-CD147 is diagnostic for the presence of β1,6-branched structures, found on tri- and tetra-antennary N-glycans and formed only through the action of GnT-V (Cummings and Kornfeld, 1982). Swainsonine inhibition of HG-CD147 appearance is also indicative of β1,6 branching and polylactosamine extensions. Swainsonine inhibits Golgi mannosidase II, blocking the biosynthetic pathway before GnT-V action and β1,6 branching (Dennis et al., 1999). The GnT-V product is the preferred substrate for extension with polylactosamine chains (Galβ1,4GlcNAcβ1,3 repeating units). These units have the potential to yield a considerable increase in the overall size of N-glycans (Dennis et al., 1999), especially if there is prolonged transit time in the trans-Golgi network (Wang et al., 1991) and/or limited competition by chain-terminating enzymes (Prieto et al., 1997).
Among transmembrane proteins that reside mostly on the cell surface plasma membrane, we could find no other published example of N-glycan polylactosamine additions as widespread (i.e., on many cell types) and extensive as those observed for HG-CD147. Proteins known to contain extensive polylactosamine, such as Lamp-1, Lamp-2, and CD63 (Lamp-3), reside mostly in lysosomes (Fukuda, 1991). Integrins can carry polylactosamine (Chakraborty et al., 2001), but it has not been reported to make a large contribution to glycoprotein size. Indeed, although swainsonine had a major effect on the size of CD147, it had no detectable effect on the size of β1-integrins obtained from the same cell in the same experiment.
Caveolin-1 Influence on CD147 Glycosylation
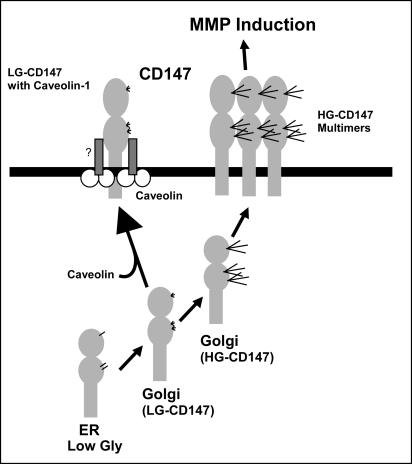
We previously demonstrated that caveolin-1 can associate with CD147 in a temperature- and cholesterol-dependent manner, with Ig domain 2 being required (Tang and Hemler, 2004). Now we show that caveolin-1 is closely linked to CD147 glycosylation. First, stable expression of caveolin-1 shifted the HG/LG ratio downward. Second, small interfering RNA depletion of caveolin-1 levels substantially increased the HG/LG ratio. Third, removal of glycosylation, with tunicamycin treatment or asparagine mutagenesis, led to increased caveolin-1 association. Fourth, caveolin-1 bound exclusively to LG-CD147, rather than HG-CD147. It was shown previously that caveolin-1 can cause reorganization of the Golgi complex, such that GnT-III activity prevents subsequent GnT-V-mediated β1,6 branching (Sasai et al., 2003). That would indeed help explain why caveolin-1 limits the appearance of β1,6 branching and polylactosamine on CD147. We hypothesize that caveolin-1 not only affects the organization of GnT in the Golgi complex but also physically associates with LG-CD147 and escorts it out of the Golgi complex to the plasma membrane (Figure 9). In this regard, although LG-CD147 can be a biosynthetic precursor of HG-CD147, we can detect both LG-CD147 and LG-CD147-caveolin complexes on the cell surface, as shown here and previously (Tang and Hemler, 2004). We do not suggest that caveolin-1 is the only factor regulating CD147 glycosylation. Indeed, CD147 appears in multiple glycosylated forms on cells such as lymphocytes, which lack caveolins. Nonetheless, in cells where caveolin-1 is present, it may play an important role in suppressing the appearance of HG-CD147.
Figure 9.
Schematic diagram of CD147 maturation and regulation by caveolin-1. After CD147 enters the Golgi complex, it can mature via two possible pathways. First, it associates with caveolin-1, fails to acquire polylactosamines, and is escorted to the cell surface while still at least partly associated with caveolin-1, in the context of a larger, caveolin-containing complex (Tang and Hemler, 2004). This LG-CD147 does not self-aggregate and does not induce MMP production. Second, with initiation by the action of GnT-V, CD147 acquires β1,6-branched polylactosamines on its N-glycans as it matures to HG-CD147. This molecule has a tendency to self-aggregate and is active in the stimulation of MMP production by neighboring cells. ER, endoplasmic reticulum; Gly, glycosylation.
Functional Relevance of CD147 Glycosylation and Regulation by Caveolin-1
Previously, we showed that caveolin-1 associates with CD147, thereby reducing CD147-dependent MMP induction (Tang and Hemler, 2004). A mutant form of CD147, lacking caveolin-1 association, exhibited increased MMP-1 induction. Conversely, caveolin-1 overexpression caused a decrease in MMP-1 induction in tumor cell-fibroblast coculture assays (Tang and Hemler, 2004). The present results provide mechanistic insights into how caveolin-1 regulation of CD147-dependent MMP production is achieved. Caveolin-1 associates with LG-CD147 and suppresses its conversion to HG-CD147. Because HG-CD147 is the form that induces MMP production (Guo et al., 1997; Sun and Hemler, 2001), we now better understand how caveolin-1 suppresses CD147-dependent MMP production. In support of a link between HG-CD147 and MMP induction, the same anti-CD147 antibody (mAb AAA6) that blocks CD147-dependent MMP induction (Sun and Hemler, 2001) selectively binds to HG-CD147.
The negative effect of caveolin-1 on HG-CD147 not only explains how caveolin-1 can suppress CD147-dependent MMP induction but also shows how caveolin-1 decreases CD147 self-association (Tang and Hemler, 2004). In this regard, HG-CD147 (and not LG-CD147) was preferentially covalently cross-linked, consistent with this form of CD147 undergoing self-association. When caveolin-1 removes HG-CD147, it simultaneously impairs self-association. Furthermore, the same low-affinity antibody (mAb AAA6) that preferentially recognizes self-associated CD147 (Koch et al., 1999) selectively binds to HG-CD147. In summary, CD147 self-aggregation, MMP induction, and HG-CD147 are all linked because 1) they are all simultaneously down-regulated by caveolin-1, 2) they are all detected/affected by mAb AAA6, and 3) it is HG-CD147 that triggers MMP induction and undergoes self-aggregation. Conversely, LG-CD147 is enhanced by caveolin-1, does not bear the AAA6 epitope, does not trigger MMP induction, and does not self-aggregate.
GnT-V activity and β1,6-branched polylactosamines are well documented to be linked to tumor progression (Dennis et al., 1999; Chakraborty and Pawelek, 2003). Now we show that CD147, which itself has been well documented to be up-regulated on cancer cells and to promote tumor progression (see Introduction), is a major carrier (if not the only major cell surface transmembrane carrier) of β1,6-branched polylactosamines. Therefore, it is attractive to speculate that the negative effects of swainsonine on HG-CD147 (shown here) are largely responsible for swainsonine having antitumor activity (Goss et al., 1995), markedly inhibiting MMP-2 production by three different astrocytoma cell lines (Rooprai et al., 2001), and inhibiting the production of other MMPs by melanoma cells (Seftor et al., 1991) and brain tumor cells (Rooprai et al., 2001). Furthermore, it is likely not simply a coincidence that caveolin-1, a well-established tumor suppressor (Wiechen et al., 2001; Aldred et al., 2003; Hino et al., 2003; Williams et al., 2003), down-regulates HG-CD147 production.
In addition to inducing MMPs, CD147 has a host of other possible functions (see Introduction). Notably, neither integrin association (Berditchevski et al., 1997) nor MCT-1 association (Kirk et al., 2000) is affected by N-glycosylation or caveolin-1. However, it remains to be seen whether CD147 functions during hyaluronan induction (Marieb et al., 2004), female fertilization (Kuno et al., 1998), spermatogenesis (Igakura et al., 1998), cyclophilin A binding and signaling (Pushkarsky et al., 2001; Yurchenko et al., 2002), and T cell activation and development (Igakura et al., 1996; Koch et al., 1999; Renno et al., 2002; Staffler et al., 2003) are affected by N-glycosylation or caveolin-1 association.
Acknowledgments
We thank Dr. Xiuwei Yang in our laboratory for helpful discussions and experimental assistance. This work was supported by a grant from the National Institutes of Health (grant CA-86712).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-05-0402. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0402.
Abbreviations used: Asn, asparagine; endo H, endoglycosidase H; GFP, green fluorescent protein; GnT, N-acetylglucosaminyltransferase; HUVEC, human umbilical vein endothelial cells; MCT, monocarboxylate transporter; MMP, matrix metalloproteinase; HG, highly glycosylated; LG, less glycosylated; l-PHA, l-phytohemagglutinin.
References
- Aldred, M.A., et al. (2003). Caveolin-1 and caveolin-2, together with three bone morphogenetic protein-related genes, may encode novel tumor suppressors down-regulated in sporadic follicular thyroid carcinogenesis. Cancer Res. 63, 2864-2871. [PubMed] [Google Scholar]
- Berditchevski, F., Chang, S., Bodorova, J., and Hemler, M.E. (1997). Generation of monoclonal antibodies to integrin-associated proteins: evidence that α3β1 complexes with EMMPRIN/basigin/OX47/M6. J. Biol. Chem. 272, 29174-29180. [DOI] [PubMed] [Google Scholar]
- Biswas, C., Zhang, Y., DeCastro, R., Guo, H., Nakamura, T., Kataoka, H., and Nabeshima, K. (1995). The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 55, 434-439. [PubMed] [Google Scholar]
- Bordador, L.C., Li, X., Toole, B., Chen, B., Regezi, J., Zardi, L., Hu, Y., and Ramos, D.M. (2000). Expression of emmprin by oral squamous cell carcinoma. Int. J. Cancer 85, 347-352. [PubMed] [Google Scholar]
- Caudroy, S., Polette, M., Nawrocki-Raby, B., Cao, J., Toole, B.P., Zucker, S., and Birembaut, P. (2002). EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin. Exp. Metastasis 19, 697-702. [DOI] [PubMed] [Google Scholar]
- Chakraborty, A.K., Pawelek, J., Ikeda, Y., Miyoshi, E., Kolesnikova, N., Funasaka, Y., Ichihashi, M., and Taniguchi, N. (2001). Fusion hybrids with macrophage and melanoma cells up-regulate N-acetylglucosaminyltransferase V, β1-6 branching, and metastasis. Cell Growth Differ. 12, 623-630. [PubMed] [Google Scholar]
- Chakraborty, A.K., and Pawelek, J.M. (2003). GnT-V, macrophage and cancer metastasis: a common link. Clin. Exp. Metastasis 20, 365-373. [DOI] [PubMed] [Google Scholar]
- Cummings, R.D., and Kornfeld, S. (1982). Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J. Biol. Chem. 257, 11230-11234. [PubMed] [Google Scholar]
- Dennis, J.W., Granovsky, M., and Warren, C.E. (1999). Glycoprotein glycosylation and cancer progression. Biochim. Biophys. Acta 1473, 21-34. [DOI] [PubMed] [Google Scholar]
- Ellis, S.M., Nabeshima, K., and Biswas, C. (1989). Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 49, 3385-3391. [PubMed] [Google Scholar]
- Fadool, J.M., and Linser, P.J. (1996). Evidence for the formation of multimeric forms of the 5A11/HT7 antigen. Biochem. Biophys. Res. Commun. 229, 280-286. [DOI] [PubMed] [Google Scholar]
- Fadool, J.M., and Linser, P.J. (1993). Differential glycosylation of the 5A11/HT7 antigen by neural retina and epithelial tissues in the chicken. J. Neurochem. 60, 1354-1364. [DOI] [PubMed] [Google Scholar]
- Fukuda, M. (1991). Lysosomal membrane glycoproteins: structure, biosynthesis, and intracellular trafficking. J. Biol. Chem. 266, 21327-21330. [PubMed] [Google Scholar]
- Goss, P.E., Baker, M.A., Carver, J.P., and Dennis, J.W. (1995). Inhibitors of carbohydrate processing: a new class of anticancer agents. Clin. Cancer Res. 1, 935-944. [PubMed] [Google Scholar]
- Guo, H., Zucker, S., Gordon, M.K., Toole, B.P., and Biswas, C. (1997). Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J. Biol. Chem. 272, 24-27. [PubMed] [Google Scholar]
- Hino, M., Doihara, H., Kobayashi, K., Aoe, M., and Shimizu, N. (2003). Caveolin-1 as tumor suppressor gene in breast cancer. Surg. Today 33, 486-490. [DOI] [PubMed] [Google Scholar]
- Hori, K., Katayama, N., Kachi, S., Kondo, M., Kadomatsu, K., Usukura, J., Muramatsu, T., Mori, S., and Miyake, Y. (2000). Retinal dysfunction in basigin deficiency. Invest. Ophthalmol. Vis. Sci. 41, 3128-3133. [PubMed] [Google Scholar]
- Igakura, T., et al. (1998). A null mutation in basigin, an immunoglobulin superfamily member, indicates its important roles in peri-implantation development and spermatogenesis. Dev. Biol. 194, 152-165. [DOI] [PubMed] [Google Scholar]
- Igakura, T., Kadomatsu, K., Taguchi, O., Muramatsu, H., Kaname, T., Miyauchi, T., Yamamura, K., Arimura, K., and Muramatsu, T. (1996). Roles of basigin, a member of the immunoglobulin superfamily, in behavior as to an irritating odor, lymphocyte response, and blood-brain barrier. Biochem. Biophys. Res. Commun. 224, 33-36. [DOI] [PubMed] [Google Scholar]
- Jiang, J.L., Zhou, Q., Yu, M.K., Ho, L.S., Chen, Z.N., and Chan, H.C. (2001). The involvement of HAb18G/CD147 in regulation of store-operated calcium entry and metastasis of human hepatoma cells. J. Biol. Chem. 276, 46870-46877. [DOI] [PubMed] [Google Scholar]
- Kanekura, T., Chen, X., and Kanzaki, T. (2002). Basigin (CD147) is expressed on melanoma cells and induces tumor cell invasion by stimulating production of matrix metalloproteinases by fibroblasts. Int. J. Cancer 99, 520-528. [DOI] [PubMed] [Google Scholar]
- Kanekura, T., Miyauchi, T., Tashiro, M., and Muramatsu, T. (1991). Basigin, a new member of the immunoglobulin superfamily: genes in different mammalian species, glycosylation changes in the molecule from adult organs and possible variation in the N-terminal sequences. Cell Struct. Funct. 16, 23-30. [DOI] [PubMed] [Google Scholar]
- Kasinrerk, W., Fiebiger, E., Stefanova, I., Baumruker, T., Knapp, W., and Stockinger, H. (1992). Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J. Immunol. 149, 847-854. [PubMed] [Google Scholar]
- Kataoka, H., DeCastro, R., Zucker, S., and Biswas, C. (1993). Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res. 53, 3154-3158. [PubMed] [Google Scholar]
- Kirk, P., Wilson, M.C., Heddle, C., Brown, M.H., Barclay, A.N., and Halestrap, A.P. (2000). CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J. 19, 3896-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, C., Staffler, G., Huttinger, R., Hilgert, I., Prager, E., Cerny, J., Steinlein, P., Majdic, O., Horejsi, V., and Stockinger, H. (1999). T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 11, 777-786. [DOI] [PubMed] [Google Scholar]
- Kuno, N., Kadomatsu, K., Fan, Q.W., Hagihara, M., Senda, T., Mizutani, S., and Muramatsu, T. (1998). Female sterility in mice lacking the basigin gene, which encodes a transmembrane glycoprotein belonging to the immunoglobulin superfamily. FEBS Lett. 425, 191-194. [DOI] [PubMed] [Google Scholar]
- Li, R., Huang, L., Guo, H., and Toole, B.P. (2001). Basigin (murine EMMPRIN) stimulates matrix metalloproteinase production by fibroblasts. J. Cell. Physiol. 186, 371-379. [DOI] [PubMed] [Google Scholar]
- Marieb, E.A., Zoltan-Jones, A., Li, R., Misra, S., Ghatak, S., Cao, J., Zucker, S., and Toole, B.P. (2004). Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res. 64, 1229-1232. [DOI] [PubMed] [Google Scholar]
- Muraoka, K., Nabeshima, K., Murayama, T., Biswas, C., and Koono, M. (1993). Enhanced expression of a tumor-cell-derived collagenase-stimulatory factor in urothelial carcinoma: its usefulness as a tumor marker for bladder cancers. Int. J. Cancer 55, 19-26. [DOI] [PubMed] [Google Scholar]
- Nichols, B.J. (2002). A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat. Cell Biol. 4, 374-378. [DOI] [PubMed] [Google Scholar]
- Polette, M., Gilles, C., Marchand, V., Lorenzato, M., Toole, B., Tournier, J.M., Zucker, S., and Birembaut, P. (1997). Tumor collagenase stimulatory factor (TCSF) expression and localization in human lung and breast cancers. J. Histochem. Cytochem. 45, 703-709. [DOI] [PubMed] [Google Scholar]
- Prieto, P.A., Larsen, R.D., Cho, M., Rivera, H.N., Shilatifard, A., Lowe, J.B., Cummings, R.D., and Smith, D.F. (1997). Expression of human H-type α1,2-fucosyltransferase encoding for blood group H(O) antigen in Chinese hamster ovary cells: evidence for preferential fucosylation and truncation of polylactosamine sequences. J. Biol. Chem. 272, 2089-2097. [DOI] [PubMed] [Google Scholar]
- Pushkarsky, T., Zybarth, G., Dubrovsky, L., Yurchenko, V., Tang, H., Guo, H., Toole, B., Sherry, B., and Bukrinsky, M. (2001). CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc. Natl. Acad. Sci. USA 98, 6360-6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renno, T., Wilson, A., Dunkel, C., Coste, I., Maisnier-Patin, K., Benoit, D.C., Aubry, J.P., Lees, R.K., Bonnefoy, J.Y., MacDonald, H.R., and Gauchat, J.F. (2002). A role for CD147 in thymic development. J. Immunol. 168, 4946-4950. [DOI] [PubMed] [Google Scholar]
- Rooprai, H.K., Kandanearatchi, A., Maidment, S.L., Christidou, M., Trillo-Pazos, G., Dexter, D.T., Rucklidge, G.J., Widmer, W., and Pilkington, G.J. (2001). Evaluation of the effects of swainsonine, captopril, tangeretin and nobiletin on the biological behaviour of brain tumour cells in vitro. Neuropathol. Appl. Neurobiol. 27, 29-39. [DOI] [PubMed] [Google Scholar]
- Sameshima, T., Nabeshima, K., Toole, B.P., Yokogami, K., Okada, Y., Goya, T., Koono, M., and Wakisaka, S. (2000a). Expression of emmprin (CD147), a cell surface inducer of matrix metalloproteinases, in normal human brain and gliomas. Int. J. Cancer 88, 21-27. [DOI] [PubMed] [Google Scholar]
- Sameshima, T., Nabeshima, K., Toole, B.P., Yokogami, K., Okada, Y., Goya, T., Koono, M., and Wakisaka, S. (2000b). Glioma cell extracellular matrix metalloproteinase inducer (EMMPRIN) (CD147) stimulates production of membrane-type matrix metalloproteinases and activated gelatinase A in co-cultures with brain-derived fibroblasts. Cancer Lett. 157, 177-184. [DOI] [PubMed] [Google Scholar]
- Sasai, K., Ikeda, Y., Ihara, H., Honke, K., and Taniguchi, N. (2003). Caveolin-1 regulates the functional localization of N-acetylglucosaminyltransferase III within the Golgi apparatus. J. Biol. Chem. 278, 25295-25301. [DOI] [PubMed] [Google Scholar]
- Seftor, R.E., Seftor, E.A., Grimes, W.J., Liotta, L.A., Stetler-Stevenson, W.G., Welch, D.R., and Hendrix, M.J. (1991). Human melanoma cell invasion is inhibited in vitro by swainsonine and deoxymannojirimycin with a concomitant decrease in collagenase IV expression. Melanoma Res. 1, 43-54. [DOI] [PubMed] [Google Scholar]
- Staffler, G., Szekeres, A., Schutz, G.J., Saemann, M.D., Prager, E., Zeyda, M., Drbal, K., Zlabinger, G.J., Stulnig, T.M., and Stockinger, H. (2003). Selective inhibition of T cell activation via CD147 through novel modulation of lipid rafts. J. Immunol. 171, 1707-1714. [DOI] [PubMed] [Google Scholar]
- Sun, J., and Hemler, M.E. (2001). Regulation of MMP production through homophilic CD147/EMMPRIN interaction. Cancer Res. 61, 2276-2281. [PubMed] [Google Scholar]
- Suzuki, S., Sato, M., Senoo, H., and Ishikawa, K. (2004). Direct cell-cell interaction enhances pro-MMP-2 production and activation in co-culture of laryngeal cancer cells and fibroblasts: involvement of EMMPRIN and MT1-MMP. Exp. Cell Res. 293, 259-266. [DOI] [PubMed] [Google Scholar]
- Tang, W., and Hemler, M.E. (2004). Caveolin-1 regulates MMP-1 induction and CD147/EMMPRIN cell surface clustering. J. Biol. Chem. 279, 11112-11118. [DOI] [PubMed] [Google Scholar]
- Taylor, P.M., Woodfield, R.J., Hodgkin, M.N., Pettitt, T.R., Martin, A., Kerr, D.J., and Wakelam, M.J. (2002). Breast cancer cell-derived EMMPRIN stimulates fibroblast MMP2 release through a phospholipase A2 and 5-lipoxygenase catalyzed pathway. Oncogene 21, 5765-5772. [DOI] [PubMed] [Google Scholar]
- Toole, B.P. (2003). Emmprin (CD147), a cell surface regulator of matrix metalloproteinase production and function. Curr. Top. Dev. Biol. 54, 371-389. [DOI] [PubMed] [Google Scholar]
- van den Oord, J.J., Paemen, L., Opdenakker, G., and de Wolf-Peeters, C. (1997). Expression of gelatinase B and the extracellular matrix metalloproteinase inducer EMMPRIN in benign and malignant pigment cell lesions of the skin. Am. J. Pathol. 151, 665-670. [PMC free article] [PubMed] [Google Scholar]
- Wang, W.C., Lee, N., Aoki, D., Fukuda, M.N., and Fukuda, M. (1991). The poly-N-acetyllactosamines attached to lysosomal membrane glycoproteins are increased by the prolonged association with the Golgi complex. J. Biol. Chem. 266, 23185-23190. [PubMed] [Google Scholar]
- Wiechen, K., et al. (2001). Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am. J. Pathol. 159, 1635-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, T.M., Cheung, M.W., Park, D.S., Razani, B., Cohen, A.W., Muller, W.J., Di Vizio, D., Chopra, N.G., Pestell, R.G., and Lisanti, M.P. (2003). Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol. Biol. Cell 14, 1027-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, S., Shibata, M., Yamamoto, S., Hagihara, M., Asai, N., Takahashi, M., Mizutani, S., Muramatsu, T., and Kadomatsu, K. (2000). Homo-oligomer formation by basigin, an immunoglobulin superfamily member, via its N-terminal immunoglobulin domain. Eur. J. Biochem. 267, 4372-4380. [DOI] [PubMed] [Google Scholar]
- Yurchenko, V., et al. (2002). Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J. Biol. Chem. 277, 22959-22965. [DOI] [PubMed] [Google Scholar]
- Zucker, S., Hymowitz, M., Rollo, E.E., Mann, R., Conner, C.E., Cao, J., Foda, H.D., Tompkins, D.C., and Toole, B.P. (2001). Tumorigenic potential of extracellular matrix metalloproteinase inducer. Am. J. Pathol. 158, 1921-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]