Abstract
The small GTPase Arf and coatomer (COPI) are required for the generation of retrograde transport vesicles. Arf activity is regulated by guanine exchange factors (ArfGEF) and GTPase-activating proteins (ArfGAPs). The ArfGAPs Gcs1 and Glo3 provide essential overlapping function for retrograde vesicular transport from the Golgi to the endoplasmic reticulum. We have identified Glo3 as a component of COPI vesicles. Furthermore, we find that a mutant version of the Glo3 protein exerts a negative effect on retrograde transport, even in the presence of the ArfGAP Gcs1. Finally, we present evidence supporting a role for ArfGAP protein in the generation of COPI retrograde transport vesicles.
INTRODUCTION
The integrity and function of eukaryotic organelles requires the regulated transport of protein and membrane cargoes among these compartments. This trafficking function is achieved through the generation of protein-coated transport vesicles, with specific coat complexes functioning at different stages of vesicle trafficking. For example, the clathrin coat complex is involved in endocytosis, the COPII coat complex functions for endoplasmic reticulum (ER)-to-Golgi anterograde traffic, and the COPI coat complex allows intra-Golgi and Golgi-to-ER retrograde transport (Kirchhausen, 2000). Each coat complex is recruited and regulated by specific sets of proteins and their associated regulatory factors (Kirchhausen, 2000).
The COPI coat complex (also known as coatomer) consists of seven subunits (α-, β-, β′-, δ-, ε-, γ-, and ζ-COP) and is controlled for vesicle biogenesis by the monomeric GTP-binding protein Arf1 (Serafini et al., 1991). Binding of GTP activates Arf1, whereas hydrolysis of GTP to GDP results in an inactive conformation for Arf1 (Serafini et al., 1991). Thus, Arf1 activity is dependent on a cycle of GTP binding and hydrolysis, which in turn regulates coatomer function. During the early stages of vesicle formation, GDP-Arf1 is thought to be recruited to the site of vesicle emergence by members of the p23 protein family in mammalian cells, or by SNARE proteins in Saccharomyces cerevisiae that may act as primers for vesicle budding (Gommel et al., 2001; Rein et al., 2002). Once at the membrane, GDP-Arf1 undergoes guanine nucleotide exchange mediated by a guanine exchange factor (ArfGEF) (Peyroche et al., 1996). On nucleotide exchange, a myristate residue at the amino terminus of Arf1 becomes exposed and stably anchors GTP-Arf1 to the membrane (Helms et al., 1993). Subsequently, coatomer proteins are recruited to the site, protein cargo is selected and packaged, and a COPI-coated vesicle is formed. Before vesicle fusion with a target membrane the protein coat is shed. Coat removal is thought to be mediated by hydrolysis of Arf1-bound GTP, which is stimulated by an Arf GTPase activating protein (ArfGAP) (Tanigawa et al., 1993; Cukierman et al., 1995).
The role of ArfGAPs in COPI-mediated vesicular transport has received considerable attention recently. The classical model for the role of ArfGAP activity in vesicular transport states that ArfGAP-stimulated GTP-Arf1 hydrolysis results in GDP-Arf1, which dissociates from the vesicle membrane and thus removes the protein coat from the vesicle (Tanigawa et al., 1993). Indeed, a recent report (Reinhard et al., 2003) concludes from in vitro studies that ArfGAP activity may only be required for vesicle uncoating, lending support for the classical model. In contrast, other investigations have revealed a role for ArfGAP activity in the packaging of protein cargo into COPI vesicles (Nickel et al., 1998; Pepperkok et al., 2000; Lanoix et al., 2001; Rein et al., 2002). These latter findings suggest that ArfGAP activity is required for steps in vesicular transport other than removal of the vesicle coat. Furthermore, new evidence suggests that ArfGAP activity may be required for the generation of COPI vesicles. Yang et al. (2002) showed that mammalian ARFGAP1 is a component of COPI-coated vesicles and that ArfGAP activity might promote COPI vesicle generation. Thus, new evidence from a number of sources presents a somewhat more complex picture of ArfGAP function, leading us to assess the extent of ArfGAP function for vesicular transport.
The budding yeast S. cerevisiae has a family of six ArfGAP proteins. Two of these ArfGAPs, Gcs1 and Glo3, provide essential overlapping function for retrograde transport from the Golgi to the ER (Poon et al., 1999). A yeast two-hybrid analysis of yeast ArfGAP interactions with coatomer subunits has shown that Glo3 interacts directly with Sec21 (γ-COP) and Sec27 (β′-COP), whereas Gcs1 fails to bind to coatomer in a two-hybrid assay (Eugster et al., 2000). These observations raise the possibility that the ArfGAP Glo3 is a component of the COPI vesicle coat and may even be required for the generation of COPI-coated transport vesicles as has been suggested for the mammalian ARFGAP1 (Yang et al., 2002).
We have assessed the biological role of Glo3-coatomer interactions and the role of ArfGAP function in retrograde vesicular transport. Here, we show that Glo3 is indeed a component of COPI-coated vesicles, whereas the related Gcs1 is not. We also have generated a mutant version of Glo3 that has a strong inhibitory effect on growth and retrograde vesicular transport for cells relying on Gcs1. Furthermore, this mutant version of the Glo3 protein prevents the generation of COPI-coated transport vesicles in vitro, indicating a role for the Glo3 protein in the generation of COPI vesicles.
MATERIALS AND METHODS
Yeast Strains, Plasmid Construction, and Growth Conditions
All strains used are isogenic with the wild-type diploid yeast strain W303 (leu2-3112 ura3-1 his3-11,15 trp1-1 ade2-1) and its isogenic haploid derivatives W303-1A (MATa) and W303-1B (MATα) (Archambault et al., 1992). Standard procedures were used for cell growth and transformation. The glo3Δ::HIS3 disruption-deletion has been described previously (Poon et al., 1999).
To control expression, the GLO3 gene was placed downstream of the weak but inducible MET3 promoter sequence (Mao et al., 2002). A 1-kb BamHI-ApaI fragment from the plasmid p314-MET that contains the MET3 promoter sequences (including the start codon for the MET3 open reading frame) was subcloned into the multiple cloning site of pRS315 to generate pSL439. The GLO3 coding sequence lacking the start codon was polymerase chain reaction-amplified using the oligonucleotides 5′-cccaagcttagtaacgatgaaggagaaaca and 5′-gggggcccgaaccaaatgctacctcgtct, and inserted in frame with the MET3 start codon in pSL439 by using the HindIII and ApaI sites to generate pSL440. To generate glo3-R59K, pSL440 was subjected to site-directed mutagenesis by using the QuikChange XL kit (Stratagene, La Jolla, CA) and the mutagenic oligonucleotides 5′-caatgctctgctgtgcataaaaacatgggtgttcatatc and 5′-gatatgaacacccatgtttttatgcacagcagagcattg. The resulting plasmid was named pSL443.
To induce expression of GLO3 and glo3-R59K from the MET3 promoter in liquid culture, transformed yeast cells were shifted to liquid medium lacking methionine and cysteine and incubated for 6 h before analysis.
Bacterial Expression of Proteins and ArfGAP Assays
Recombinant Glo3 was expressed in Escherichia coli as described previously (Poon et al., 1999). For expression of recombinant Glo3-R59K in E. coli, the GLO3 expression plasmid pPPL42 was subjected to site-directed mutagenesis by using the QuikChange XL kit (Stratagene) and the mutagenic oligonucleotides described above to generate pSL460. Arf1 protein was produced as described previously (Poon et al., 1996). ArfGAP activity was assayed as described previously (Poon et al., 2001).
Light Microscopy
Cells expressing a Myc-tagged version of Emp47 were induced to express either the wild-type GLO3 or mutant glo3-R59K gene from the MET3 promoter by incubation in medium lacking methionine. Six hours after transfer to medium lacking methionine, cells were fixed by addition of 5% formalin for 1 h, washed with phosphate-buffered saline (PBS), and treated with zymolyase in buffered sorbitol to remove cell wall material. Cells were harvested by centrifugation, placed on polylysine-coated slides, and treated with mouse monoclonal anti-myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA). After washing with PBS, cells were then exposed to goat anti-mouse antibody conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR). Cells were visualized with a motorized Axioplan II microscope equipped with an Axiocam HRc digital camera and Plan Apochromat 100× 1.4 numerical aperture (all from Carl Zeiss, Jena, Germany).
Electron Microscopy
Cells were grown overnight in selective medium to early- to mid-log phase, harvested, washed twice with water, and incubated in selective medium lacking methionine for 6 h at 30°C. The cells were high-pressure frozen and treated as described previously (Sandmann et al., 2003).
Coimmunoprecipitation
Cells were grown to mid-log phase in rich medium, NaN3 was added to a final concentration of 10 mM, and then cells were collected by centrifugation, resuspended in lysis buffer (50 mM Tris-Cl [pH 7.4], 15 mM EGTA, 100 mM NaCl, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, protease inhibitors) containing glass beads, and vortexed at 4°C for 15 min. The supernatant was collected and clarified by centrifugation at 12,000 × g for 15 min. Protein concentration was assessed by Bio-Rad protein assay (Bio-Rad, Hercules, CA). Coimmunoprecipitation was performed as described previously (Elion, 1999). Briefly, 0.5 mg of total protein was incubated in coimmunoprecipitation buffer with anti-Sec21 polycolonal antibody at 1:250 titer for 90 min at 4°C. Prewashed protein A-agarose beads (Invitrogen, Carlsbad, CA) were added, and samples were incubated an additional 60 min at 4°C with rotation. After incubation, protein A-agarose beads were washed three times in 1 ml of coimmunoprecipitation buffer, resuspended in 40 μl of Laemmli buffer, and boiled for 5 min.
Carboxypeptidase Y (CPY) Assay
Cells were grown to mid-log phase at 30°C, collected by centrifugation, washed twice in medium lacking methionine, resuspended in medium lacking methionine at ∼1 × 106 cells/ml, and grown at 30°C for an additional 6 h. Due to the use of the MET3 promoter to control expression of glo3 genes, cells were subjected only to a 7-min “pulse” with Redivue ProMix [35S] Trans label (Amersham Biosciences, Piscataway, NJ), but without a “chase” with excess unlabeled methionine and cysteine, to prevent repression of the MET3 promoter. Samples were collected at 0, 30, and 60 min and subjected to immunoprecipitation of CPY as described previously (Poon et al., 1999). Additionally, cells were also treated for CPY analysis as described previously before being subjected to CPY immunoprecipitation (Poon et al., 1999).
Analysis of Kar2 Secretion
Cells were grown overnight in HC-Leu medium at 30°C, collected by centrifugation, washed twice with water, resuspended in 25 ml of HC-Leu-Met medium at ∼1 × 106 cells/ml, and incubated for 6 h at 30°C. Cells were then sedimented, 20 ml of the growth medium was collected, and secreted proteins were precipitated according to Wessel and Flugge (1984). The protein pellet was resuspended in 100 μl of 5× Laemmli buffer and analyzed by immunoblot with antibodies directed against Kar2.
In Vitro Vesicle Budding Assay
Golgi membranes were prepared and the Golgi budding assay was performed as described by Spang and Schekman (1998) with the following modifications. For the Golgi budding reactions, membranes were incubated with 0.1 mM GTP or guanosine 5′-O-(3-thio)triphosphate (GTPγS), coatomer (125 μg/ml), and Arf1 protein (25 μg/ml) at 30°C for 30 min in a total volume of 400 μl. After chilling on ice, samples were loaded on a Ficoll-sucrose gradient consisting of 0.3 ml of 60% (wt/vol) sucrose, 0.8 ml of 7.5%, 1 ml of 5, 4, and 3%, and 0.8 ml of 2% (wt/wt) Ficoll in 15% (wt/vol) sucrose in 20 mM HEPES, pH 6.8, 5 mM Mg(OAc)2, 150 mM KOAc (B88*). The vesicles were separated from the Golgi apparatus by centrifugation for 2 h at 35,000 rpm (SW50.1 rotor; Beckman Coulter, Fullerton, CA). Fractions (400 μl) were collected from the top. Fractions 4-6 of the gradients were pooled, mixed with an equal volume of 80% Nycodenz in B88*, and overlaid with 600 μl of 35, 25, 20, and 15% and 400 μl of 10% Nycodenz in B88*. The gradients were centrifuged for 16 h at 40,000 rpm (SW50.1 rotor; Beckman Coulter). Fractions (300 μl) were collected from the top, TCA precipitated, resolved on SDS-PAGE, and analyzed by immunoblot.
RESULTS
The ArfGAP Glo3 Interacts In Vivo with Coatomer Components
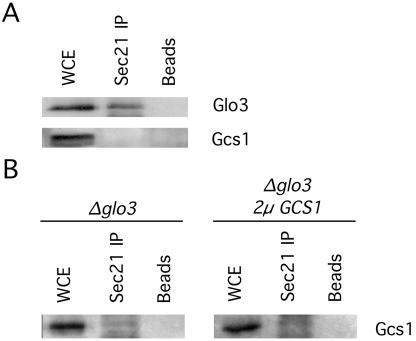
The ArfGAPs Gcs1 and Glo3 have been shown to provide essential overlapping function for retrograde vesicular transport (Poon et al., 1999). A previous study using the yeast two-hybrid system demonstrated direct protein-protein interactions between the ArfGAP Glo3 and the coatomer proteins Sec21 and Sec27, but it failed to identify any interaction between coatomer and the ArfGAP Gcs1 (Eugster et al., 2000). To evaluate the in vivo biological relevance of these ArfGAP-coatomer interactions, we immunoprecipitated the coatomer complex from a yeast protein extract by using anti-Sec21 antibodies and assessed the coprecipitation of Glo3 and Gcs1. As shown in Figure 1A, Glo3 was coprecipitated along with Sec21, whereas Gcs1 failed to coprecipitate with Sec21 from the same extract.
Figure 1.
The ArfGAP Glo3, but not Gcs1, coimmunoprecipitates with the coatomer complex. (A) Protein extracts from a wild-type yeast strain were incubated with anti-Sec21 antibody, followed by incubation with protein A-agarose beads. Beads were pelleted by centrifugation, washed, and resuspended in 1× Laemmli buffer. Samples were run on a 10% SDS-PAGE, transferred to polyvinylidene difluoride membrane, and analyzed with antibodies against Gcs1 and Glo3. (B) The coimmunoprecipitation experiment was repeated in strains lacking Glo3p. The samples were analyzed as in A with antibodies against Gcs1. WCE, whole cell extract.
The ArfGAP Gcs1 is able to provide function for retrograde transport in the complete absence of Glo3 (Poon et al., 1999), so it is possible that Gcs1 may only associate with coatomer in the absence of Glo3 protein. To test this hypothesis, we subjected a protein extract from glo3Δ mutant cells, which lack Glo3 protein, to immunoprecipitation with anti-Sec21 antibodies. We failed to coprecipitate Gcs1 along with Sec21 from these glo3Δ mutant cells (Figure 1B). Furthermore, even when we subjected a protein extract from glo3Δ mutant cells expressing increased levels of Gcs1 from a high-copy plasmid to immunoprecipitation with anti-Sec21 antibodies, we also failed to coprecipitate any Gcs1 along with Sec21 (Figure 1B). These results lead us to conclude that Glo3 physically associates with the coatomer complex in vivo but that Gcs1 does not display any physical association with coatomer.
The ArfGAP Glo3 Is Found on COPI-coated Vesicles Generated In Vitro
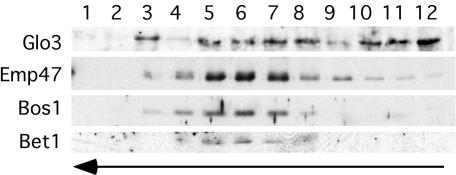
The identification of a Glo3-coatomer interaction in vivo raised the possibility that such an interaction may take place on Golgi membranes and/or on the COPI vesicles themselves. To determine whether Glo3 is incorporated into the coat of COPI vesicles through association with coatomer, Golgi membranes were isolated from wild-type yeast cells and used for an in vitro vesicle-budding reaction (Spang and Schekman, 1998). Enriched Golgi membranes were incubated with purified coatomer, Arf1, and GTP to stimulate COPI vesicle formation. The vesicles were then separated from the Golgi membrane by a velocity-sedimentation gradient, collected, and further purified by buoyant density centrifugation. The vesicle fraction was then subjected to Western blot analysis, revealing that Glo3 is indeed found on COPI vesicles generated in vitro from Golgi membranes (Figure 2). The identity of COPI vesicles was verified by the comigration in the gradient of the ER-Golgi shuttle v-SNAREs Bet1 and Bos1 and the COPI-vesicle cargo Emp47 (Figure 2, fractions 5-7). During the COPI vesicle enrichment procedure, part of the coat is shed due to GTP hydrolysis. Therefore, Glo3 is detected in the load fractions (fractions 10-12) of the buoyant density gradient. This also was observed when the gradient was analyzed for coatomer and Arf1 (unpublished data). We also detected Glo3 in one fraction that does not correlate with COPI vesicles (fraction 3), which may represent either a vesicle population other than COPI-coated vesicles or fragmented Golgi membranes. Nevertheless, we find that the ArfGAP Glo3 is a component of COPI-coated vesicles.
Figure 2.
The Glo3 ArfGAP is found on the coat of vesicles budded from Golgi membranes in vitro. Golgi membranes from wild-type cells were incubated with Arf1p, coatomer, and GTP to generate COPI vesicles. The vesicles were separated from the Golgi membranes by velocity sedimentation centrifugation. The vesicle peak was collected and floated on a buoyant density gradient. Fractions were collected, precipitated, and analyzed by immunoblot. Bet1 and Bos1 are v-SNAREs in the ER-Golgi shuttle and Emp47 is a vesicle cargo. The arrows indicate the movement of the lipid particles in the gradient.
Expression of a Mutant Glo3 Protein Impairs Growth
Because Glo3 is incorporated into the COPI vesicle coat, we assessed the effect of a mutant version of Glo3. We eliminated the ArfGAP activity in Glo3 by substitution of a lysine residue for the arginine residue at position 59 (Glo3-R59K). This arginine residue is invariant in the ArfGAP protein family and has previously been shown to be required for Gcs1 ArfGAP activity (Yanagisawa et al., 2002). Indeed, the R59K mutation causes a marked defect in Glo3 function as measured by an in vitro ArfGAP assay (Figure 3A).
Figure 3.
Expression of the mutant Glo3-R59K protein causes lethality. (A) Glo3-R59K-His6 was expressed in E. coli, purified using Ni2+/NTA-agarose, and used for an in vitro ArfGAP assay. The maximum activity observed corresponds to the hydrolysis of 82% of the total pool of radioactively labeled GTP-Arf per assay sample. Wild-type Glo3: (▪), Glo3-R59K: (•) (B) glo3Δ yeast cells harboring low-copy plasmid with the MET3pr-glo3-R59K gene were streaked for single colonies on medium containing methionine (Met+) to repress glo3-R59K expression or on medium lacking methionine (Met-) to induce glo3-R59K expression, and incubated at 30°C for 2 d. (C) Wild-type yeast cells harboring a low-copy plasmid with the MET3pr-glo3-R59K gene were streaked for single colonies on medium containing methionine (Met+) to repress glo3-R59K expression or on medium lacking methionine (Met-) to induce glo3-R59K expression, and incubated at 30°C for 2 days.
Because the Glo3-R59K protein does not exhibit measurable ArfGAP activity in vitro, we assessed the effect of this mutant Glo3 on cell growth. To accomplish this, we placed the glo3-R59K mutant allele under control of the inducible MET3 promoter (MET3pr-glo3-R59K) (Mao et al., 2002). Expression of this glo3-R59K gene is repressed in the presence of methionine, whereas in the absence of methionine glo3-R59K expression is induced. As assessed by Western blot analysis, the relative abundance of Glo3-R59K protein expressed from the MET3 promoter was similar to the level of Glo3 protein expressed from the endogenous GLO3 promoter (unpublished data). Therefore, the Glo3-R59K protein is expressed at a level that reflects the wild-type situation.
As shown in Figure 3B, the presence of an empty vector or a plasmid harboring the wild-type GLO3 gene under control of the MET3 promoter (MET3pr-GLO3) had little effect on the growth of cells lacking a chromosomal copy of the wild-type GLO3 gene. Likewise, expression of the glo3-R59K in wild-type cells (with an intact copy of the GLO3 gene) had no effect on cell growth and viability, even when glo3-R59K was expressed from a high-copy plasmid (Figure 3C; unpublished data). Therefore, the Glo3-R59K mutant protein does not exert a dominant negative effect over wild-type Glo3. In contrast, expression of the glo3-R59K mutant allele (MET3prglo3-R59K) was detrimental to the growth of cells lacking a chromosomal copy of the wild-type GLO3 gene (Figure 3B), indicating that Glo3-R59K impairs growth when this mutant version is the only Glo3 protein present in the cell. The growth defect exhibited by mutant cells expressing the glo3-R59K mutant gene may be due to the Glo3-R59K protein interfering with Gcs1-mediated retrograde vesicular transport.
Glo3-R59K Interacts with Coatomer In Vivo
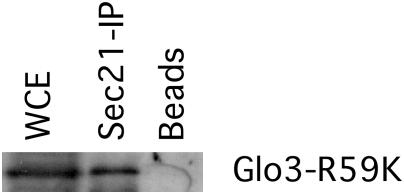
We have determined that the ArfGAP Glo3 interacts with the Sec21 component of the COPI coat and that this interaction leads to the incorporation of Glo3 into the COPI vesicle coat. Furthermore, we have found that a mutant version of the Glo3 protein asserts a negative effect on cell growth when it is the only version of Glo3 present in the cell. These observations raise the possibility that the lethality imposed by the Glo3-R59K mutant protein may be due to a loss of association with Sec21 and thus a failure to incorporate Glo3-R59K into the COPI vesicle. To test this hypothesis, we subjected a protein extract from glo3Δ mutant cells expressing glo3-R59K to immunoprecipitation with anti-Sec21 antibodies and assessed the coprecipitation of Glo3-R59K. We find that Glo3-R59K does indeed coprecipitate along with Sec21 (Figure 4), indicating that the mutation present in Glo3-R59K does not disrupt the association of Glo3-R59K with coatomer.
Figure 4.
Glo3-R59K coimmunoprecipitates with the coatomer complex. Protein extracts from glo3Δ mutant cells expressing Glo3-R59K were incubated with anti-Sec21 antibody, followed by incubation with protein A-agarose beads. Beads were pelleted by centrifugation, washed, and resuspended in 1× Laemmli buffer. Samples were run on a 10% SDS-PAGE and transferred to polyvinylidene difluoride membrane. The blot was decorated with Glo3 antibodies.
Glo3-R59K Impairs Vesicular Transport
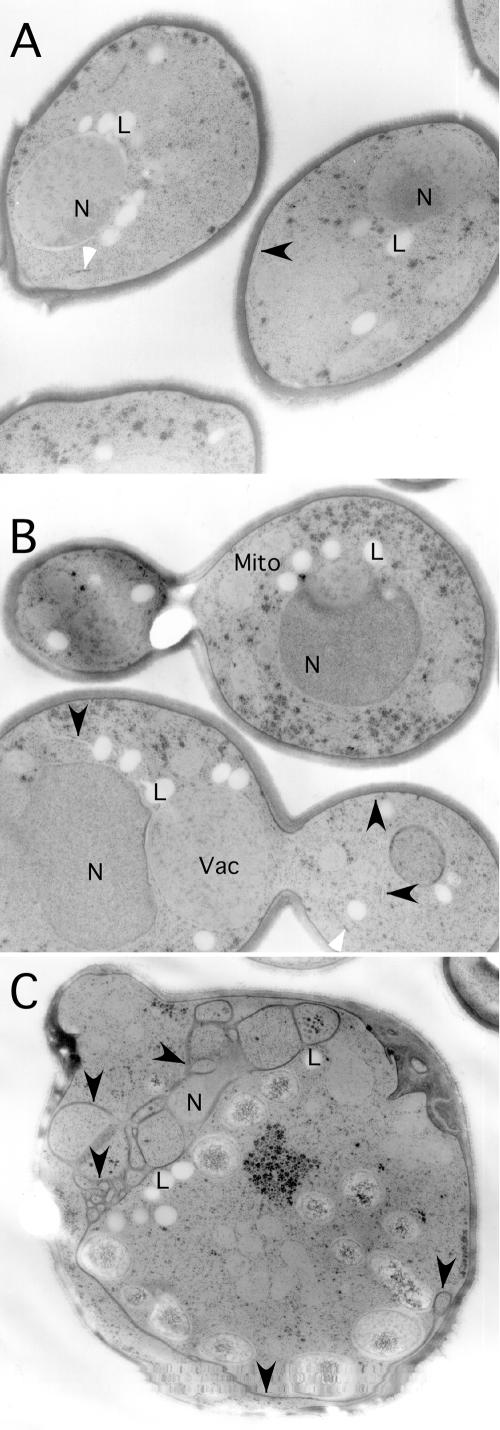
To investigate the effect of defective Glo3-R59K on vesicular transport in yeast cells, we first examined cells by electron microscopy. We have previously observed an accumulation of ER membranes in cells in which both Gcs1 and Glo3 ArfGAP function is impaired (Poon et al., 1999). Such ER accumulation is a hallmark of defective vesicular transport and is indicative of an indirect effect of perturbations in retrograde vesicular transport on anterograde vesicle traffic. As shown in Figure 5C, glo3Δ mutant cells expressing the glo3-R59K allele accumulated a significant amount of ER membranes. In contrast, glo3Δ mutant cells expressing wild-type GLO3, or glo3Δ mutant cells carrying an empty vector, did not show any accumulation of ER membrane (Figure 5, A and B). These data indicate that expression of the Glo3-R59K mutant protein may cause a block in retrograde vesicular transport, even in the presence of the other ArfGAP, Gcs1, capable of performing retrograde transport.
Figure 5.
Glo3-R59K causes accumulation of ER membrane. glo3Δ cells carrying the MET3pr-GLO3 plasmid (A), an “empty” vector plasmid (B), or the MET3pr-glo3-R59K plasmid (C) were shifted to medium lacking methionine and grown for 6 h at 30°C. Cells were collected and fixed for examination by electron microscopy. N, nucleus; Mito, mitochondria; and Vac, vacuole. The black arrow-heads point to peripheral ER in all three panels. The white arrow-heads indicate Golgi cisternae. Lipid bodies (L) are present in all strains due to growth in minimal media.
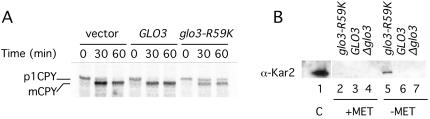
To further characterize the effects of mutant Glo3-R59K on vesicular transport, the ability of mutant cells to process CPY was assessed. The p1 precursor form of CPY is found in the ER; upon transport to the Golgi apparatus p1CPY is glycosylated to become the p2 form; finally, proteolytic processing of p2CPY in the vacuole leads to the production of the mature form, mCPY. Alterations in vesicular transport have been shown to lead to the accumulation of the precursor forms of CPY due to slowed CPY transport through the secretory pathway (Stevens et al., 1982). Cells lacking a chromosomal copy of the wild-type GLO3 gene that harbor an empty vector, a plasmid carrying the wild-type GLO3 gene, or a plasmid carrying the glo3-R59K mutant allele were exposed to radiolabeled methionine and cysteine, and samples were taken thereafter for immunoprecipitation of CPY (see Materials and Methods). glo3Δ mutant cells harboring a vector or a plasmid carrying the wild-type GLO3 gene processed p1CPY to mCPY within 30 min of exposure to radiolabel (Figure 6A, lanes 2 and 5). In contrast, cells expressing the glo3-R59K mutant gene were inefficient in converting the p1 precursor form of CPY to mCPY (Figure 6A). Even after 60 min, a significant amount of p1CPY was detectable (Figure 6A, lane 9). Similar results were seen when cells were subjected to a chase with excess unlabeled methionine and cysteine before CPY immunoprecipitation (unpublished data). Therefore, mutant Glo3-R59K impairs the transport of CPY through the secretory pathway, even in the presence of the ArfGAP Gcs1.
Figure 6.
Glo3-R59K causes defects in CPY maturation and secretion of the ER-resident protein Kar2. (A) Proliferating cells were exposed to [35S]methionine and cysteine for 7 min and sampled without a chase with unlabeled amino acids at 0, 30, and 60 min. CPY was immunoprecipitated, resolved by SDS-PAGE, and detected by autoradiography. This panel is representative of three independent experiments. (B) Expression of MET3pr-glo3-R59K, MET3pr-GLO3, or MET3pr-empty vector was induced by incubation in medium lacking methionine for 6 h at 30°C. The medium was collected, proteins were precipitated, and analyzed by immunoblot. Kar2 was secreted only by cells expressing the ArfGAP-dead Glo3-R59K.
Next, we tested for secretion of the ER-resident protein Kar2. Secretion of Kar2 is consistent with an impairment of retrograde transport from the Golgi to the ER because the failure to retrieve Kar2 back from the Golgi to the ER results in secretion of the Kar2 protein into the growth medium (Semenza et al., 1990). Cells lacking a chromosomal copy of the wild-type GLO3 gene harboring an empty vector, a plasmid carrying the wild-type GLO3 gene, or a plasmid carrying the glo3-R59K mutant allele were grown to mid-log phase and then shifted to medium lacking methionine and grown for an additional 6 h. The cells were then removed by centrifugation, and the growth medium was analyzed for the presence of Kar2. Whereas cells expressing the wild-type GLO3 gene or harboring the empty vector did not secrete Kar2 into the growth medium, cells expressing the glo3-R59K mutant allele secreted a significant amount of Kar2 (Figure 6B). These results provide further evidence that, even in the presence of the retrograde-transport ArfGAP Gcs1, Glo3-R59K impairs retrograde transport.
The data presented above demonstrate that when mutant Glo3-R59K is the only version of Glo3 present in the cell, retrograde vesicular transport is impaired. Furthermore, this impairment likely reflects the interference by Glo3-R59K with the ability of Gcs1 to mediate retrograde transport, because cells lacking any Glo3 are normally able to perform retrograde transport (albeit less efficiently) with only Gcs1 present.
Glo3-R59K Causes Mislocalization of the Retrograde Cargo Protein Emp47
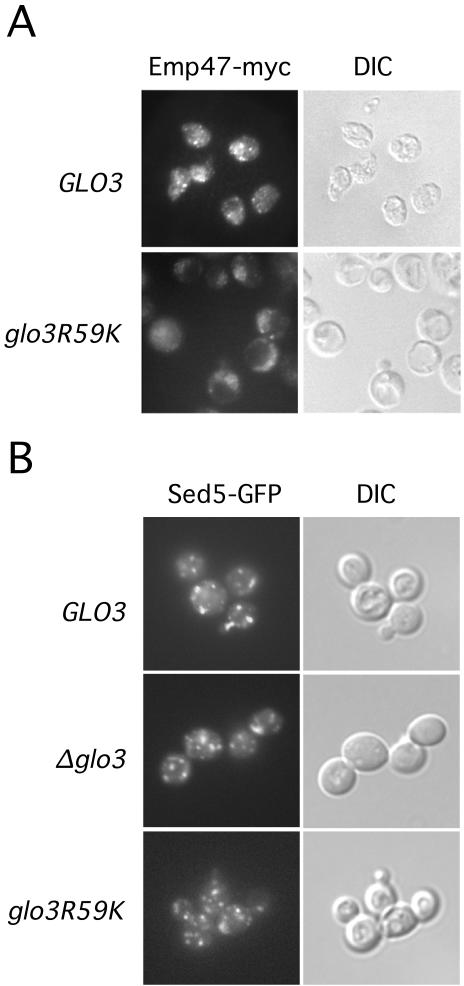
To assess the effect of defective Glo3 function on localization of a retrograde cargo protein, we replaced the endogenous EMP47 gene with a gene expressing Emp47 fused to the myc epitope. These cells also lacked a chromosomal copy of the wild-type GLO3 gene and harbored either the MET3pr-GLO3 or the MET3pr-glo3-R59K plasmid. Cells were transferred to methionine-free medium to induce expression of either wild-type or mutant Glo3 and were incubated for an additional 6 h, and then fixed and processed for light microscope visualization of Emp47 protein. As shown in Figure 7, cells expressing wild-type Glo3 protein exhibited the expected punctate distribution of Emp47, consistent with localization in the Golgi compartment. Cells expressing the mutant Glo3-R59K displayed a dramatically deranged localization for Emp47, which did not seem to be transported to the vacuole for degradation. In addition, cells expressing the mutant Glo3-R59K protein were enlarged compared with wild-type cells and exhibited prominent vacuoles (Figure 7A). Thus, defective Glo3 function causes the retrograde protein Emp47 to mislocalize. This mislocalization is consistent with the finding that in coatomer-retention mutants, Emp47 is lost from the Golgi apparatus and transported to the vacuole (Schroder-Kohne et al., 1998). In Glo3-R59K cells, Emp47 does not reach the vacuole, which might be due to indirect effects on post-Golgi sorting.
Figure 7.
Retrograde cargo localization requires intact Glo3 function. (A) Cells harboring the MET3pr-GLO3 or MET3pr-glo3-R59K genes were transferred to medium lacking methionine to induce gene expression. After 6 h, cells were processed for fluorescence microscopy as described previously. The retrograde cargo protein Emp47-myc was detected by Alexa 488 fluorescence (left). Cell morphology (differential interference contrast) is displayed in panels on the right. (B) Sed5-GFP is localized to the Golgi. glo3Δ cells carrying the MET3pr-GLO3 plasmid, an empty vector plasmid or the MET3pr-glo3-R59K plasmid and plasmid expressing Sed5-GFP were shifted to medium lacking methionine and grown for 6 h at 30°C. Cells were immediately examined for Sed5-GFP localization.
The diffuse staining pattern of Emp47 in cells expressing the mutant Glo3 made us wonder whether Golgi integrity is compromised; thus, we examined a Golgi-resident protein, the cis-Golgi t-SNARE Sed5, as one measure of Golgi integrity. Cells lacking a chromosomal copy of the wild-type GLO3 gene and harboring an empty vector, a plasmid carrying the wild-type GLO3 gene, or a plasmid carrying the glo3-R59K mutant allele were transformed with a Sed5-GFP construct. We grew the resulting cells to early-log phase, and then the cells were shifted to medium lacking methionine, grown for an additional 6 h, and immediately inspected for the localization of Sed5-GFP (Figure 7B). Sed5-GFP localization in glo3Δ and GLO3 cells was indistinguishable from that in cells containing the Glo3-R59K mutant version of the ArfGAP Glo3. This finding is consistent with our results from the electron microscopy, where we did observe normal Golgi morphology (unpublished data). Thus, the glo3-R59K mutant affects retrograde transport from the Golgi to the ER, but it does not seem to dramatically affect Golgi morphology or composition.
Increased Abundance of the ArfGAP Gcs1 Overcomes the Lethality Caused by Glo3-R59K
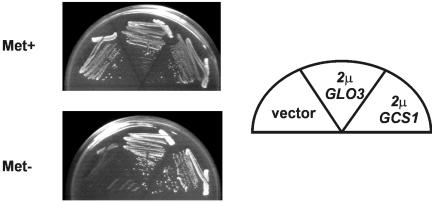
Because the mutant Glo3-R59K protein interferes with the ability of Gcs1 to mediate retrograde vesicular transport, we assessed the effect of increased abundance of Gcs1 on the lethality imposed by glo3-R59K expression. Cells lacking a chromosomal copy of the wild-type GLO3 gene and harboring the MET3pr-glo3-R59K plasmid were transformed with a high-copy GLO3 plasmid, a high-copy GCS1 plasmid, and an empty vector. We found that cells induced to express the mutant Glo3-R59K protein were able to grow if these cells also had increased abundance of either wild-type Glo3 or Gcs1. Cells with only the mutant Glo3-R59K protein failed to grow (Figure 8). These results demonstrate that increasing the abundance of Gcs1 overcomes the lethality caused by glo3-R59K expression, suggesting that increased Gcs1 abundance likely allows retrograde transport to proceed in the presence of Glo3-R59K by competing more effectively for necessary components of vesicular transport.
Figure 8.
Increased abundance of the Gcs1 ArfGAP rescues Glo3-R59K lethality. glo3Δ yeast cells harboring a low-copy plasmid with the MET3pr-glo3-R59K gene were transformed with either an empty high-copy plasmid, a high-copy plasmid carrying the GLO3 gene, or a high-copy plasmid carrying the GCS1 gene. Cells were streaked for single colonies on either Met+ or Met- media and grown at 30°C for 2 days.
Functional Glo3 Is Required for the Generation of COPI Vesicles In Vitro
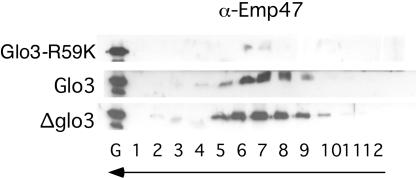
Mutant Glo3-R59K protein may impair retrograde vesicular transport either by affecting COPI-vesicle uncoating or by interfering with the generation of COPI-coated vesicles. To determine whether the loss of function imposed by Glo3-R59K prevents COPI-coated vesicle generation, we assessed the ability of Glo3-R59K to mediate the production of COPI vesicles by using the in vitro vesicle-budding reaction (Spang and Schekman, 1998). Actively proliferating cells lacking a chromosomal copy of the wild-type GLO3 gene and harboring the MET3pr-glo3-R59K plasmid were transferred to medium lacking methionine to induce expression of the mutant Glo3 protein. After a 6-h incubation, cells were harvested and Golgi membranes were isolated. Vesicles were generated from the enriched Golgi membranes, and COPI vesicles were separated from the Golgi by velocity sedimentation and further purified by buoyant density flotation. As expected, Golgi membranes isolated from cells carrying an empty vector or a MET3pr-GLO3 plasmid were able to form COPI-coated vesicles in the presence of GTP in vitro as judged by the presence of the cargo Emp47 (Figure 9). The SNARE proteins were also present in the vesicle fractions (unpublished data). However, Golgi membranes isolated from cells carrying the MET3pr-glo3-R59K plasmid failed to produce COPI vesicles in the presence of GTP (Figure 9). This ability to generate COPI vesicles from Glo3-R59K Golgi was restored by the addition of exogenous Glo3. These data demonstrate that intact Glo3 function is required for the generation of COPI vesicles in vitro and that this function is independent of the stimulation of the GTPase activity of Arf1, because in the presence of the nonhydrolyzable analog, GTPγS, COPI-coated vesicles were formed from wild-type Golgi membranes (Spang and Schekman, 1998; Todorow et al., 2000; Spang et al., 2001).
Figure 9.
Glo3-R59K prevents the generation of COPI vesicles in vitro. COPI vesicles were generated from Golgi membranes isolated from yeast cells carrying the MET3pr-glo3-R59K, MET3pr-GLO3, and MET3pr-empty plasmids, which were incubated in the presence of GTP and COPI components. The vesicles were purified over a velocity gradient and subsequently floated on a Nycodenz gradient. Fractions were collected from the top, separated by SDS-PAGE, and analyzed by immunoblot. The arrows indicate the direction of movement of lipid particles within the gradient.
DISCUSSION
We have investigated the role of Glo3 ArfGAP function in S. cerevisiae for COPI-mediated retrograde vesicular transport. We have found that the protein-protein interaction between Glo3 and Sec21, a component of the COPI vesicle coat, previously identified by yeast two-hybrid analysis (Eugster et al., 2000), also occurs in vivo and that Glo3 is a component of COPI vesicles. The replacement of wild-type Glo3 with a mutant Glo3 protein that lacks measurable ArfGAP activity (Glo3-R59K) impairs cell growth, even though the mutant Glo3-R59K protein can still interact with the Sec21 component of coatomer. The inhibition caused by mutant Glo3-R59K is due to a block in retrograde vesicular transport. Furthermore, Glo3-R59K impairs vesicular transport by interfering with the ability of Gcs1 ArfGAP to mediate retrograde transport, because increased abundance of Gcs1 can overcome the lethality caused by glo3-R59K expression. Finally, we demonstrate that the mutant Glo3-R59K protein prevents the generation of COPI vesicles as determined by an in vitro vesicle budding assay, indicating that intact Glo3 (or unimpeded Gcs1) is required for the generation of COPI vesicles.
Both the Gcs1 and Glo3 ArfGAPs can facilitate retrograde vesicular transport (Poon et al., 1999). In contrast to what we found for Glo3, however, we were unable to detect Gcs1 in association with coatomer components, suggesting that Gcs1 is not part of a COPI coat complex. This finding therefore suggests that Gcs1 and Glo3 proteins normally play distinct roles for vesicular transport but that in the absence of one of these ArfGAPs, the other member of this protein pair is able to provide enough ArfGAP function to maintain viability and vesicular transport activity. However, Gcs1 is capable of providing only a minor contribution to the retrograde transport process: glo3Δ cells with only Gcs1 for retrograde transport are impaired for growth, whereas cells lacking Gcs1 protein but with intact Glo3 are proficient for retrograde functions (Poon et al., 1999). Similarly, Dogic et al. (1999) found that glo3Δ mutant cells are defective for cargo retrieval from the Golgi to the ER, whereas deletion of any of the other five genes encoding ArfGAP proteins (including Gcs1) does not result in any defect in Golgi-to-ER cargo retrieval. These findings suggest that Gcs1 performs Glo3-related activities poorly. Our finding that the Glo3-R59K mutant protein exerts a negative effect on retrograde vesicular transport even in the presence of Gcs1 indicates that Gcs1 may be able to supply effective Glo3-like function only in the complete absence of Glo3 protein. The inhibitory effect of the Glo3-R59K mutant protein on cell growth and retrograde transport likely reflects interactions between Glo3-R59K and components of the vesicular-transport machinery, which in turn lead to the formation of inactive complexes that effectively exclude Gcs1 and prevent the provision of ArfGAP function. However, an increased abundance of the Gcs1 protein overcomes the Glo3-R59K inhibition of growth, which suggests that the associations formed through Glo3-R59K interactions are reversible. This view also accommodates our observations that the presence of wild-type Glo3 protein eliminates the inhibitory effects of Glo3-R59K mutant protein. In any case, these observations indicating that Glo3 and Gcs1 have distinct roles in vesicular transport lead us to suggest that Glo3 is predominantly involved in retrograde transport, whereas the Gcs1 may normally play only a minor role, if any.
The classical model for COPI-mediated vesicular transport proposed by Tanigawa et al. (1993) suggests that hydrolysis of Arf-bound GTP causes the dissociation of Arf and coatomer from the vesicle membrane, and therefore GTP hydrolysis may only be required for the uncoating of COPI vesicles. ArfGAPs thus have been thought to function primarily to induce shedding of the vesicle coat by stimulating GTP hydrolysis by Arf. Nevertheless, several studies have implied that ArfGAP proteins may provide additional activities for vesicular transport. Mounting evidence indicates that ArfGAPs may be required for the proper selection and packaging of cargo proteins and SNAREs into nascent COPI vesicles (Nickel et al., 1998; Goldberg, 2000; Pepperkok et al., 2000; Lanoix et al., 2001; Rein et al., 2002). Indeed, ArfGAP proteins have been hypothesized to facilitate cargo packaging through the action of a “discard pathway” in which vesicle generation is aborted via ArfGAP function if improper cargo is packaged (Goldberg, 2000). These observations implicate ArfGAPs in transport vesicle production.
Two reports have addressed a role for ArfGAP proteins in the generation of COPI vesicles. Yang et al. (2002) suggested that the mammalian ARFGAP1 is a constituent of COPI vesicles and that GTP hydrolysis (and thus ArfGAP activity) is necessary for COPI vesicle generation. Reinhard et al. (2003) found no such requirement for ArfGAP activity during the generation of COPI vesicles in an in vitro reconstitution system, but instead they found that ArfGAP activity is necessary and sufficient for the uncoating of COPI vesicles. Therefore, there is conflicting evidence concerning a role for ArfGAP function in COPI-mediated vesicular transport.
In light of our findings that the Glo3 protein is a component of the COPI vesicle coat, that the mutant Glo3-R59K protein has a negative effect on retrograde vesicular transport, and that Glo3-R59K prevents the generation of COPI vesicles in vitro, we propose that functional Glo3 is indeed required for the generation of COPI-coated vesicles. Springer et al. (1999) hypothesized that Arf-mediated GTP hydrolysis early in vesicle biogenesis releases Arf protein for subsequent rounds of coatomer recruitment. This hypothesis is known as the “priming complex” model for COPI-mediated vesicular transport. In support of this hypothesis, experiments have shown that coatomer remains on membranes long after Arf-bound GTP hydrolysis has taken place (Presley et al., 2002). These findings therefore support a role for ArfGAP activity in COPI vesicle generation. Our finding that a mutant Glo3-R59K protein without ArfGAP function prevents the generation of COPI-coated vesicles in vitro lends further weight to the priming complex model.
Our in vitro vesicle budding assay demonstrates that vesicles can form even in the presence of GTPγS, a nonhydrolyzable analogue of GTP (unpublished data; Spang and Schekman, 1998; Todorow et al., 2000; Spang et al., 2001). Thus, GTPase activity (and therefore ArfGAP activity) is not necessary for the generation of transport vesicles in vitro. Most likely, the components necessary for vesicle generation, including GTP-Arf, are in excess in our in vitro assay so that, unlike the in vivo situation, there is no need to provide ArfGAP activity for the release Arf proteins through GTP hydrolysis. Nevertheless, we also find that the presence of the mutant Glo3 protein Glo3-R59K severely inhibits vesicle production in vitro. Thus, other functions of Glo3 in addition to the stimulation of GTPase activity may be required to generate transport vesicles. We suggest that the Glo3 protein may provide at least two functions for effective vesicular transport through interactions with coat proteins and cargo and through stimulation of the GTPase activity of Arf. Although we find that the mutant Glo3-R59K protein retains the ability to interact with coat components, a productive interaction between Glo3-R59K and cargo may well be disrupted. Therefore, it is conceivable that without effective interaction of ArfGAP with some component (such as cargo) no priming complex can be stabilized at the membrane to allow successful polymerization of coat components. Thus, ArfGAP may be required for both the generation and the removal of the COPI coat on vesicles in vivo.
Our findings support a role for ArfGAP function in the generation of COPI vesicles. However, many questions remain unanswered, such as which proteins regulate ArfGAP function, and exactly how this ArfGAP function is involved in the early and late stages of the vesicular transport process. The identification of ArfGAP-SNARE interactions involved in the recruitment of Arf and coatomer to membranes may represent a mechanistic explanation for the requirement for ArfGAP function during vesicle generation (Rein et al., 2002). The requirement for intact ArfGAP function in the SNARE-mediated recruitment of Arf and coatomer to the membrane awaits further investigation.
Acknowledgments
We thank Kendra Gillis for expert technical assistance, Gary Faulkner and Heinz Schwarz for electron microscopy, and Steven Whitefield for fluorescence microscopy. Jeffrey Gerst is acknowledged for the gift of the Sed5-GFP plasmid. This research was supported by funds from the Canadian Cancer Society through a grant from the National Cancer Institute of Canada (to G.C.J. and R.A.S.) and the Max Planck Society (to A.S.). A.S. is a European Molecular Biology Organization Young Investigator.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-04-0316. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-04-0316.
References
- Archambault, J., M.A. Drebot, J.C. Stone, and J.D. Friesen. (1992). Isolation and phenotypic analysis of conditional-lethal, linker-insertion mutations in the gene encoding the largest subunit of RNA polymerase II in Saccharomyces cerevisiae. Mol. Gen. Genet. 232, 408-414. [DOI] [PubMed] [Google Scholar]
- Cukierman, E., Huber, I., Rotman, M., and Cassel, D. (1995). The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex localization. Science 270, 1999-2002. [DOI] [PubMed] [Google Scholar]
- Dogic, D., de Chassey, B., Pick, E., Cassel, D., Lefkir, Y., Hennecke, S., Cosson, P., and Letourneur, F. (1999). The ADP-ribosylation factor GTPase-activating protein Glo3p is involved in ER retrieval. Eur J. Cell Biol. 78, 305-310. [DOI] [PubMed] [Google Scholar]
- Elion, E.A. (1999). Detection of protein-protein interactions by coprecipitation. Curr. Protoc. Mol. Biol. 20.5.1.-20.5.9. [DOI] [PubMed]
- Eugster, A., Frigerio, G., Dale, M., and Duden, R. (2000). COP I domains required for coatomer integrity, and novel interactions with ARF and ARFGAP. EMBO J. 19, 3905-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg, J. (2000). Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell 100, 671-679. [DOI] [PubMed] [Google Scholar]
- Gommel, D.U., Memon, A.R., Heiss, A., Lottspeich, F., Pfannstiel, J., Lechner, J., Reinhard, C., Helms, J.B., Nickel, W., and Wieland, F.T. (2001). Recruitment to Golgi membranes of ADP-ribosylation factor 1 is mediated by the cytoplasmic domain of p23. EMBO J. 20, 6751-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms, J.B., Palmer, D.J., and Rothman, J.E. (1993). Two distinct populations of ARF bound to Golgi membranes. J. Cell Biol. 121, 751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen, T. (2000). Three ways to make a vesicle. Nat. Rev. Mol. Cell Biol. 1, 187-198. [DOI] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Stark, A., Szafer, E., Cassel, D., Dejgaard, K., Weiss, M., and Nilsson, T. (2001). Sorting of Golgi resident proteins into different subpopulations of COPI vesicles: a role for ArfGAP1. J. Cell Biol. 155, 1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, X., Hu, Y., Liang, C., and Lu, C. (2002). MET3 promoter: a tightly regulated promoter and its application in construction of conditional lethal strain. Curr. Microbiol. 45, 37-40. [DOI] [PubMed] [Google Scholar]
- Nickel, W., Malsam, J., Gorgas, K., Ravazzola, M., Jenne, N., Helms, J.B., and Wieland, F.T. (1998). Uptake by COPI-coated vesicles of both anterograde and retrograde cargo is inhibited by GTPgammaS in vitro. J. Cell Sci. 111, 3081-3090. [DOI] [PubMed] [Google Scholar]
- Pepperkok, R., Whitney, J.A., Gomez, M., and Kreis, T.E. (2000). COPI vesicles accumulating in the presence of a GTP restricted arf1 mutant are depleted of anterograde and retrograde cargo. J. Cell Sci. 113, 135-144. [DOI] [PubMed] [Google Scholar]
- Peyroche, A., Paris, S., and Jackson, C.L. (1996). Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature 384, 479-481. [DOI] [PubMed] [Google Scholar]
- Poon, P.P., Cassel, D., Spang, A., Rotman, M., Pick, E., Singer, R.A., and Johnston, G.C. (1999). Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 18, 555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, P.P., Nothwehr, S.F., Singer, R.A., and Johnston, G.C. (2001). The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network. J. Cell Biol. 155, 1239-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, P.P., Wang, X., Rotman, M., Huber, I., Cukierman, E., Cassel, D., Singer, R.A., and Johnston, G.C. (1996). Saccharomyces cerevisiae Gcs1 is an ADP-ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA. 93, 10074-10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley, J.F., Ward, T.H., Pfeifer, A.C., Siggia, E.D., Phair, R.D., and Lippincott-Schwartz, J. (2002). Dissection of COPI and Arf1 dynamics in vivo and role in Golgi membrane transport. Nature 417, 187-193. [DOI] [PubMed] [Google Scholar]
- Rein, U., Andag, U., Duden, R., Schmitt, H.D., and Spang, A. (2002). ARFGAP-mediated interaction between the ER-Golgi v-SNAREs and the COPI coat. J. Cell Biol. 157, 395-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard, C., Schweikert, M., Wieland, F.T., and Nickel, W. (2003). Functional reconstitution of COPI coat assembly and disassembly using chemically defined components. Proc. Natl. Acad. Sci. USA 100, 8253-8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann, T., Herrmann, J.M., Dengjel, J., Schwarz, H., and Spang, A. (2003). Suppression of coatomer mutants by a new protein family with COPI and COPII binding motifs in Saccharomyces cerevisiae. Mol. Biol. Cell 14, 3097-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder-Kohne, S., Letourneur, F., and Riezman, H. (1998). alpha-COP can discriminate between distinct, functional di-lysine signals in vitro and regulates access into retrograde transport. J. Cell Sci. 111, 3459-3470. [DOI] [PubMed] [Google Scholar]
- Semenza, J.C., Hardwick, K.G., Dean, N., and Pelham, H.R. (1990). ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61, 1349-1357. [DOI] [PubMed] [Google Scholar]
- Serafini, T., Orci, L., Amherdt, M., Brunner, M., Kahn, R.A., and Rothman, J.E. (1991). ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell 67, 239-253. [DOI] [PubMed] [Google Scholar]
- Spang, A., Herrmann, J.M., Hamamoto, S., and Schekman, R. (2001). The ADP ribosylation factor-nucleotide exchange factors Gea1p and Gea2p have overlapping, but not redundant functions in retrograde transport from the Golgi to the endoplasmic reticulum. Mol. Biol. Cell. 12, 1035-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang, A., and Schekman, R. (1998). Reconstitution of retrograde transport from the Golgi to the ER in vitro. J. Cell Biol. 143, 589-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, S., Spang, A., and Schekman, R. (1999). A primer on vesicle budding. Cell 97, 145-148. [DOI] [PubMed] [Google Scholar]
- Stevens, T., Esmon, B., and Schekman, R. (1982). Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell 30, 439-448. [DOI] [PubMed] [Google Scholar]
- Tanigawa, G., Orci, L., Amherdt, M., Ravazzola, M., Helms, J.B., and Rothman, J.E. (1993). Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J. Cell Biol. 123, 1365-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorow, Z., Spang, A., Carmack, E., Yates, J., and Schekman, R. (2000). Active recycling of yeast Golgi mannosyltransferase complexes through the endoplasmic reticulum [In Process Citation]. Proc. Natl. Acad. Sci. USA 97, 13643-13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel, D., and Flugge, U.I. (1984). A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141-143. [DOI] [PubMed] [Google Scholar]
- Yanagisawa, L.L., Marchena, J., Xie, Z., Li, X., Poon, P.P., Singer, R.A., Johnston, G.C., Randazzo, P.A., and Bankaitis, V.A. (2002). Activity of specific lipid-regulated ADP ribosylation factor-GTPase-activating proteins is required for Sec14p-dependent Golgi secretory function in yeast. Mol. Biol. Cell 13, 2193-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J.S., Lee, S.Y., Gao, M., Bourgoin, S., Randazzo, P.A., Premont, R.T., and Hsu, V.W. (2002). ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J. Cell Biol. 159, 69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]