Abstract
Background
To examine the effects of type 2 diabetes mellitus (DM) on the variables associated with prostatic growth including serum prostate-specific antigen (PSA), serum testosterone, and prostate volume, and to correlate these variables with the duration of diabetes treatment.
Methods
Our study was conducted over 3 months recruiting 501 men aged ≥ 55 years; of whom 207 had type 2 DM. Exclusion criteria were active urinary tract infection, suspicious rectal examination, urologic cancer, end-organ damage, and recent urological manipulations. Serum PSA and serum testosterone were measured. Prostate volume was determined by abdominal ultrasonography using an ellipsoid formula.
Results
The mean patient age was 60.21 ± 5.95 years. The mean PSA, testosterone, and prostate volume for diabetic men were 2.3 ng/mL, 3 ng/mL, and 56 g, respectively. The corresponding values for nondiabetic men were 3.5 ng/mL, 4 ng/mL, and 51 g, respectively (P = 0.001, P = 0.001, P = 0.03, respectively). The mean PSA density was 0.049 ± 0.043 ng/mL/cm3 in diabetics versus 0.080 ± 0.056 ng/mL/cm3 in non-diabetics (P < 0.001).
Conclusion
Type 2 DM is significantly associated with lower serum PSA and testosterone, and larger prostate volume.
Keywords: Diabetes mellitus, Prostate, Prostate-specific antigen, Prostate volume, Testosterone
1. Introduction
Diabetes mellitus (DM) is a serious problem in male health. A positive association exists between clinical markers of benign prostatic hyperplasia (BPH) and DM.1, 2 Subnormal serum free testosterone is detected in diabetic men.3 Kasper et al suggested an inverse correlation between DM and the risk of prostate cancer.4
The aim of this work was to determine the effects of type 2 DM on serum total prostate-specific antigen (PSA), serum total testosterone, and prostate volume.
2. Materials and methods
The study was prospectively conducted over 3 months and recruited male patients aged ≥ 55 years who presented to our hospital with different benign urological conditions. The study included 501 men and 207 of them had type 2 DM. Exclusion criteria were patients with active urinary tract infection, urological cancer, end-organ damage, abnormal digital rectal examination findings, and recent urological manipulations.
This study was approved by the ethical committee of our institution and informed consent was obtained from participating patients.
All men were subjected to detailed history taking and physical examination. Body mass index was calculated. Six milliliters of venous blood were drawn at 8:00 am, then serum was separated and stored at −20°C. Serum PSA and testosterone were assessed using electro-chemiluminescence immunoassay. Prostate size was calculated using abdominal ultrasonography. The ellipsoid formula was applied.
Data were analyzed using SPSS version 18.0 (SPSS, IBM Corporation, Chicago, IL, USA). The P value was assumed to be significant at ≤ 0.05.
3. Results
The mean age of patients was 60.21 ± 5.95 years (55.0–93.0 years). For diabetic patients, the mean PSA, testosterone, and prostate volume were 2.3 ng/mL, 3 ng/mL, and 56 g, respectively. The corresponding values for nondiabetic individuals were 3.5 ng/mL, 4 ng/mL and 51 g, respectively. The mean body mass index (BMI) was 32.23 ± 5.04 and 29.32 ± 4.20 for diabetic patients and nondiabetic individuals, respectively (Table 1).
Table 1.
Comparison between the two studied groups according to PSA, testosterone, prostate volume, PSA density, and BMI.
| Variable | Diabetics (207) | Nondiabetics (294) | P value | |
|---|---|---|---|---|
| PSA | 2.3 ± 1.5 | 3.5 ± 1.9 | 0.001 | |
| PSA | < 4 | 179 (86.5%) | 193 (65.6%) | < 0.0001 |
| > 4 | 28 (13.5) | 101 (34.4%) | ||
| Testosterone | 3 ± 1.8 | 4 ± 2.1 | 0.001 | |
| Prostate volume | 56 ± 18 | 51 ± 23 | 0.03 | |
| PSA density | 0.05 ± 0.04 | 0.08 ± 0.05 | 0.001 | |
| BMI | 32.23 ± 5.04 | 29.32 ± 4.20 | 0.001 | |
BMI, body mass index; PSA, prostate-specific antigen.
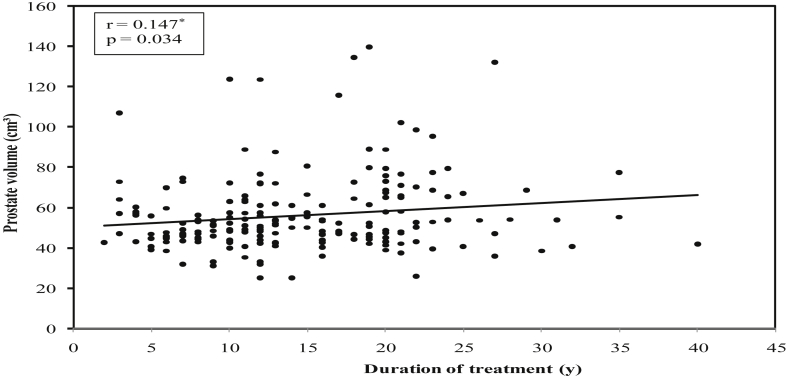
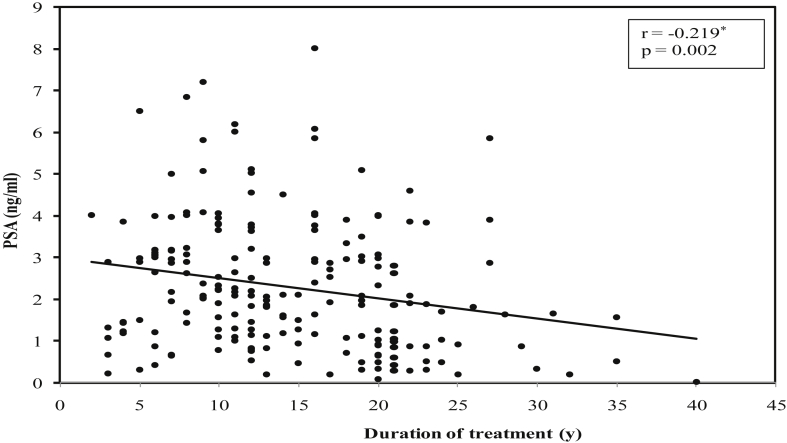
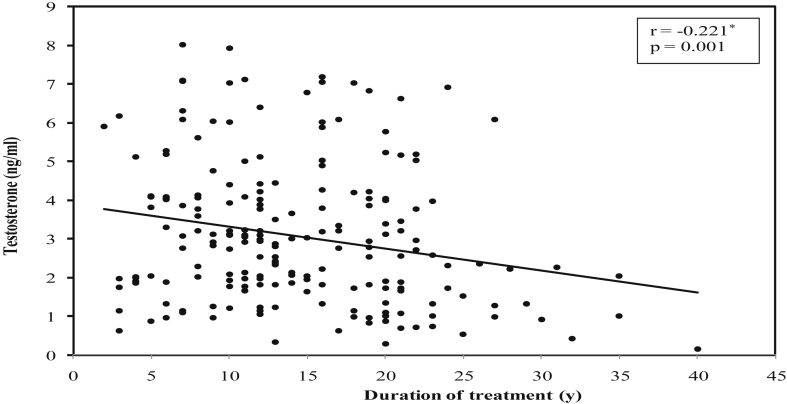
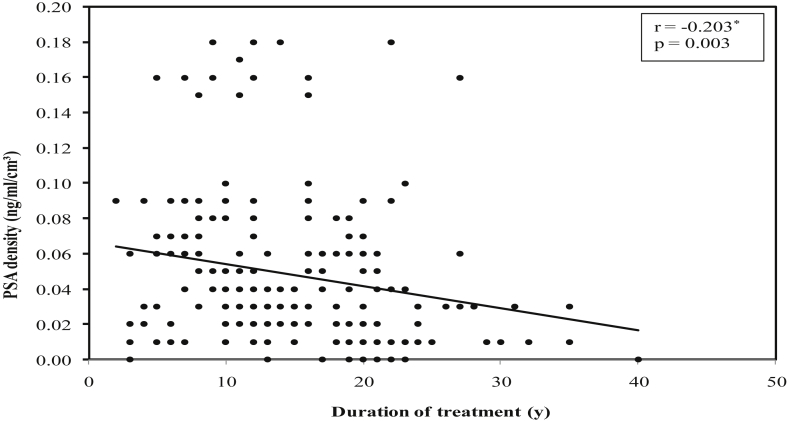
There was a significant positive correlation between duration of treatment of DM and mean prostate volume (r = 0.147, P = 0.034) (Fig. 1), while significant negative correlations were found between duration of DM treatment and mean serum PSA values (r = −0.219, P = 0.002) (Fig. 2), mean serum testosterone values (r = −0.221, P = 0.001) (Fig. 3), and mean PSA density values (r = −0.203, P = 0.003) (Fig. 4).
Fig. 1.
Correlation between duration of treatment of diabetes mellitus with prostate volume in diabetic group.
Fig. 2.
Correlation between duration of treatment of diabetes mellitus with PSA in diabetic group. PSA, prostate-specific antigen.
Fig. 3.
Correlation between duration of treatment of diabetes mellitus with testosterone in diabetic group.
Fig. 4.
Correlation between duration of treatment of diabetes mellitus with PSA density in diabetic group. PSA, prostate-specific antigen.
High BMI in diabetic patients was a confounding factor, therefore, multiple regression analysis was done, confirming the true significant correlation of DM with the studied parameters (Table 2).
Table 2.
Multiple regression analysis for PSA, testosterone, prostate volume and PSA density, in relation to DM, studying BMI as a confounding factor
| B | SE | t | p | 95% CI |
|||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| PSA | DM | 0.743 | 0.155 | 4.795∗ | < 0.001 | 0.439 | 1.048 |
| BMI | −0.166 | 0.016 | 10.359∗ | < 0.001 | −0.197 | −0.134 | |
| F = 87.977*, P < 0.001*, R = 0.511, R2 = 0.261 | |||||||
| Testosterone | DM | 0.400 | 0.165 | 2.420* | 0.016 | 0.075 | 0.724 |
| BMI | −0.210 | 0.017 | 12.305* | < 0.001 | −0.243 | −0.176 | |
| F = 96.237*, P < 0.001*, R = 0.528, R2 = 0.279 | |||||||
| Prostate volume | DM | 4.629 | 1.633 | 2.835* | 0.005 | 1.420 | 7.837 |
| BMI | 3.011 | 0.168 | 17.883* | < 0.001 | 2.680 | 3.341 | |
| F = 136.430*, P < 0.001*, R = 0.629, R2 = 0.396 | |||||||
| PSA density | DM | 0.020 | 0.004 | 4.853* | < 0.001 | 0.012 | 0.028 |
| BMI | −0.006 | 0.000 | 14.784* | < 0.001 | −0.007 | −0.005 | |
| F = 156.697*, P < 0.001*, R = 0.621, R2 = 0.386 | |||||||
BMI, body mass index; CI, confidence interval; PSA, prostate-specific antigen; SE, standard error.
4. Discussion
The overall mean PSA in the present study was 3.02 ± 1.89 ng/mL. Comparable levels were observed among Middle Eastern men showing higher PSA with no evidence of prostate cancer. This was attributed to increased incidence of BPH, alone or with concomitant prostatitis.5
Our study showed that type 2 DM is associated with a significantly lower serum total PSA, than in nondiabetic individuals; a finding that has been shown in other studies. This may be attributed to lower androgen levels found in diabetic men.3 Muller et al6 reported that men with elevated and highly elevated hemoglobin A1c levels had 15% and 29% lower serum PSA levels, respectively. Our results also suggested that men with type 2 DM have slower increases in serum PSA levels over time. Similarly, Parekh et al7 reported lower prostate-cancer risk and serum PSA levels in later stages of diabetes, which are characterized by insulin resistance and lower levels of circulating insulin. Moreover, diabetes might alter PSA values through impaired kidney function.8 Low serum PSA in diabetic men may also be explained, in our cohort, by the significantly lower serum testosterone level in men with type 2 DM, confirming the direct role of hypogonadism in PSA values reported by many authors.3, 9, 10 Accordingly, an endocrine society currently recommends the measurement of testosterone in patients with type 2 DM on a routine basis.11
Large prostate volume has been associated with components of metabolic syndrome.12, 13 This was consistent with our study, in which a positive correlation between the duration of diabetes treatment and prostate size was observed. In fact, both type 2 DM and BPH seem to share similar epidemiological features, possibly related to aging and diet.14 Barnard et al15 connected the reduction of growth of stem epithelial prostate cells with the reduction of insulin. Other possible mechanisms have been proposed to associate the development of BPH with type 2 DM, such as the increase in peripheral sympathetic nerve tone and activity of the autonomic nervous system caused by hyperinsulinemia,16 and hypoxia caused by DM-induced vascular damage.17
We have previously shown that high BMI is associated with the same observed changes in prostate-related parameters.18 However, in the present study, multiple regression analysis confirmed that type 2 DM was significantly associated with a change in prostatic parameters, independent from the effect of BMI.
We believe the importance of this study lies in studying the effect of type 2 DM on the main parameters related to prostatic growth, and being novel in correlating the effect of duration of DM on these parameters.
A meta-analysis has proved the inverse correlation between DM and prostate cancer.4 Baradaran et al19 studied 511 patients and concluded that longer duration of DM may be protective against prostate cancer. We did not study the association with prostate cancer as an endpoint in our study. We tried to study how DM can affect the prostate. This possibly refers to the protective effect against prostate cancer or possibly just underdiagnosis of prostate cancer in diabetic patients because of lower PSA and even larger prostate volume that affects transrectal ultrasound biopsy outcomes. Our study may extend further to hypothetically show that diabetic patients may be liable to develop early castration-resistant prostate cancer, due to larger prostate volume in the presence of lower testosterone, pointing to the role of early androgen-independent growth of the prostate. Many theories concerning prostate cancer could be understood from this study, but will need further studies to prove how practical they are.
In conclusion, patients with type 2 DM tend to have significantly lower serum total PSA, lower serum testosterone, and larger prostate volume compared to nondiabetic individuals.
Conflicts of interest
All authors have no conflict of interest to declare.
Contributor Information
Mohamed M. Hashad, Email: mohie_hashad@yahoo.com.
Ahmed F. Kotb, Email: drahmedfali@gmail.com.
References
- 1.Safarinejad M.R. Prevalence of benign prostatic hyperplasia in a population-based study in Iranian men 40 years old or older. Int Urol Nephrol. 2008;40:921–931. doi: 10.1007/s11255-008-9338-7. [DOI] [PubMed] [Google Scholar]
- 2.Sarma A.V., Burke J.P., Jacobson D.J., McGree M.E., St Sauver J., Girman C.J. Associations between diabetes and clinical markers of benign prostatic hyperplasia among community-dwelling black and white men. Diabetes Care. 2008;31:476–482. doi: 10.2337/dc07-1148. [DOI] [PubMed] [Google Scholar]
- 3.Rhoden E.L., Ribeiro E.P., Teloken C., Souto C.A. Diabetes mellitus is associated with subnormal serum levels of free testosterone in men. BJU Int. 2005;96:867–870. doi: 10.1111/j.1464-410X.2005.05728.x. [DOI] [PubMed] [Google Scholar]
- 4.Kasper J.S., Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 5.Kehinde E.O., Sheikh M., Mojimoniy O.A., Francis I., Anim J.T., Nkansa-Dwamena D. High serum prostate-specific antigen levels in the absence of prostate cancer in Middle-Eastern men: the clinician's dilemma. Br J Urol. 2003;91:618–622. doi: 10.1046/j.1464-410x.2003.04199.x. [DOI] [PubMed] [Google Scholar]
- 6.Muller H., Raum E., Rothenbacher D., Stegmaier C., Brenner H. Association of diabetes and body mass index with levels of prostate-specific antigen: implications for correction of prostate-specific antigen cutoff values? Cancer Epidemiol Biomarkers Prev. 2009;18:1350–1356. doi: 10.1158/1055-9965.EPI-08-0794. [DOI] [PubMed] [Google Scholar]
- 7.Parekh N., Lin Y., Marcella S., Kant A.K., Lu-Yao G. Associations of lifestyle and physiologic factors with prostate-specific antigen concentrations: evidence from the National Health and Nutrition Examination Survey (2001-04) Cancer Epidemiol Biomarkers Prev. 2008;17:2467–2472. doi: 10.1158/1055-9965.EPI-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruun L., Ekberg H., Bjork T., Lilja H., Høglund P., Christensson A. Rapid elimination by glomerular filtration of free prostate specific antigen and human kallikrein 2 after renal transplantation. J Urol. 2004;171:1432–1435. doi: 10.1097/01.ju.0000118580.19344.68. [DOI] [PubMed] [Google Scholar]
- 9.Corona G., Mannucci E., Petrone L., Ricca V., Balercia G., Mansani R. Association of hypogonadism and type II diabetes in men attending an outpatient erectile dysfunction clinic. Int J Impot Res. 2006;18:190–197. doi: 10.1038/sj.ijir.3901391. [DOI] [PubMed] [Google Scholar]
- 10.Grossmann M., Thomas M.C., Panagiotopoulos S., Sharpe K., Macisaac R.J., Clarke S. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S., Cunningham G.R., Hayes F.J., Matsumoto A.M., Snyder P.J., Swerdloff R.S. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 12.Hammarsten J., Ogstedt B.H., Holthuis N., Mellstrom D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1:157–162. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 13.Ozden C., Ozdal O.L., Urgancioglu G., Koyuncu H., Gokkaya S., Memis A. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007;51:199–206. doi: 10.1016/j.eururo.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 14.Gades N.M., Jacobson D.J., Girman C.J., Roberts R.O., Lieber M.M., Jacobsen S.J. Prevalence of conditions potentially associated with lower urinary tract symptoms in men. BJU Int. 2005;95:549–553. doi: 10.1111/j.1464-410X.2005.05337.x. [DOI] [PubMed] [Google Scholar]
- 15.Barnard R.J., Obayashi N.K., Aronson W.J. Effect of diet and exercise intervention on the growth of prostate epithelial cells. Prostate Cancer Prostatic Dis. 2008;11:362–366. doi: 10.1038/pcan.2008.6. [DOI] [PubMed] [Google Scholar]
- 16.Kasturi S., Russell S., McVary K.T. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr Urol Rep. 2006;7:288–292. doi: 10.1007/s11934-996-0008-y. [DOI] [PubMed] [Google Scholar]
- 17.Berger A.P., Deibl M., Halpern E.J., Lechleitner M., Bektic J., Horninger W. Vascular damage induced by type 2 diabetes mellitus as a risk factor for benign prostatic hyperplasia. Diabetologia. 2005;48:784–789. doi: 10.1007/s00125-005-1678-6. [DOI] [PubMed] [Google Scholar]
- 18.Elrifai A., Kotb A., Sharaki O., Abdelhady M. Correlation of body mass index with serum total PSA, total testosterone and prostatic volume in a sample of men. Polish Ann Med. 2016;223:1–5. [Google Scholar]
- 19.Baradaran N., Ahmadi H., Salem S., Lotfi M., Jahani Y., Baradaran N. The protective effect of diabetes mellitus against prostate cancer: role of sex hormones. Prostate. 2009;69:1744–1750. doi: 10.1002/pros.21023. [DOI] [PubMed] [Google Scholar]