Abstract
Cisplatin is a widely used chemotherapeutic drug showing high efficiency in the treatment of primary tumors such as ovarian, testicular and cervical cancers. The major drawback of cisplatin is tumor resistance either acquired or intrinsic. Many mechanisms are involved in the resistance, among them is the Nrf2 pathway which regulates glutathione related enzymes. Caffeic acid, a non-toxic polyphenol which is abundant in many foods modulates glutathione S-transferase (GST) and glutathione reductase (GSR) activity, these enzymes were shown to be involved in resistance of cells towards cisplatin. Caffeic acid induces the Nrf2 pathway and can also inhibit the activity of GST and GSR.
Our findings demonstrate that the co-treatment of cancer cells with cisplatin and caffeic acid can enhance cisplatin cytotoxicity and increases the amount of platinum bound to nuclear DNA. However, 6 h of pre incubation with caffeic acid prior to cisplatin treatment led to acquired resistance to cisplatin and reduced DNA binding.
In conclusion, the enzyme inhibitory action of caffeic acid is dominant when the two agents are co-administered leading to increased cytotoxicity, and the Nrf2 induction is dominant when the cells are treated with caffeic acid prior to cisplatin treatment leading to resistance.
The use of caffeic acid as adjuvant for cisplatin should be carefully examined due to different pharmacokinetic profiles of caffeic acid and cisplatin. Thus, it is questionable if the two agents can reach the tumors at the right time frame in vivo.
Highlights
-
•
Caffeic acid acts as a dual agent, as NRf2-keap1 pathway inducer and GST inhibitor.
-
•
Pre treatment with caffeic acid prior to cisplatin increased the resistance of ovarian carcinoma cells to cisplatin.
-
•
Co administration of caffeic acid and cisplatin enhanced ovarian carcinoma cells cytotoxicity to cisplatin.
Graphical abstract
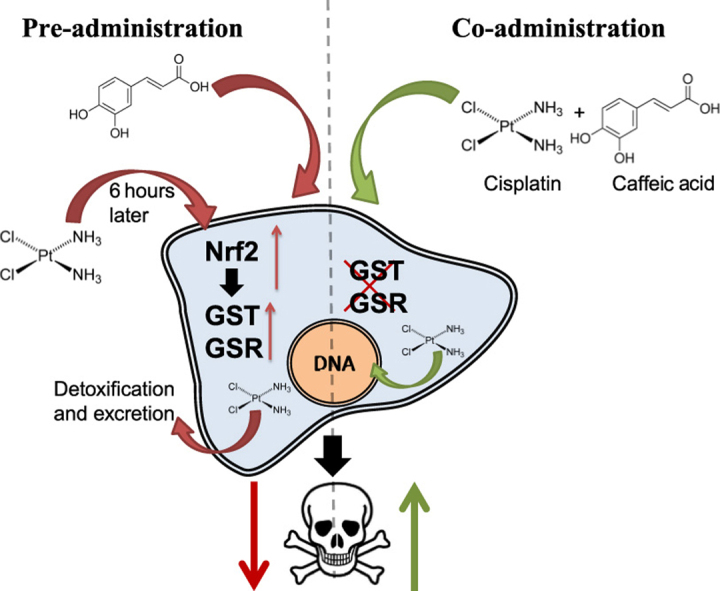
1. Introduction
Since its discovery in 1965 and entrance to the clinic in 1978, cisplatin became one of the most important and efficient chemotherapeutic drugs [1], [2], [3].
Cisplatin [cis-diamminedichloroplatinum(II)] is administered in the clinic with other drugs to treat ovarian, testicular, cervical cancer and additional more cancer types [4], [5], [6]. Cisplatin binds DNA preferably to adjacent guanines on the same strand, leading to DNA lesions, distortion of the DNA structure and consequently to cell death via apoptosis [7]. The efficacy of cisplatin in the clinic is limited by severe side effects in some cases but more prominently by tumor resistance [8]. The side effects of cisplatin include nephrotoxicity, ototoxicity, neurotoxicity and other side effects common to chemotherapy [9], [10], [11].
Cisplatin resistance can be either intrinsic (e.g. as observed in patients with colorectal, lung and prostate cancer) [12], [13], [14] or acquired following cisplatin chemotherapy (as often seen in patients with ovarian cancer) [15]. The mechanisms of cisplatin resistance had been studied in several types of cisplatin resistant cell lines and appear to be multifactorial. It has been shown that cancer cells can develop cisplatin resistance through (1) decreasing cisplatin concentration within the cells by reducing its influx (via CRT1 copper transporters) [16] and increasing its efflux (via ATP7A transporters) [17], (2) changing the balance of pro-apoptotic and anti-apoptotic factors [18], (3) inducing changes in DNA repair system that results in increased nucleotide excision repair [19], interstrand crosslink repair or loss of mismatch repair [20], [21], (4) affecting the DNA damage tolerance mechanisms [22] and finally (5) enhancing the drug detoxification system by elevating the levels of intracellular scavengers such as glutathione (GSH) [23].
While the detoxification of cisplatin by its interaction with glutathione may be due to spontaneous binding [24], it is probably catalyzed in the cells by glutathione S-transferases (GSTs) [25]. Indeed, high GST levels are found to correlate to cisplatin resistance in the clinic [26].
Other GSH related enzymes may play a role in this resistance pathway. GSH is synthesized by γ-glutamate cysteine ligase and GSH synthase. GSH reductase (GSR) is recycling the oxidized GSSG back to its reduced form. Those enzymes have been also linked to cisplatin resistance [27], [28]. GSH related enzymes are part of the phase II enzymes family, which are under the control of the Nrf2/Keap1 pathway.
Nrf2/Keap1 pathway is one of the key cellular pathways regulating cell defenses against oxidative stress and reactive electrophilic xenobiotics [29]. Nrf2 (NF-E2 p45-related Factor 2) is a bZip transcription factor and a member of the Cap ‘n’ Collar family which is bound to the suppressor Keap1 homodimer in the cytoplasm. The association between Nrf2 and Keap1 facilitates ubiquitination of the Nrf2 protein and its degradation in the proteasome as part of the Nrf2 basal activity control [30]. Nrf2 is activated upon changes in the redox state in the cell or in response to electrophiles. Thiol groups of the cysteine residues of Keap1 are oxidized to form disulphide bonds in response to oxidative stress or modified by electrophiles, these modifications lead to conformational change of the protein and release of Nrf2 [31], [32].
Free Nrf2 undergoes kinase mediated phosphorylations and translocates into the nucleus. In the nucleus Nrf2 binds together with small Maf proteins to the antioxidant response element (ARE) in the regulatory regions of target genes and promotes the induction of the phase II enzymes [33].
Caffeic acid is a polyphenol from the hydrocinnamic acid family which is found in many foods including coffee, fruits, cereals and more [34].
In our previous work we found that caffeic acid acts in a dual way as an inducer of the Nrf2 pathway and as an inhibitor of GST and GSR [35].
We demonstrated that GST and GSR activity in cisplatin sensitive cell line (A2780) and in cisplatin resistant cell line (A2780cisR) is affected in a different way following caffeic acid treatment. While A2780 cells demonstrate bell-shaped activity of GST following caffeic acid treatment, in A2780cisR cells the GST activity is U-shaped.
These results suggest that there is a competition between the induction and the inhibitory effects of caffeic acid.
Therefore, we hypothesize that co-administration of cisplatin with caffeic acid may affect the cells sensitivity to cisplatin. Particularly, the Nrf2 induction by caffeic acid might contribute to the acquired resistance through the induction of protective phase II enzymes. While the inhibition of GST and GSR can partly circumvent the resistance and allow cisplatin reach the nucleus in higher amounts and therfore be more potent.
2. Materials and methods
2.1. Materials
Caffeic acid (CA), trigonelline, potassium iodide, potassium tetrachloroplatinate, silver nitrate,70% nitric acid (redistilled), 3-(4,5-dimethylthiazole-2-yl)−2,5-diphenyl tetrazolium bromide(MTT) and other common reagents were purchased from Sigma Chemical Co., St. Louis, MO. Ammonium hydroxide was purchased from Bio-Lab Jerusalem, Israel. Cell culture medium, L-glutamine, gentamycin and fetal calf serum were purchased from BioInd Bet Dagan, Israel.
2.2. Cell culture
Human ovarian carcinoma A2780 and daughter line A2780cisR were obtained from ATCC, USA. The cells were cultured in RPMI 1640 medium supplemented with10% fetal bovine serum, 2 mM L-glutamine, and 50 μg/mL gentamycin. The cultures were maintained in a humidified 5% CO2 incubator at 37 °C. Cells were subcultured every 3–4 days to maintain logarithmic growth and were allowed to grow for 24 h in the experiment wells before use.
2.3. Cytotoxicity MTT assay
The cytotoxic effects of cisplatin and caffeic acid against the A2780 and A2780cisR tumor cells were assessed via MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. The cells were seeded into 96- wells at a density of 6×103 cells per well. Cells were permitted to adhere for 24 h, and then treated with the various concentrations of cisplatin and caffeic acid for 24 and 48 h. The cultured medium was removed and replaced with 150 μL MTT (0.5 mg/mL) per well before termination at 2 h. After removal of the MTT solution, 200 μL DMSO was added to each well. The absorbance was recorded on a Biotek microplate reader (Biotek Instruments, Inc., Winooski, VT) at the wavelength of 540 nm.
All experiments were performed independently in triplicate and data were presented as mean±S.E.M.
2.4. Caspase 3 activity assay
Treated cells were incubated for 1 h in PBS containing 2.5 μM Ac-DEVD-AMC, a fluorogenic substrate specific of caspase 3 (Calbiochem, Darmstadt, Germany), with 0.02% Triton X-100, 10 mM dithiothreitol, and 20 mM Tris pH 7.4, at 37 °C. Fluorescence was measured at 355 nm/460 nm on a Citation 3 fluorometer (BioTek Instruments, Inc., VT, USA) for 40 min, and the activity was calculated in the linear range of the slope and normalized to cell count in each well.
2.5. Pt-DNA adducts quantification
Cells were seeded in 6-well 24 h prior treatment at a density of 3×105 cells per well. Cells were treated for 24 h with cisplatin and caffeic acid then the medium was removed and the cell trypsinizid. Trypsin was removed after centrifugation and the DNA was extracted using DNeasy Blood & Tissue kit (QIAGEN, Germany). DNA concentration in each sample was quantified by absorbance measuring using ND-1000 UV–Vis Spectrophotometer (NanoDrop, Thermo Scientific, Waltham, MA).
The samples were diluted with 1% redistilled nitric acid (without metal traces) to the amounts of 5-20 ng per sample.
195Pt content in the DNA samples was measured by ICP-MS (Agilent 7500cx, Santa Carla, CA) and normalized to µg of DNA for comparing.
All experiments were performed independently in triplicate and data were presented as mean±SD.
2.6. Statistical analysis
Differences between groups were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett's t-test. Difference of P<0.05 was considered statistically significant compared to the untreated control group, or as defined in the figure legends.
3. Results
3.1. Cell viability after cisplatin and caffeic acid treatment
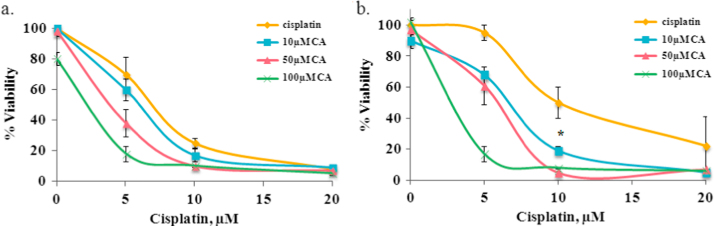
In order to establish the effect of caffeic acid treatment in combination with cisplatin, we treated both cisplatin sensitive and resistant cells and measured the viability by the MTT assay. Following the treatments for 48 h the IC50 of cisplatin was 6.5 and 10 µM in sensitive and resistant cells, respectively (Fig. 1). Caffeic acid alone was not toxic to the cells. At 100 µM it reduced sensitive cells viability only by 20% (Fig. 1a).
Fig. 1.
Viability of A2780 (a) and A2780cisR cells (b) following treatment of cisplatin and caffeic acid for 48 h as measured by MTT assay. Cisplatin treatment without caffeic acid (yellow); cisplatin in combination with 10 µM caffeic acid (blue); cisplatin in combination with 15 µM caffeic acid (red); cisplatin in combination with 100 µM caffeic acid (green). Results are presented as the means of cytotoxicity±SD. * indicate statistically-significant differences (p<0.05) compared to the control. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the sensitive cells (A2780) the addition of caffeic acid resulted in the decrease in the IC50 values from 6.5 µM to 6, 4 and 2.5 µM when cisplatin was combined with 10, 50 and 100 µM of caffeic acid, respectively (Fig. 1a). A more prominent effect was observed in the resistant cells. In these cells the IC50 values decreased from 10 µM to 6.5, 6 and 3.5 µM when treated with the same combination as the sensitive cells (Fig. 1b). When resistant cells were treated with 10 µM of caffeic acid and 5 µM of cisplatin (2:1 ratio) the cell viability was the same as for cisplatin treated sensitive cells (60% viability).
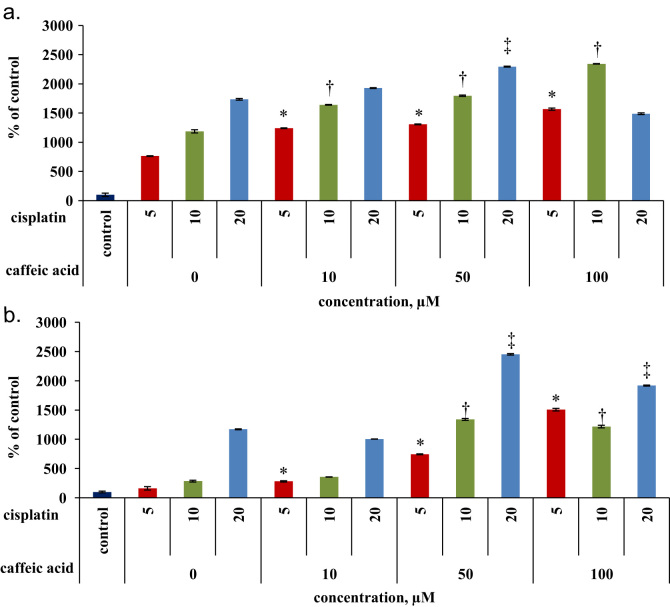
Apoptosis of the cells following the treatments was mesured via the activity of caspase 3. The activity of caspase 3 in the sensetive cells was up to 4.7 fold higher than in the resistant cells following cisplatin treatment alone (Fig. 2). Exposure of the sensetive cells to the combinations of cisplatin and caffeic acid as mentioned above resulted in a dose response activation of the apoptotic machinary. For example co treatment of 5 µM cisplatin with 50 µM of caffeic acid increased the activity of caspase by 1.7 fold compairing to 5 µM of cisplatin (Fig. 2a). Similar trend was obtained in the resistant cells, when the most prodominant effect was observed at cisplatin concentration of 5 µM. In this case the combination of 5:50 µM (cisplatin/caffeic acid) increased the activity of caspase by 4.3 fold (Fig. 2b).
Fig. 2.
Caspase 3 activity following treatment of cisplatin in combination with caffeic acid for 48 h (a) Caspase 3 activity in A2780 cells (b) and in A2780cisR cells. Data represented as % control (untreated cells)±SD; *, †, ‡ indicates signnificance (p<0.05) comparing to treatment of cisplatin alone at concentrations of 5,10,20 µM, respectivley.
3.2. Effects of timing of CA treatment on cisplatin toxicity
We showed in our previous work that caffeic acid induces phase II enzymes through the activation of the Nrf2 pathway but also inhibits the enzyme activity of GSTP1 and GSR1 [20]. Those effects take place at different time frames. Therefore, we tested the effect of the timing of caffeic acid treatment with cisplatin on its cytotoxicity.
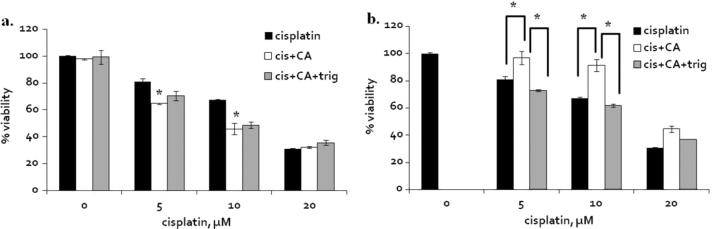
The black bars (Fig. 3) represent A2780 cells viability following 24 h of cisplatin treatment. When 50 µM of caffeic acid was co- administrated with cisplatin at 5 and 10 µM, A2780 cells viability was reduced by additional 20% compared to cisplatin concentrations of 5 and 10 µM without caffeic acid (Fig. 3a-white).
Fig. 3.
The effect of the timing of caffeic acid treatment in combination with cisplatin in A2780 cells. (a) Co-administration of cisplatin and 50 µM of caffeic acid or caffeic acid and trigonelline for 24 h. (b) 6 h pre treatment of caffeic acid or caffeic acid and trigonelline followed by washout and cisplatin treatment for additional 24 h. Cisplatin treatment (black); combination of cisplatin and caffeic acid (white); combination of cisplatin, caffeic acid and trigonelline (grey). Results are presented as the means of cytotoxicity±SD. * indicates signnificance (p<0.05) comparing to treatment of cisplatin control of the same concentration or as indicated in the figure.
It was recently reported that trigonelline, an alkaloid which is also found in coffee, can inhibit the translocation of Nrf2 into the nucleus thus, inhibiting the induction of phase II enzymes [36]. The addition of 1 µM trigonelline with 50 µM caffeic acid and cisplatin (5, 10, 20 µM) had no effect on the observed cell viability comparing to co-treatment of cisplatin with caffeic acid and without trigonelline (Fig. 3a-grey). Next, cells were pretreated with 50 µM of caffeic acid 6 h prior the cisplatin treatments, then the cells were washed twice and the cell media was replaced. In this experiment the pretreatment with caffeic acid resulted in an increase in cells viability by up to 25% compared to cisplatin control (Fig. 3b-white).
In this case, the addition of trigonelline annulled completely the effect of caffeic acid pretreatment (Fig. 3b-grey).
3.3. DNA platination
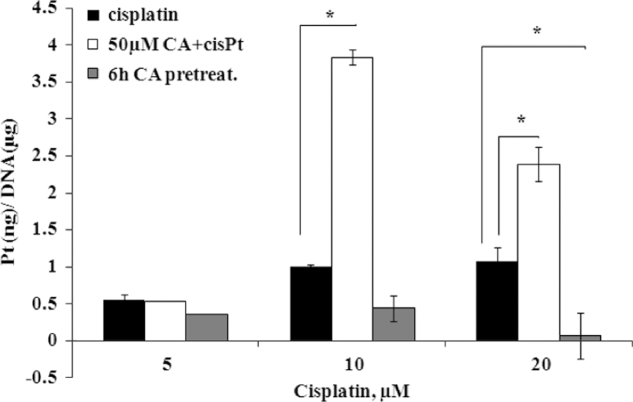
In order to elucidate the mechanism involved in the enhancement of cisplatin toxicity in the presence of caffeic acid, we repeated the previous experiments and quantified the platinum-DNA adducts by ICP-MS as the DNA is the ultimate target of cisplatin. Following cisplatin treatment by 5, 10 and 20 µM, we measured 0.55, 1 and 1.08 ng of 195Pt per 1 µg of DNA respectively (Fig. 4-black). When 50 µM caffeic acid co-administrated with cisplatin, a significant increase in DNA platination was observed for cisplatin concentrations of 10 and 20 µM (Fig. 3-white). 3.38 ng of 195Pt per 1 µg of DNA was bound to DNA, approximately a 4-fold increase was obtained as compared to 10 µM cisplatin treatment. However, a 2.2-fold increase in binding occurred upon co-treatment of 20 µM cisplatin with caffeic acid (2.4 ng 195Pt).
Fig. 4.
DNA platination of A2780 cells following cisplatin treatment, co-treatment with caffeic acid and pre-treated with caffeic acid for 24 h. 195Pt (ng)/DNA (µg) following cisplatin treatment (black); 195Pt (ng)/DNA (µg) following cisplatin co-treatment with caffeic acid (white); 195Pt (ng)/DNA (µg) following cisplatin treatment after 6 h pre-treatment with 50 µM caffeic acid(grey). Results are presented as the means of cytotoxicity±SD. * indicate statistically-significant differences (p<0.05) compared to the cisplatin treated cells.
On the contrary, when the cells were pretreated with caffeic acid, DNA platination significantly decreased (Fig. 4-grey), a 50% and 95% decrease in platination compared to 10 and 20 µM of cisplatin.
4. Discussion
Cisplatin is one of the most widely used chemotherapeutic agents in the clinic for treatment of testicular, ovarian, cervical, head and neck, non-small cell lung cancer and more types of cancer. Although cisplatin is highly effective, its dosage is limited by severe side effects and more importantly by tumor resistance (acquired or intrinsic). For many years the research had focused on finding solutions to overcome the resistance by either designing novel platinum based drugs or looking for alternative dosage regiment protocols [37], [38]. Many molecules were tested in combination to improve cisplatin efficacy in resistant tumors. For example, Ma et. al. found that emodin, a natural anthraquinone derivative, enhanced cisplatin cytotoxicity in human ovarian carcinoma cell line (COC1) [39]. Also, EGCG (Epigallocatechin gallate) significantly reduced cell viability in eight biliary tract cancer (BTC) cell lines and additionally displayed a synergistic cytotoxic effect with cisplatin in tested BTC cell lines [40]. Caffeic acid also displayed some positive effect on cisplatin toxicity [41].
Here we demonstrated that the effect of caffeic acid on cisplatin toxicity depends on the timing of its administration with cisplatin. Caffeic acid is a polyphenol found in coffee and is very ubiquitous in human diet. It is not toxic even at high doses of 0.5-1gr of daily consumption [42]. In our work we worked with sub-toxic micromolar concentrations and found a synergistic effect with cisplatin.
When the A2780 and A2780cisR cells were treated with cisplatin and caffeic acid at various concentrations, the more predominant effect of caffeic acid on cisplatin were observed in the resistant cells, see Fig. 1. In these cells, caffeic acid administration was able to almost completely circumvent resistance. In the sensitive cells, the combinations (at all caffeic acid concentrations) also lead to a further sensitization of the cells to cisplatin. This effect was achieved due to the inhibition of the detoxification enzymes by caffeic acid.
GST can potentially increase the resistance to cisplatin via the binding of glutathione making the platinum complex a substrate for the MDR2 pumps. Besides its direct dependence on GST activity, this detoxification pathway is also dependant upon glutathione concentrations. Thus other glutathione related enzymes that are also under the Nrf2 control can contribute indirectly to the resistance.
Caffeic acid is an inducer of the Nrf2 pathway on one hand but an inhibitor of GST and GSR on the other hand, thus acting as an agent with dual activity affecting both enzymes concentrations and their activity [35].
Fig. 1, Fig. 3 show that while co-administration of caffeic acid with cisplatin increased cells sensitivity, a 6 h pretreatment with caffeic acid led to cells resistance (Fig. 3). Caffeic acid can enter the cells to and inhibit GST and GSR present at basal levels in the cells [35]. The translation of induced GST and GSR mRNA to proteins following Nrf2 pathway activation by caffeic acid can take 6–12 h and more [43]. Thus, when caffeic acid is administered with cisplatin, the inhibitory effect is much faster than the induction of phase II enzymes.
The inhibitory effect of caffeic acid on GST and GSR allows cisplatin to enter to the nucleus and bind to the DNA. Indeed, DNA binding increased by 4 and 2.2 fold when caffeic acid was added to 10 and 20 µM cisplatin, respectively. The increased DNA binding of cisplatin lead to the activation of the apoptitic response in cells, as shown in Fig. 2, and sequentially to cell death.
The 6 h pre treatment of the cells with caffeic acid followed by cells washout exhibited an opposite effect to the co-treatment, as cells became resistant. At this period of time, Nrf2 and phase II enzymes are induced by caffeic acid [35]. RT-PCR results from our previous work confirm that at this time period, Nrf2 and GST levels induced by caffeic acid increased by 2 and 3 fold, respectively. As a result, cisplatin administered to the Nrf2 activated cells is detoxified more efficiently than in the naive sensitive cells. As seen in Fig. 3, the DNA binding of cisplatin in caffeic acid pre-treated cells is reduced by 50% and 90% of control when tested at 10 and 20 µM, respectively.
The involvement of the Nrf2 pathway is demonstrated by the addition of trigonelline together with caffeic acid and cisplatin. Trigonelline is an inhibitor of Nrf2 translocation into the nucleus, thus aborting the induction of phase II enzymes. The co-treatment of the cells with trigonelline, caffeic acid and cisplatin did not change the cells viability observed, indicating that this effect is not Nrf2 dependant. However, when the cells were treated with trigonelline and caffeic acid 6 h prior to cisplatin administration, this annulled the resistance of the cells resulting from acquired pretreatment by of caffeic acid alone. This indicates that Nrf2 pathway is involved in the resistance acquired from the pre-treatment.
When the cells were treated with 5 and 10 µM of cisplatin the binding of cisplatin to the DNA increased linearly. However, treatment of 20 µM did not further increase the amount of DNA bound cisplatin.
Both in co and pre treatment experiments, at 20 µM cisplatin the DNA binding was lower in comparison to treatment of caffeic acid and 10 µM cisplatin, this can be possibly explained by the multi factorial resistance to cisplatin. The involved mechanisms may be nucleotide excision repair (NER), interstrand crosslink repair or loss of mismatch repair are DNA repair mechanisms coping with DNA damage caused by cisplatin binding.
It is highly probable that high concentrations of cisplatin induce not only GST mediated resistance in cells but also other resistance factors, such as NER that act additively or synergistically to detoxify and remove the DNA lesions.
Taken together, the use of caffeic acid and other Nrf2 inducers as adjuvants to increase cisplatin efficacy particularly in resistant tumors can lead to adverse effects, and if taken together with cisplatin can lead to resistance.
We suggest that careful design of the timing of the co-treatment of cisplatin and caffeic acid is very important and may have opposite outcomes on cell survival. Administration of mixture of cisplatin and caffeic acid (or oral intake of caffeic acid) to cancer patients can improve the treatment if the two compound will reach their target simultaneously. However, it is more reasonable that due to different pharmacokinetic profiles of both agents that they will reach the target cells at different time points. This may lead to a rapidly acquiring resistance to cisplatin.
It should be considered that patients undergoing chemotherapeutic therapy will avoid caffeic acid consumption, and other known Nrf2 inducers, few days prior to the treatment. In recently published work, coffee consumption is linked to reduced risk of breast cancer [44], however other study indicated that breast cancer patients consuming more than 3 cups/day of coffee had higher overall death rates [45].
Recently it was discovered that the Nrf2 pathway (Nrf2, phase II enzymes and GSH levels) is rhythmically activated by the circadian clock [46]. For example, in rats increased GSH and GST were observed at the beginning of the active period [47]. In mice, Nrf2 and GSH were highest in the morning, however, GST was at the peak at early dark period [48]. It was also found that curcumin (polyphenol) activity was dependant on time of its administration [49]. Hybertson and Gao suggested that the natural circadian variations in Nrf2 activity could impact the scheduling of drugs or other agents intended to activate or inhibit the Nrf2 pathway for therapeutic benefit [50]. Pekovic-Vaughan et al. raised the question whether Nrf2-activating agents should be administered to coincide with and support the daily high point of Nrf2 function, or to coincide with, and perhaps counteract [48], the daily low point of Nrf2 function. Therefore, it can be speculated that carful timing of administration of caffeic acid with cisplatin according to the circadian rhythm can further improve the potency of the latter.
In conclusion, caffeic acid is a agent with dual activity that can sensitize or habituate the cells to cisplatin treatment depending on the timing of its administration. As favorable results were obtained when caffeic acid and cisplatin were co administered, there is a rational to prepare a new platinum-caffeic acid compound.
Conflict of interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported, in part, by the Alex Grass Center for Drug Design and Synthesis of Novel Therapeutics (Grant no. 0364794 at HUJI). RK is affiliated with the Bloom Center of Pharmacy. R.K is the incumbent of the Richard and Jean Zarbin Chair in Medical Studies at the Hebrew University of Jerusalem.
References
- 1.Price K.A.R., Cohen E.E. Current treatment options for metastatic head and neck cancer. Curr. Treat. Options Oncol. 2012;13(1):35–46. doi: 10.1007/s11864-011-0176-y. [DOI] [PubMed] [Google Scholar]
- 2.Carballido E.M., Rosenberg J.E. Optimal treatment for metastatic bladder cancer. Curr. Oncol. Rep. 2014;16:9. doi: 10.1007/s11912-014-0404-2. [DOI] [PubMed] [Google Scholar]
- 3.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong D.K., Bundy B., Wenzel L. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New Engl. J. Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong R.N. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem. Res. Toxicol. 1997;10(1):2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 6.Rose P.G., Bundy B.N., Watkins E.B. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New Engl. J. Med. 1999;340(15):1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson E.R., Lippard S.J. Structure, recognition, and processing of cisplatin-DNA adducts. Chem. Rev. 1999;99(9):2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 8.Siddik Z.H. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 9.Wang H., Jia Z., Sun J. Nitrooleic acid protects against cisplatin nephropathy: role of COX-2/mPGES-1/PGE(2) cascade. Mediat. Inflamm. 2015 doi: 10.1155/2015/293474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S.H., Kim H.S., An Y.S., Chang J., Choi J., Im G.J. Protective effect of resveratrol against cisplatin-induced ototoxicity in HEI-OC1 auditory cells. Int. J. Pediatr. Otorhinolaryngol. 2015;79(1):58–62. doi: 10.1016/j.ijporl.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Barlinn K., Lehrach H., Siepmann T., Braeuer D., Buntrock U., Sassim N. Temporary loss of moral behavior in a patient undergoing chemotherapy with cisplatin – breaking bad. Bmc Psychiatry. 2015:15. doi: 10.1186/s12888-015-0386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng-Rong N., Paterson J., Alpert L., Tsao M.-S., Viallet J., Alaoui-Jamali M.A. Elevated DNA repair capacity is associated with intrinsic resistance of lung cancer to chemotherapy. Cancer Res. 1995;55(21):4760–4764. [PubMed] [Google Scholar]
- 13.Kawaguchi J., Adachi S., Yasuda I. Cisplatin and ultra-violet-C synergistically down-regulate receptor tyrosine kinases in human colorectal cancer cells. Mol. Cancer. 2012;11:45. doi: 10.1186/1476-4598-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumulec J., Balvan J., Sztalmachova M. Cisplatin-resistant prostate cancer model: differences in antioxidant system, apoptosis and cell cycle. Int J. Oncol. 2014;44(3):923–933. doi: 10.3892/ijo.2013.2223. [DOI] [PubMed] [Google Scholar]
- 15.Gifford G., Paul J., Vasey P.A., Kaye S.B., Brown R., Scottish Gynaecological C. The acquisition of hMLH1 methylation in plasma DNA after chemotherapy predicts poor survival for ovarian cancer patients. Clin. Cancer Res. 2004;10(13):4420–4426. doi: 10.1158/1078-0432.CCR-03-0732. [DOI] [PubMed] [Google Scholar]
- 16.Holzer A.K., Manorek G.H., Howell S.B. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharmacol. 2006;70(4):1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 17.Tadini-Buoninsegni F., Bartolommei G., Moncelli M.R. Translocation of platinum anticancer drugs by human copper ATPases ATP7A and ATP7B. Angew. Chem.-Int. Ed. 2014;53(5):1297–1301. doi: 10.1002/anie.201307718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiota M., Yokomizo A., Tada Y. P300/CBP-associated factor regulates Y-box binding protein-1 expression and promotes cancer cell growth, cancer invasion and drug resistance. Cancer Sci. 2010;101(8):1797–1806. doi: 10.1111/j.1349-7006.2010.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Usanova S., Piée-Staffa A., Sied U. Cisplatin sensitivity of testis tumour cells is due to deficiency in interstrand-crosslink repair and low ERCC1-XPF expression. Mol. Cancer. 2010;9:248. doi: 10.1186/1476-4598-9-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kudo K., Gavin E., Das S., Amable L., Shevde L.A., Reed E. Inhibition of Gli1 results in altered c-Jun activation, inhibition of cisplatin-induced upregulation of ERCC1, XPD and XRCC1, and inhibition of platinum-DNA adduct repair. Oncogene. 2012;31(44):4718–4724. doi: 10.1038/onc.2011.610. [DOI] [PubMed] [Google Scholar]
- 21.Kothandapani A., Dangeti V.S., Brown A.R. Novel role of base excision repair in mediating cisplatin cytotoxicity. J. Biol. Chem. 2011;286(16):14564–14574. doi: 10.1074/jbc.M111.225375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson S.W., Swiggard P.A., Handel L.M. Relationship between platinum-DNA adduct formation and removal and cisplatin cytotoxicity in cisplatin-sensitive and -resistant human ovarian cancer cells. Cancer Res. 1994;54(22):5911–5916. [PubMed] [Google Scholar]
- 23.Ishikawa T., Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J. Biol. Chem. 1993;268(27):20116–20125. [PubMed] [Google Scholar]
- 24.Kasherman Y., Sturup S., Gibson D. Is glutathione the major cellular target of cisplatin? A study of the interactions of cisplatin with cancer cell extracts. J. Med. Chem. 2009;52(14):4319–4328. doi: 10.1021/jm900138u. [DOI] [PubMed] [Google Scholar]
- 25.Goto S., Iida T., Cho S., Oka M., Kohno S., Kondo T. Overexpression of glutathione S-transferase pi enhances the adduct formation of cisplatin with glutathione in human cancer cells. Free Radic. Res. 1999;31(6):549–558. doi: 10.1080/10715769900301121. [DOI] [PubMed] [Google Scholar]
- 26.Surowiak P., Materna V., Kaplenko I. Augmented expression of metallothionein and glutathione S-transferase pi as unfavourable prognostic factors in cisplatin-treated ovarian cancer patients. Virchows Arch. 2005;447(3):626–633. doi: 10.1007/s00428-005-1228-0. [DOI] [PubMed] [Google Scholar]
- 27.Stordal B., Hamon M., McEneaney V. Resistance to paclitaxel in a cisplatin-resistant ovarian cancer cell line is mediated by P-glycoprotein. PLoS One. 2012;7(7):e40717. doi: 10.1371/journal.pone.0040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujimori S., Abe Y., Nishi M. The subunits of glutamate cysteine ligase enhance cisplatin resistance in human non-small cell lung cancer xenografts in vivo. Int J. Oncol. 2004;25(2):413–418. [PubMed] [Google Scholar]
- 29.Murakami S., Motohashi H. Roles of Nrf2 in cell proliferation and differentiation. Free Radic. Biol. Med. 2015 doi: 10.1016/j.freeradbiomed.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi A., Kang M.I., Okawa H. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004;24(16):7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levonen A.L., Landar A., Ramachandran A. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem J. 2004;378(Pt 2):373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinkova-Kostova A.T., Holtzclaw W.D., Cole R.N. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. Usa. 2002;99(18):11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 35.Sirota R., Gibson D., Kohen R. The role of the catecholic and the electrophilic moieties of caffeic acid in Nrf2/Keap1 pathway activation in ovarian carcinoma cell lines. Redox Biol. 2015;4:48–59. doi: 10.1016/j.redox.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arlt A., Sebens S., Krebs S. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene. 2013;32(40):4825–4835. doi: 10.1038/onc.2012.493. [DOI] [PubMed] [Google Scholar]
- 37.Sun W., Wang T., Shi F. Randomized phase III trial of radiotherapy or chemoradiotherapy with topotecan and cisplatin in intermediate-risk cervical cancer patients after radical hysterectomy. BMC Cancer. 2015;15:353. doi: 10.1186/s12885-015-1355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najajreh Y., Perez J.M., Navarro-Ranninger C., Gibson D. Novel soluble cationic trans-diaminedichloroplatinum(II) complexes that are active against cisplatin resistant ovarian cancer cell lines. J. Med. Chem. 2002;45(24):5189–5195. doi: 10.1021/jm0201969. [DOI] [PubMed] [Google Scholar]
- 39.Ma J., Yang J., Wang C. Emodin augments cisplatin cytotoxicity in platinum-resistant ovarian cancer cells via ROS-dependent MRP1 downregulation. Biomed. Res. Int. 2014;2014:107671. doi: 10.1155/2014/107671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayr C., Wagner A., Neureiter D. The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complement Altern. Med. 2015;15:194. doi: 10.1186/s12906-015-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai J., Cheung S., Chan E., Hasman D. Antiproliferation effect of commercially brewed coffees on human ovarian cancer cells in vitro. Nutr. Cancer. 2010;62(8):1044–1057. doi: 10.1080/01635581.2010.492083. [DOI] [PubMed] [Google Scholar]
- 42.Olthof M.R., Hollman P.C., Katan M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001;131(1):66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 43.K. Zimmermann, J. Baldinger, B. Mayerhofer , A. Atanasov, V. Dirsch, E. Heiss, Activated AMPK boosts the Nrf2/HO-1 signaling axis-A role for the unfolded protein response, 88b, 2015, pp. 417–426. [DOI] [PMC free article] [PubMed]
- 44.Oh J.K., Sandin S., Ström P., Löf M., Adami H.O., Weiderpass E. Prospective study of breast cancer in relation to coffee, tea and caffeine in Sweden. Int J. Cancer. 2015;137(8):1979–1989. doi: 10.1002/ijc.29569. [DOI] [PubMed] [Google Scholar]
- 45.Lehrer S., Green S., Rosenzweig K.E. Coffee consumption associated with increased mortality of women with breast. Cancer J. Caffeine Res. 2013;3(1):38–40. doi: 10.1089/jcr.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wende A.R., Young M.E., Chatham J., Zhang J., Rajasekaran N.S., Darley-Usmar V.M. Redox biology and the interface between bioenergetics, autophagy and circadian control of metabolism. Free Radic. Biol. Med. 2016 doi: 10.1016/j.freeradbiomed.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapenna D., De Gioia S., Mezzetti A. Circadian variations in antioxidant defences and lipid peroxidation in the rat heart. Free Radic. Res Commun. 1992;17(3):187–194. doi: 10.3109/10715769209068165. [DOI] [PubMed] [Google Scholar]
- 48.Pekovic-Vaughan V., Gibbs J., Yoshitane H. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28(6):548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarma A., Sharma V.P., Sarkar A.B., Sekar M.C., Samuel K., Geusz M.E. The circadian clock modulates anti-cancer properties of curcumin. BMC Cancer. 2016;16(1):759. doi: 10.1186/s12885-016-2789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hybertson B.M., Gao B. Role of the Nrf2 signaling system in health and disease. Clin. Genet. 2014;86(5):447–452. doi: 10.1111/cge.12474. [DOI] [PubMed] [Google Scholar]