Abstract
The anti-melanoma differentiation-associated gene 5 (MDA-5) antibody is a marker of clinically amyopathic dermatomyositis (CADM) and rapidly progressive interstitial lung disease (ILD) with acute respiratory failure. A 35-year-old woman with cervical cancer showed Gottron's papules, severe hypoxemia, and diffuse ground-glass opacities on chest computed tomography. She was diagnosed with rapidly progressive ILD associated with CADM. Her serum was positive for the anti-MDA-5 antibody. Combination therapy with corticosteroids, immunosuppressants, and direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) improved her respiratory dysfunction. Eventually, surgery for the cancer was performed successfully. This is the first case to demonstrate the efficacy of PMX-DHP for rapidly progressive ILD with anti-MDA-5 antibody-positive CADM and a malignancy.
Keywords: Anti-melanoma differentiation-associated gene 5 antibody, Clinically amyopathic dermatomyositis, Rapidly progressive interstitial lung disease, Direct hemoperfusion using polymyxin B-immobilized fiber column, Cervical cancer
Abbreviations: Anti-MDA-5, anti-melanoma differentiation-associated gene 5; CADM, clinically amyopathic dermatomyositis; ILD, interstitial lung disease; PMX-DHP, direct hemoperfusion using polymyxin B-immobilized fiber column
1. Introduction
Clinically amyopathic dermatomyositis (CADM) is characterized by typical skin manifestations of dermatomyositis (DM) with amyopathy or hypomyopathy, and the acute onset of interstitial pneumonia with CADM has been known to develop into rapidly progressive interstitial lung disease (ILD) [1], [2]. Rapidly progressive ILD associated with CADM is refractory to intensive therapies such as the systemic administration of high-dose corticosteroids and immunosuppressive agents, leading to a poor prognosis [2], [3]. We have previously reported that direct hemoperfusion using polymyxin B-immobilized fiber column (PMX-DHP) therapy in combination with conventional therapy can be effective in the management of rapidly progressive ILD in patients with CADM [4]. However, the efficacy of adding PMX-DHP therapy when rapidly progressive ILD is associated with CADM and a malignancy remains unclear.
A new autoantibody, the anti-melanoma differentiation-associated gene 5 (MDA-5) antibody (originally referred to as the anti-CADM-140 antibody), has been identified in certain phenotypes of DM, especially CADM [5]. Detection of this antibody is potentially important because its presence may be closely associated with rapidly progressive ILD [5], [6], [7]. The anti-MDA-5 antibody is known to be mutually exclusive of anti-aminoacyl-tRNA synthetase (ARS) antibodies, which are representative antibodies detected in DM and polymyositis, and anti-transcriptional intermediary factor 1 gamma (TIF1-γ) antibody, which is closely linked to cancer-associated myositis [6], [7], [8]. Recent studies have reported that serial monitoring of serum anti-MDA-5 antibody levels can be useful for assessing therapeutic efficacy, suggesting that this antibody may serve as a marker for disease activity in rapidly progressive ILD with CADM [9]. Furthermore, Fiorentino et al. demonstrated that the anti-MDA-5 antibody was associated with a unique cutaneous characteristic phenotype consisting of skin ulceration and tender papules on the palms, and that the distribution of DM patients with this antibody varied between ethnic groups [10]. In contrast, according to recent findings, the anti-MDA-5 antibody seems to be associated with a relative low risk of malignancy-associated DM [7].
Here we describe a rapidly progressive ILD due to anti-MDA-5 antibody-associated CADM complicated with cervical cancer, who was successfully treated by a combination of pharmacotherapies, PMX-DHP therapy, and resection of cervical cancer.
2. Case report
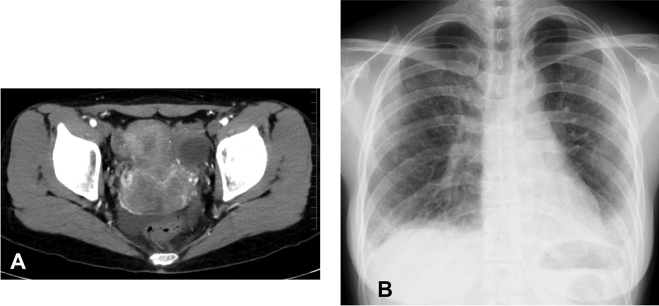
A 35-year-old woman with a 1-month history of atypical genital bleeding was diagnosed with keratinizing squamous cell carcinoma by a cervical scraping cytology examination and was referred to the Department of Gynecology in our hospital. Pelvic computed tomography (CT) revealed a heterogeneous enhancing mass (62 × 40 mm) in the cervix and the proximal part of the vagina (Fig. 1A). She also presented with dyspnea on exertion that had started 1 month earlier. She was referred to our department and admitted for evaluation and treatment.
Fig. 1.
Pelvic computed tomography (CT) scan and chest radiograph on admission. (A) The CT scan showed a 62 × 40-mm cervical lesion without parametrial invasion. The tumor was mostly localized at the anterior lip of the cervix, which was enhanced heterogeneously. (B) The chest radiograph showed a marked loss of volume and diffuse ground-glass opacities, especially in the lower lung fields.
Fine crackles were audible in the bilateral middle and lower lung fields. On examination of her hands, hyperkeratotic lesions were seen predominantly involving the palmar surface of the fingers (“mechanic's hands”), with the presence of scaly erythematous eruptions (Gottron's papules) on the extensor surface of the proximal interphalangeal and metacarpophalangeal joints. Neurological findings showed no weakness of her proximal muscles on a manual muscle test.
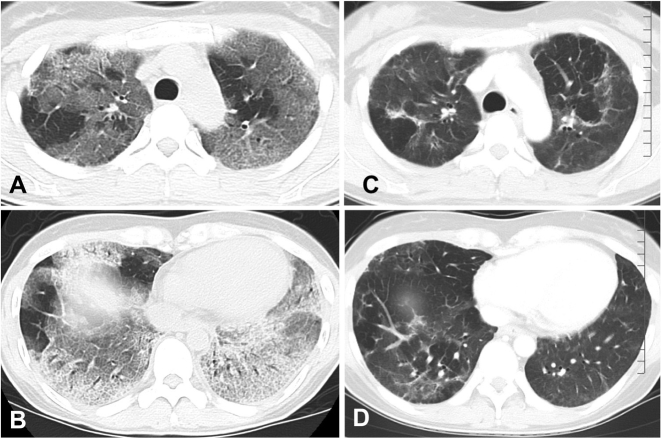
A chest radiography showed marked bilateral volume loss and a diffuse reticular pattern that was more predominant in the lung base than in the apex (Fig. 1B). Chest high-resolution CT scans demonstrated widespread ground-glass opacities with reticulations and traction bronchiectasis in both lungs, suggesting ILD (Fig. 2A and B).
Fig. 2.
Changes in chest computed tomography (CT) scan findings before and 3 months after the initial treatment. (A, B) Before the treatment, the CT scan showed bilateral diffuse ground-glass opacities, reticulation opacities, and traction bronchiectasis. (C, D) Three months after the treatment, the ground-glass opacities and traction bronchiectasis had decreased dramatically.
Laboratory findings showed elevated serum lactate dehydrogenase (LDH) and KL-6 levels, at 418 U/l (112–213 U/l) and 906 U/ml (105.3–401.2 U/ml), respectively. In contrast, the levels of creatine kinase and myoglobin were not elevated. The ferritin level was also within the normal range. Blood levels of endotoxin, procalcitonin, and β–D glucan were below the detectable limits. Antinuclear and anti-ARS antibodies, including the anti-Jo-1 antibody, were negative. Interestingly, the anti-MDA-5 antibody was detected by immunoprecipitation assay, although the anti-TIF1-γ antibody was negative. Bronchoalveolar lavage (BAL) cellular analysis revealed that lymphocytes had increased to 24.5% and neutrophils to 8.5%, whereas the CD4/CD8 ratio in BAL lymphocytes had decreased to 0.15. Arterial blood gas analysis and pulmonary function tests on admission showed decreased PaO2 (66.4 Torr), decreased vital capacity (60.5% predicted value), and low diffusion capacity of the lung for carbon monoxide (43.3% predicted value). Skin biopsy of a papule developing on the hand showed perivascular infiltrates of inflammatory cells in the dermis, consistent with the characteristic histological changes associated with DM. We therefore diagnosed the patient as having rapidly progressive ILD due to anti-MDA-5 antibody-associated CADM and cervical cancer.
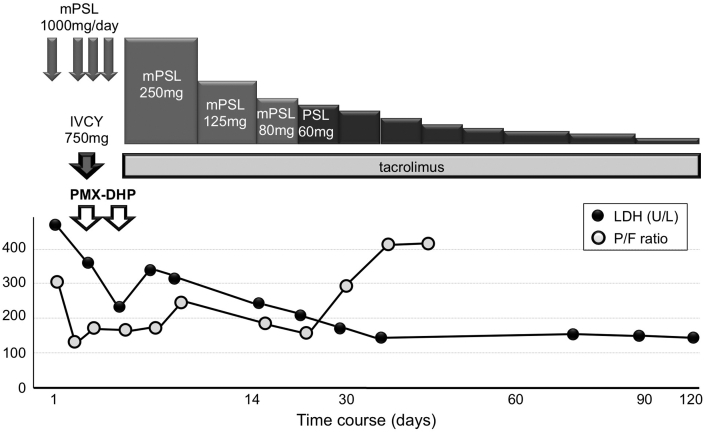
Medical treatment for the rapidly progressive ILD with respiratory failure was needed before surgery for cervical cancer. The patient was administered a combination of a 3-day course of pulse methylprednisolone (1000 mg/day), pulse cyclophosphamide (750 mg/day), and tacrolimus (3–4 mg/day, dose-adjusted for a target trough level of 5–10 ng/ml). However, her respiratory symptoms and signs deteriorated rapidly, and PaO2/FiO2 (P/F) ratio decreased from 316.1 (PaO2 of 66.4 Torr on room air) to 147.6 (PaO2 of 73.5 Torr on venturi mask at FiO2 0.5). PMX-DHP was therefore administered at a flow rate of 100 ml/min for 4 h once daily for 2 successive days (Fig. 3). One week after the PMX-DHP therapy, the P/F ratio improved to 223.8 and serum levels of LDH decreased to 200 U/l. Thereafter, maintenance treatment with tapered corticosteroids and tacrolimus were continued. The patient gradually recovered. Three months after the PMX-DHP therapy, a chest CT showed a remarkable improvement in the bilateral opacities (Fig. 2C and D), and oxygen inhalation was discontinued 3 months later, even during exercise.
Fig. 3.
The clinical course of the patient. mPSL, methylprednisolone; PSL, prednisolone; IVCY, intravenous cyclophosphamide; PMX-DHP, direct hemoperfusion using polymyxin B-immobilized fiber column; LDH, lactate dehydrogenase; P/F, PaO2/FiO2.
Four months after the initial therapy, the dose of prednisolone was reduced to 25 mg/day, and tacrolimus tough level was 8 ng/ml at the dose of 2 mg/day. Thereafter, the patient underwent radical hysterectomy and pelvic lymphadenectomy for cervical cancer. Surgical pathology demonstrated a stage 2b carcinoma. The patient then received postoperative adjuvant chemoradiotherapy. She ultimately recovered to an excellent clinical condition, with no signs of active ILD.
3. Discussion
We reported here a case of anti-MDA-5 antibody-positive and anti-TIF1-γ antibody-negative rapidly progressive ILD complicated with CADM and cervical cancer, which was successfully treated by multimodal therapy, including a combination of high-dose corticosteroids, immunosuppressants, and PMX-DHP, followed by resection of cervical cancer.
Rapidly progressive ILD with acute respiratory failure is one of the most severe and refractory complications of CADM patients, with extremely poor prognosis [1], [2]. A triple therapy approach with high-dose corticosteroids, cyclophosphamide, and cyclosporin has emerged as a recommended therapy for refractory interstitial pneumonias associated with CADM [3], but its efficacy remains limited. Previous reports have shown the efficacy of PMX-DHP on acute-onset and refractory ILD, such as acute respiratory distress syndrome and acute exacerbation of idiopathic pulmonary fibrosis [11], [12]. The present case suggests that PMX-DHP treatment may assist the effects of the combination therapy of pulse and high-dose corticosteroids and immunosuppressants in a patient with CADM-associated rapidly progressive ILD even when this complicated by a malignancy. PMX-DHP has been used for the treatment of sepsis to adsorb and eliminate blood endotoxins [11], [13], [14]. Interestingly, it has been shown to improve pulmonary oxygenation as well as hemodynamic status in patients with acute respiratory distress syndrome in both the presence or absence of blood endotoxins [11], [14]. Animal and clinical studies have suggested that another mechanism of the action of PMX-DHP is the direct or indirect removal of activated leukocytes in peripheral blood and/or inflammatory mediators, including proinflammatory cytokines [13], [14]. Interestingly, Zou et al. demonstrated that the disease activities of CADM-ILD are related to the serum level of interleukin-8 and the mRNA level of CD11b in circulating neutrophils [15]. Several case reports and retrospective studies have demonstrated that applying PMX-DHP in addition to conventional therapies was effective for the treatment of rapidly progressive interstitial pneumonias with respiratory failure, including CADM [4], [12], [14]. However, the efficacy of PMX-DHP therapy in combination with conventional therapies remains unclear.
The anti-MDA-5 antibody was initially identified as a novel autoantibody against 140-kDa proteins in CADM patients (hence its original name of “anti-CADM-140 antibody”) [5]. The present case was positive for anti-MDA-5 antibody. This antibody is associated with rapidly progressive ILD and severe cutaneous vasculopathy in patients with DM, particularly in those with CADM [5], [6], [7]. A retrospective study by Muro et al. showed that the anti-MDA-5 antibody may be a useful biomarker for ILD with CADM [9]. Another study showed that the 2-year survival rate for anti-MDA-5 antibody-positive patients was lower than that for anti-ARS antibody-positive patients or antibody-negative patients [16]. Thus, the early detection of anti-MDA-5 antibodies in ILD patients with CADM may allow for an early aggressive intervention to decrease fatalities [6], [7], [16].
The patient in the present case had a combination of cervical cancer, CADM, and rapidly progressive ILD. The anti-TIF1-γ antibody (originally known as the anti-p155 or anti-p155/140 antibody) has been identified as a myositis-specific autoantibody in DM [17]. This antibody is detected in approximately 20%–30% DM patients and 50%–100% cancer-associated DM patients, and is useful for disease management [7], [8], [17]. Furthermore, the anti-MDA-5 and anti-TIF1-γ antibodies are mutually exclusive, and the anti-MDA-5 antibody identifies a relatively low risk of malignancy-associated DM. Therefore, DM-specific antibodies such anti-MDA-5 and anti-TIF1-γ antibody define clinically distinct subtypes, and are valuable in predicting outcomes in DM. The present case was negative for the anti-TIF1-γ antibody despite having cervical cancer. To date, case reports on the association between CADM with cervical cancer have been rare [18], and it remains controversial whether this patient's cancer should be considered as relevant or accidental. We are aware of only two other reports of anti-MDA-5 antibody-positive patients with malignancy, CADM, and active ILD [19], [20]. One case did not recover from the respiratory failure after the complete removal of thyroid cancer, and the other case (with advanced gastric cancer) could not be treated because of acute respiratory failure. Unfortunately, there was no information regarding the anti-TIF1-γ antibody in either case. In patients with DM as a paraneoplastic syndrome, it is critically important to control the underlying malignancy [17]. In patients with a malignancy and rapidly progressive ILD with CADM, surgical resection of the malignancy is nearly impossible; however, in the present case, a combination therapy of high-dose corticosteroid, immunosuppressants, and PMX-DHP improved the rapidly progressive ILD, and surgical resection of the cancer was successful.
In summary, this report indicates the efficacy of PMX-DHP in combination with corticosteroids and immunosuppressants for the treatment of rapidly progressive ILD in a patient with CADM and cervical cancer. In this case, the anti-MDA-5 antibody suggested rapidly progressive ILD in a patient with CADM, but the anti-TIF1-γ antibody was negative despite the patient's cervical cancer. There are no established treatments for CADM with rapidly progressive ILD and malignancy; thus, further studies are required to determine the diagnostic and therapeutic strategies for CADM with serious complications.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgment
The authors thank Dr. Ran Sasai (Department of Department of Rheumatology and Clinical Immunology, Kyoto University Hospital) for the measurements of anti-MDA-5 and TIF1-γ antibody titers by immunoprecipitation assay in the patient.
References
- 1.Gerami P., Schope J.M., McDonald L., Walling H.W., Sontheimer R.D. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis siné myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J. Am. Acad. Dermatol. 2006;54:597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 2.Hallowell R.W., Ascherman D.P., Danoff S.K. Pulmonary manifestations of polymyositis/dermatomyositis. Semin. Respir. Crit. Care Med. 2014;35:239–248. doi: 10.1055/s-0034-1371528. [DOI] [PubMed] [Google Scholar]
- 3.Kameda H., Nagasawa H., Ogawa H., Sekiguchi N., Takei H., Tokuhira M. Combination therapy with corticosteroids, cyclosporin A, and intravenous pulse cyclophosphamide for acute/subacute interstitial pneumonia in patients with dermatomyositis. J. Rheumatol. 2005;32:1719–1726. [PubMed] [Google Scholar]
- 4.Ichiyasu H., Horio Y., Tsumura S., Hirosako S., Sakamoto Y., Sakata S. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod. Rheumatol. 2014;24:361–365. doi: 10.3109/14397595.2013.852847. [DOI] [PubMed] [Google Scholar]
- 5.Sato S., Hirakata M., Kuwana M., Suwa A., Inada S., Mimori T. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 2005;52:1571–1576. doi: 10.1002/art.21023. [DOI] [PubMed] [Google Scholar]
- 6.Gono T., Kawaguchi Y., Satoh T., Kuwana M., Katsumata Y., Takagi K. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology. 2010;49:1713–1719. doi: 10.1093/rheumatology/keq149. [DOI] [PubMed] [Google Scholar]
- 7.SL1 Tansley, Betteridge Z.E., McHugh N.J. The diagnostic utility of autoantibodies in adult and juvenile myositis. Curr. Opin. Rheumatol. 2013;25:772–777. doi: 10.1097/01.bor.0000434664.37880.ac. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino K., Muro Y., Sugiura K., Tomita Y., Nakashima R., Mimori T. Anti-MDA5 and anti-TIF1-gamma antibodies have clinical significance for patients with dermatomyositis. Rheumatology. 2010;49:1726–1733. doi: 10.1093/rheumatology/keq153. [DOI] [PubMed] [Google Scholar]
- 9.Muro Y., Sugiura K., Hoshino K., Akiyama M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology. 2012;51:800–804. doi: 10.1093/rheumatology/ker408. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentino D., Chung L., Zwerner J., Rosen A., Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J. Am. Acad. Dermatol. 2011;65:25–34. doi: 10.1016/j.jaad.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsushima K., Kubo K., Yoshikawa S., Koizumi T., Yasuo M., Furuya S. Effects of PMX-DHP treatment for patients with directly induced acute respiratory distress syndrome. Ther. Apher. Dial. 2007;11:138–145. doi: 10.1111/j.1744-9987.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 12.Abe S., Azuma A., Mukae H., Ogura T., Taniguchi H., Bando M. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med. 2012;51:1487–1491. doi: 10.2169/internalmedicine.51.6965. [DOI] [PubMed] [Google Scholar]
- 13.Ronco C., Klein D.J. Polymyxin B hemoperfusion: a mechanistic perspective. Crit. Care. 2014;18:e309. doi: 10.1186/cc13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz D.N. New trends in polymyxin B hemoperfusion: from 2006 to 2013. Blood Purif. 2014;37:9–13. doi: 10.1159/000356986. [DOI] [PubMed] [Google Scholar]
- 15.Zou J., Chen J., Yan Q., Guo Q., Bao C. Serum IL8 and mRNA level of CD11b in circulating neutrophils are increased in clinically amyopathic dermatomyositis with active interstitial lung disease. Clin. Rheumatol. 2016;35:117–125. doi: 10.1007/s10067-015-3080-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z., Cao M., Plana M.N., Liang J., Cai H., Kuwana M. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res. 2013;65:1316–1324. doi: 10.1002/acr.21985. [DOI] [PubMed] [Google Scholar]
- 17.Trallero-Araguás E., JÁ Rodrigo-Pendás, Selva-O'Callaghan A., Martínez-Gómez X., Bosch X., Labrador-Horrillo M. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64:523–532. doi: 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S., Mahajan B.B., Kaur S., Singh A. Paraneoplastic dermatomyositis with carcinoma cervix: a rare clinical association. Case Rep. Dermatol. Med. 2014:836246. doi: 10.1155/2014/836246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina-Ruiz A.M., Romero F., Carrasco L., Feltes F., Haro R., Requena L. Amyophatic dermatomyositis presenting as a flagellated skin eruption with positive MDA5 antibodies and thyroid cancer: a real association? Clin. Exp. Dermatol. 2015;40:887–890. doi: 10.1111/ced.12674. [DOI] [PubMed] [Google Scholar]
- 20.Yamaoka T., Doi C., Yokomi A., Tanemura A., Murota H., Tani M. Anti-MDA5 antibody-positive dermatomyositis with lethal progressive interstitial lung disease and advanced gastric cancer. Eur. J. Dermatol. 2014;24:490–491. doi: 10.1684/ejd.2014.2381. [DOI] [PubMed] [Google Scholar]