Abstract
Tubulin glycylation is a posttranslational modification found in cells with cilia or flagella. The ciliate Tetrahymena has glycylation on ciliary and cortical microtubules. We showed previously that mutating three glycylation sites on β-tubulin produces immotile 9 + 0 axonemes and inhibits cytokinesis. Here, we use an inducible glycylation domain mutation and epitope tagging to evaluate the potential of glycylation-deficient tubulin for assembly and maintenance of microtubular systems. In axonemes, the major defects, including lack of the central pair, occurred during assembly, and newly made cilia were abnormally short. The glycylation domain also was required for maintenance of the length of already assembled cilia. In contrast to the aberrant assembly of cilia, several types of cortical organelles showed an abnormally high number of microtubules in the same mutant cells. Thus, the consequences of deficiency in tubulin glycylation are organelle type specific and lead to either insufficient assembly (cilia) or excessive assembly (basal bodies and cortical microtubules). We suggest that the diverse functions of the β-tubulin glycylation domain are executed by spatially restricted microtubule-associated proteins.
INTRODUCTION
Microtubules (MTs), polymers made of α/β-tubulin, are subject to conserved secondary modifications, known as posttranslational tubulin modifications (PTMs) (Rosenbaum, 2000; Westermann and Weber, 2003). One modification, tubulin glycylation (TuG), was found mainly in cells with cilia or flagella (Adoutte et al., 1985; Bressac et al., 1995), and more recently in the mammalian brain microtubules (Banerjee, 2002). TuG occurs by addition of a glycine (monoglycylation) or a polyglycine chain (polyglycylation) to the γ-carboxyl group of multiple glutamic acids near the COOH termini of α- and β-tubulin (Redeker et al., 1994). The ciliate Tetrahymena has mono- and polyglycylated ciliary and cortical MTs, whereas monoglycylation also occurs on nuclear and intracytoplasmic MTs (Xia et al., 2000; Thazhath et al., 2002). Three major TuG sites could be eliminated from Tetrahymena α-tubulin without detectable changes (Xia et al., 2000), but a similar mutation on β-tubulin led to ciliary paralysis and arrest in cytokinesis (Thazhath et al., 2002). Cilia in the β-tubulin glycylation mutant lacked the central pairs (CPs), and some had incomplete peripheral doublets. The cytokinesis arrest was associated with incomplete severing of longitudinal cortical MTs (LMs) (Thazhath et al., 2002). Thus, TuG is important for proper function of diverse types of MTs, but the biochemical mechanism, as well as the timing of action of tubulin glycylation in the course of MT biogenesis is unknown.
PTMs are enriched in relatively stable organelles, including basal bodies (BBs) as well as cilia and flagella (Sasse and Gull, 1988; Adoutte et al., 1991; Bressac et al., 1995; Johnson, 1998). However, some of these “stable” microtubular organelles, such as cilia and flagella, are known to undergo tubulin subunit exchange (Rosenbaum and Child, 1967; Stephens, 1997; Marshall and Rosenbaum, 2001; Song and Dentler, 2001). Thus, the phenotypic changes observed in the TuG domain mutants could result either from defects during assembly of new organelles or lack of proper maintenance because these organelles exchange tubulin subunits. Here, we use tubulin epitope tagging in an inducible TuG domain mutant, to follow the fate of TuG deficient β-tubulin for many generations. We found that the mutant tubulin can be used for both assembly of new structures and replacement of polymerized tubulin in already assembled structures. Surprisingly, the TuG domain affects the earliest stages of assembly of cilia, resulting in the failure to initiate construction of the CP. The TuG domain mutation also decreased the rate of axonemal assembly and reduced the maximal length of both newly assembled cilia and preexisting cilia as a result of slow subunit exchange. In contrast to assembly and postassembly defects in cilia that resulted in the absence or short size of MTs, we observed overgrowth of several types of MT organelles in the cell cortex, which could be responsible for the cleavage furrow arrest. Thus, the TuG domain has multiple roles in the assembly and size regulation of complex microtubular organelles, but its action is determined by the organelle type context.
MATERIALS AND METHODS
Construction of Strains with Inducible Expression of Tagged β-Tubulin
The pTTMN plasmid (Shang et al., 2002) was used to construct the pBHM plasmid, by replacing the MTT1 gene coding region with that of BTU1 and inserting the hemagglutinin (HA) epitope tag sequence in front of the stop codon. The βDDDE440 heterokaryons (Thazhath et al., 2002) were mated and, after 24 h, transformed biolistically with the MTT1-BTU1-HA-MTT1 fragment obtained by SacI digestion of pBHM. Transformants were selected on SPP medium with paramomycin (pm, 120 μg/ml) and cadmium chloride (Cd, 2 μg/ml). The transformants (βDDDE440-CD) had a wild-type (WT) phenotype in the presence of Cd and developed the βDDDE440 mutant phenotype on medium lacking Cd. To obtain a WT control strain suitable for studies on the flux of tubulin, the β-tubulin knockout heterokaryons (Xia et al., 2000) were mated and subjected to biolistic transformation with the MTT1-BTU1-HAMTT1 fragment. Transformants that integrated the fragment into the MTT1 locus were selected in SPP with 120 μg/ml pm and 5 μg/ml Cd. A single transformant strain (Cd dependent for growth) was subjected to a second biolistic transformation with the WT HindIII-BglII BTU1 fragment, to replace the disrupted region of BTU1. The final transformants (named WT-CD) were selected that were able to grow without Cd.
Analyses of the βDDDE440 Phenotype
The constitutive βDDDE440 phenotype was induced by mating mutant heterokaryon strains (Thazhath et al., 2002). For Cd-dependent mutant phenotype induction in the βDDDE440-CD strain, cells were grown in medium containing Cd for several generations and starved for 8 h in 10 mM Tris, pH 7.5. The cell concentration was adjusted to 1 × 104 cells/ml in MEPP medium (Orias and Rasmussen, 1976) lacking Cd and copper salts. Cell concentration was maintained between 1 × 104 and 1 × 105 by periodic dilutions. For immunofluorescence (Thazhath et al., 2002), primary antibodies were monoclonal acetylated α-tubulin 6-11 B1 (1:10 dilution, generously provided by Dr. G. Piperno (Mount Sinai Medical School, New York, NY), monoclonal anti-α-tubulin 12G10 (1:25), polyclonal or monoclonal anti-HA (BD Biosciences Clontech, Palo Alto, CA; 1:100), and polyclonal anti-total Tetrahymena tubulin (SG) antibodies (1:200). Secondary antibodies were goat anti-mouse fluorescein isothiocyanate, and goat anti-rabbit-Cy3 (Zymed Laboratories, South San Francisco, CA) conjugates (1:100). Cells were viewed using a Leica TCS SP2 spectral confocal microscope with coherent Ti:sapphire multiphoton laser (Mira Optima 900-F). The length of cilia was measured using NIH Image software with the “Measurements” macro. Only relatively straight cilia were measured. In some experiments deciliation was carried out before immunofluorescence as described previously (Calzone and Gorovsky, 1982). Transmission electron microscopy (TEM) was done as described previously (Jerka-Dziadosz et al., 2001).
RESULTS
The β-Tubulin Glycylation Domain Is Required for Assembly of the Central Pair and Affects Both the Rate of Assembly and Length of Assembled Axonemes
Previously, we showed that replacement of the normal β-tubulin gene by a glycylation deficient β-tubulin gene in Tetrahymena leads to the presence of paralyzed 9 + 0 cilia and multiple arrests in cytokinesis (Thazhath et al., 2002). Because the TuG domain mutation was lethal, previously we used mating of heterokaryons as a means of bringing the lethal mutant phenotype to expression. Most of the microtubular systems in Tetrahymena, including cilia and BBs, are duplicated without resorption of old structures. Thus, the glycylation-deficient tubulin could be affecting the assembly of new organelles, the maintenance of old organelles, or both processes. To distinguish between the assembly and postassembly phenomena, we developed a new approach for induction of the TuG domain mutation in vegetative cells and epitope tag-based tracking of WT and mutant proteins in the same Tetrahymena cells over many generations.
The βDDDE440-CD strain (see Materials and Methods for details on strain construction) has two β-tubulin genes: a βDDDE440 TuG domain mutant gene located at the normal, β-tubulin-encoding BTU1 locus and a second Cd-inducible, WT β-tubulin coding region with a COOH-terminal HA tag in the MTT1 locus (Shang et al., 2002). In the βDDDE440 mutation, three adjacent glutamic acids homologous to the major glycylation sites in Paramecium (Vinh et al., 1999) (positions 437-439) are replaced with nonmodifiable aspartic acids (Thazhath et al., 2002). In the presence of Cd, the βDDDE440-CD cells produced a mixture of mutant and WT tubulin and exhibited a WT phenotype, in agreement with the recessive character of the βDDDE440 mutation (Thazhath et al., 2002). After removal of Cd, the βDDDE440-CD cells expressed mainly mutant tubulin and gradually developed the βDDDE440 phenotype similar to the phenotype described previously for constitutive mutants (Thazhath et al., 2002): they divided normally only two to three times (Figure 1B) before losing motility and arresting repeatedly in cytokinesis, forming characteristic subdivided chains of subcells (Figures 2 and 3). Although nearly all βDDDE440-CD cells underwent a single cytokinesis arrest, forming chains made of two subcells, only 5-10% formed chains with three or more subcells. The milder phenotype of the βDDDE440-CD mutants compared with the constitutive mutants analyzed previously (Thazhath et al., 2002) could be the result of production of some WT-HA β-tubulin even without Cd, due to the basal activity of the MTT1 promoter (Brown et al., 2003). We also have constructed a control strain (WT-CD), WT in the BTU1 locus with an additional WT-HA-tagged BTU1 coding region in the MTT1 locus. These cells showed a normal phenotype in Cd, indicating that overexpression of tagged β-tubulin was not responsible for the observed phenotypic changes in the mutant.
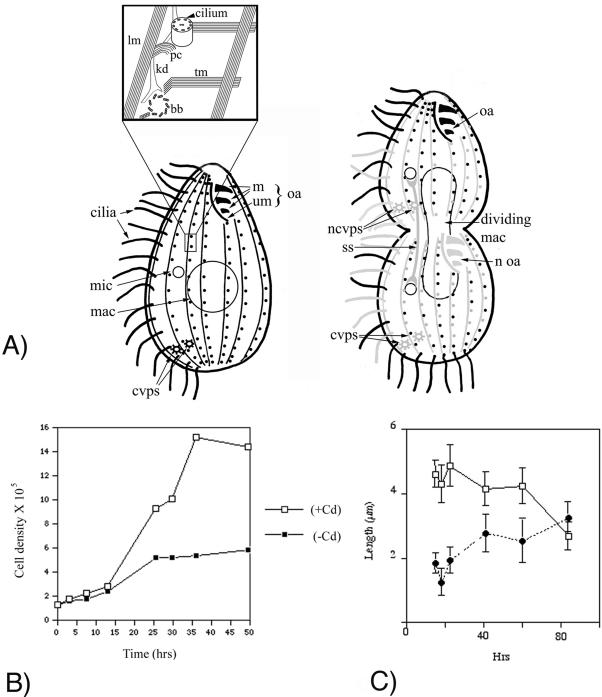
Figure 1.
(A) Schematic representation of MT-based organelles in Tetrahymena. The intracytoplasmic, macronuclear, oral fiber, and cytoproct MTs are not shown. An interphase (left) and dividing (right) cell are shown. The inset shows a magnification of a region of the cortex surrounding two adjacent BBs. In the dividing cell, we distinguish between preformed (black) and newly made organelles (gray). We also use gray to depict preformed organelles with a relatively high rate of subunit exchange, even though some of them are maintaining structural integrity (LMs and CVPs). Note the presence of an anterior and posterior invariant zone in the dividing cell. ss, separation spindle (anaphase B) of the micronucleus; mic, micronucleus; mac, macronucleus; cvps, contractile vacuole pores; ncvps, new contractile vacuole pores, m, oral membranelles; um, undulating membrane; oa, oral apparatus; noa, new oral apparatus; lm, longitudinal MT; tm, transverse MT; kd, kinetodesmal fiber (nonmicrotubular); pc, postciliary MT; bb, basal body. (B) Growth of βDDDE440-CD cells in the presence (open squares) and absence of Cd (solid squares). The mutant cells undergo only two to three complete cell divisions before undergoing a cytokinesis arrest. (C) Length of old (rectangles) and new cilia (filled circles) in the βDDDE440-CD cells as a function of time of incubation in the medium lacking Cd. Data points represent average length of cilia (±SD), calculated for 20-50 cilia per each time point.
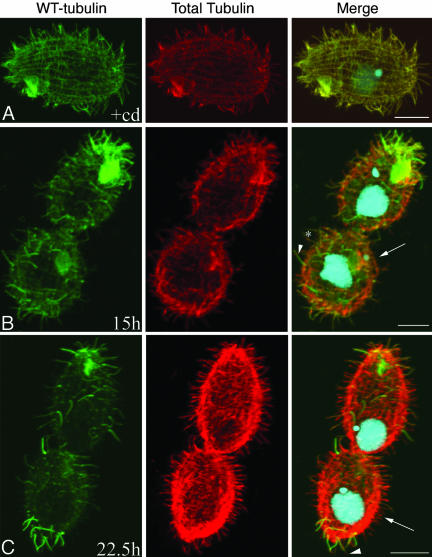
Figure 2.
Tubulin exchange in βDDDE440-CD cells after Cd removal. Cells were stained with a combination of anti-HA (green) and anti-tubulin antibodies SG (red) at 0 (A), 15 (B), and 22.5 (C) h after Cd removal. Structures with brighter green signal represent “older” organelles enriched in WT tubulin. (A) Cell grown for ∼2 wk in Cd, showing uniform distribution of WT-HA and total tubulin. (B) Note older cilia showing uniform HA label (arrowhead) and cilia with lower level of HA tubulin that were formed sometime after Cd removal (asterisk). Also, note regions devoid of cilia (arrow). (C) Note the presence of a posterior invariant zone. Arrowheads show old cilia with a distal tip made up mainly of mutant tubulin. Arrows indicate new and short cilia that are made primarily of mutant tubulin. Bar, 8 μm.
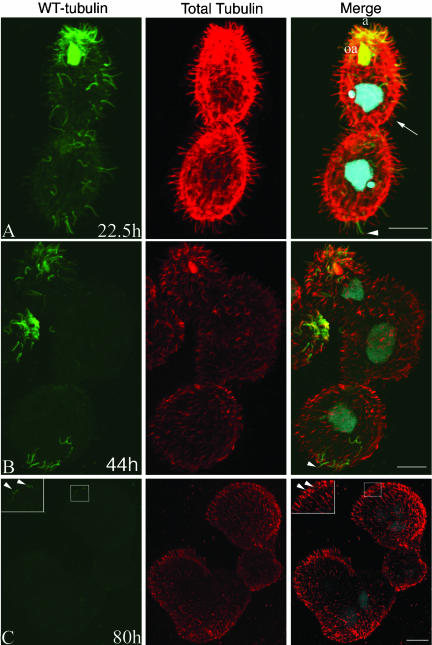
Figure 3.
Tubulin exchange in βDDDE440-CD cells after Cd removal (part of the same experimental series as in Figure 2). Cells were stained at 22 (A), 44 (B), and 80 (C) h after Cd removal. Note the presence of an anterior invariant zone in A (a) associated with conserved cilia of membranelles of the anterior oral apparatus (oa). Arrowheads show old cilia with a distal tip made up mainly of mutant tubulin. Arrows indicate new and short cilia that are made primarily of mutant tubulin. (B) Cell composed of three subcells. An anterior end of another cell is seen on the left side. Note conservation of old cilia in the anterior and posterior invariant zones of the chain. Cilia made of primarily mutant tubulin are much shorter compared with older cilia retaining WT-HA tubulin. Tubulin subunit exchange occurs at tips of older cilia (arrowhead). (C) Cell that has failed to divide twice, producing four subcells. Note the nearly complete absence of HA label, indicating nearly complete exchange with mutant tubulin. A few shortened old cilia are present at the anterior end and the HA label of old tubulin is distributed uniformly (inset in both anti-HA and merged panels). Bar, 8 μm.
Because the βDDDE440-CD mutants express both HA-tagged WT tubulin and the nontagged βDDDE440 mutant tubulin, we could trace the relative localizations of both tubulins in the same cells. We initially grew either the βDDDE440-CD mutants or WT-CD control cells in the presence of Cd for many generations to achieve uniform labeling of all MTs with WT-HA tubulin (Figure 2A for the mutant cells). To shut down the WT-HA β-tubulin production, cells were transferred to a medium lacking Cd. Both types of cells were periodically diluted into a fresh medium to maintain optimal cell density and analyzed by double immunofluorescence by using anti-HA and general anti-total tubulin antibodies.
In the βDDDE440-CD cells, at 15 h (∼3 generations) after Cd removal, we observed two types of normal length cilia, distinguishable by the intensity of the HA signal relative to the total tubulin. Some cilia showed strong (Figure 2B, arrowheads), whereas other cilia showed weaker HA labeling (Figure 2B, asterisk), in both cases uniform along the entire length. The control WT-CD strain showed a similar pattern of cilia labeling (Figure 4A). In Tetrahymena, new BBs form within rows of somatic cilia by a so-called templated mechanism in which they assemble in proximity to old BBs (Allen, 1969). After they form, new BBs are inserted anterior to old ones within the ciliary row and undergo ciliation. Neither the old BB nor its cilium is resorbed (Figure 1A). Thus, the simplest interpretation of this staining pattern is that the strongly HA-positive cilia are old cilia formed before Cd removal, whereas the weakly labeled cilia were formed after Cd removal and contain higher amounts of mutant tubulin.
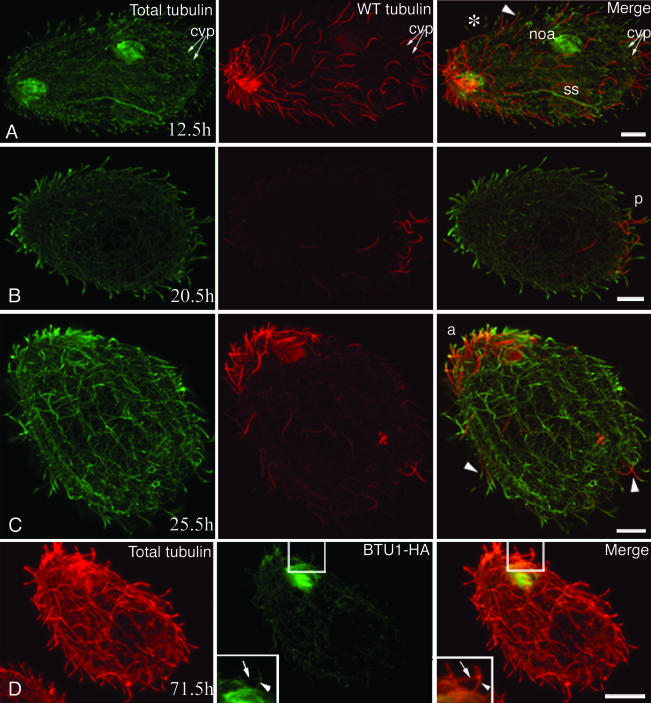
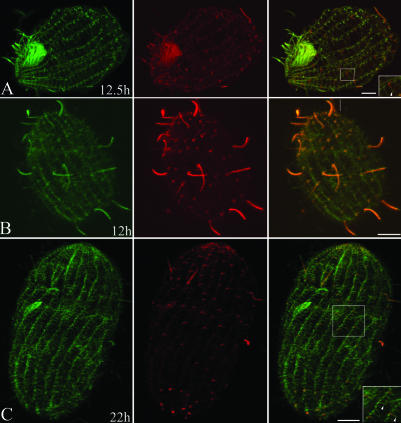
Figure 4.
Tubulin exchange in WT-CD cells. Cells in A-D were labeled by immunofluorescence by using 12G10 monoclonal antibodies (green) against total α-tubulin and anti-HA tag antibodies (red). Note that inverted colors are used to denote WT and total tubulin compared with Figures 2 and 3. After Cd washout, structures with stronger red signal represent old organelles. The cells were analyzed at 12.5 (A), 20.5 (B), 25.5 (C), and 71.5 (D) h after Cd removal. (A) Dividing cell. Note the presence of old cilia (arrowhead) assembled before Cd removal. The asterisk indicates a cilium formed after Cd removal, which has a lower HA-tagged tubulin content. The newly formed OA (noa) and the more dynamic structures such as the mitotic micronuclear separation spindle (ss) are less strongly labeled with the anti-HA antibodies (compare left and middle). The contractile vacuole pores (cvp) already show a much lower intensity of HA labeling compared with adjacent cilia. (B and C) Note the presence of a posterior invariant zone in B (p) and an anterior invariant zone in C (a). In C, arrowheads indicates cilia with reduced HA labeling at their tips, indicating tubulin exchange. (D) Cell labeled at 71.5 h with monoclonal anti-HA (green) and polyclonal anti-total tubulin (red) antibodies. This image shows that old cilia undergo subunit exchange along their entire length. The arrowhead in the inset points to a cilium that still labels with the anti-HA antibody along the length of the cilium, although weakly. For comparison, the arrow in the inset points to an adjacent cilium that does not label with the anti-HA antibody. Bar, 8 μm.
Also at 15 h, large areas devoid of cilia occurred in the mutant, mainly in the equatorial region (Figure 2B, arrow). Thus, either BB formation or ciliogenesis was affected in this region. The duplication of BBs seemed normal based on a comparison of immunofluorescence data to ultrastructure of constitutive mutants with the same number of subcells (constitutive mutants were used for TEM due to higher proportion of mutant cells with multiple subcells). A longitudinal section of the two-subcell stage showed cortical rows with densely packed BBs (Figure 5A). A transverse section of a cell in a similar stage revealed multiple adjacent BBs lacking axonemes. These BBs had a morphologically normal transitional plate, the site from which the CP normally assembles (Figure 6A). Thus, BBs in the mutant seem to form normally but experience a delay in ciliation (see below), which was not observed in the WT-CD strain (Figure 4A).
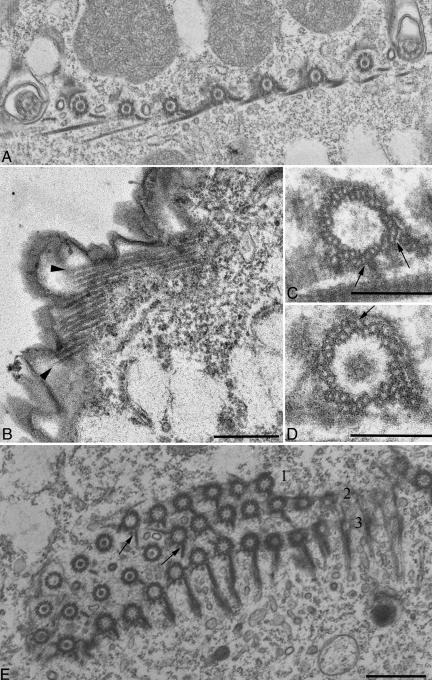
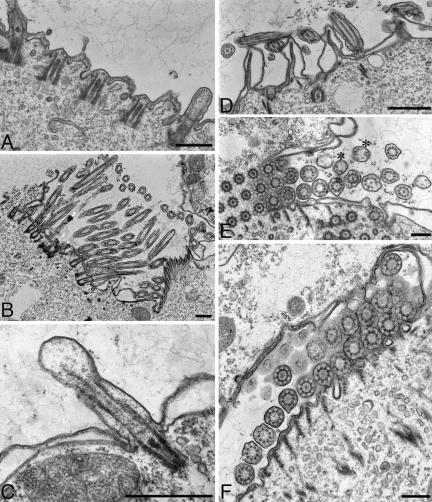
Figure 5.
TEM analysis of the βDDDE440 mutant cortex. Cells were obtained by conjugation of mutant heterokaryons, and mutant chains at the two-subcell stage were hand picked and prepared for TEM (A) Longitudinal section showing dense packing of BBs. (B) An LM bundle showing profiles of 14 MTs. (C and D) Cross sections through the BBs. Arrows point to abnormal additional MTs associated with the triplets. (E) Cross section of an OA. The membranelles of the AZM show overgrown PC ribbons (arrows) in the first and second row. The AZM rows are labeled 1, 2, and 3, respectively.
Figure 6.
TEM analysis of cilia in the βDDDE440 mutants. Cells were obtained by conjugation of mutant heterokaryons and mutant chains at the two-subcell stage were hand picked and prepared for TEM. (A) Longitudinal section of the cortex showing a delay in the ciliation of new BBs. (B) Section of the newly formed OA of the posterior mutant subcell at a two-subcell stage. Virtually all newly formed cilia lack CP. (C) Section through a short mutant cilium. Note the bulbous tip and a lack of attachment of the outer doublets to the ciliary membrane. (D) Longitudinal section through the apical region of an anterior mutant subcell at a two-cell stage. The cilia shown belong to the anterior invariant zone and seem normal in every respect. (E and F) Cross sections through mutant cilia showing defective axonemal structures lacking the CP and conversion of outer doublets into singlets (asterisks).
By 22 h (corresponding to 3-4 generations in WT), the mutant cortex was covered with new cilia made primarily of mutant tubulin (Figures 2C and 3A, arrows), which were much shorter than cilia enriched in WT tubulin. TEM of the two-subcell mutants revealed almost exclusively very short cilia lacking CP in the mid- and posterior sectors of cells (Figure 6, A-C). Particularly striking are images of newly developing ciliary oral membranelles composed exclusively of very short 9 + 0 axonemes, indicating that the CP is absent during the earliest stages of axoneme assembly (Figure 6, B, E, and F). Some new cilia had swollen tips with doublet MTs terminating prematurely (Figure 6C). Finally, some cilia had peripheral singlets with missing B-tubules (Figure 6E) as we found previously (Thazhath et al., 2002).
The new cilia made mainly of mutant tubulin elongated slowly over the period of 80 h, but their length reached only ∼60% that of normal cilia (Figure 1C). Thus, the mutant tubulin can be used to assemble new axonemes but the resulting structures are short and incomplete.
TuG Deficient β-Tubulin Reduces the Size of Assembled Cilia by Slow Subunit Renewal
In both mutant and WT cells, some cilia retained a high content of HA tubulin and normal length for many generations after Cd removal. Large clusters of such old cilia were particularly prominent at the anterior end of mutants (Figure 3A, a). Electron micrographs at the two-subcell mutant stage showed ultrastructurally normal cilia concentrated in the apical anterior region of mutant cells (Figure 6D). Thus, in the “old” anterior cilia, the presence of a normal 9 + 2 structure correlates with retention of WT tubulin. Some cells also had a smaller posterior region enriched in old cilia (Figure 2C, arrowhead). At about the same time we also observed WT-CD cells with either an anterior or posterior group of old cilia (Figure 4, B and C). Similar “invariant zones” were detected in Paramecium (Iftode et al., 1989) and result from lack of insertion of new BBs in the areas close to the cell extremities as the cell doubles its size before division (Figure 1A, dividing cell on the right).
In the mutants with an anterior invariant region, the ciliary membranelles of the oral apparatus (OA) labeled strongly with anti-HA antibodies, indicating little exchange (Figure 3A, oa). In contrast, cells with a posterior invariant zone invariably had a weak HA signal in the OA (Figure 2C). A similar correlation was seen for WT-CD cells (Figure 4, B and C). Before cell division, a new OA is formed in the equatorial region (Figure 1A). At the same time, the old OA undergoes only limited remodeling while it retains the bulk of old structures (Frankel, 1999). Thus, the cell with an anterior invariant region and largely conserved OA is likely to be the anterior daughter whereas the cell with the posterior invariant region and new OA is likely derived from the posterior daughter of the previous cell cycle (Figure 1A). A WT example of a dividing cell at a relatively early stage of epitope dilution that separates daughter cells with an anterior and a posterior invariant zone is shown in Figure 4A. Based on the similarity of patterns of regions enriched in old tubulin between mutants and WT cells, it seems that TuG has no effect on the pattern of insertion of new ciliary units and conservation of the content of old structures.
Importantly, old cilia in both the βDDDE440-CD and WT-CD strain showed incorporation of newly made tubulin. At 22-44 h (3.5-7 generations in WT), many old cilia of mutant cells had short segments at their tips that were devoid of WT-HA tubulin but were labeled by the general anti-tubulin antibody, indicating that they were made predominately of new βDDDE440 tubulin (Figures 2C and 3, A and B, arrowheads). Similar kinetics of exchange at the tips of old cilia was seen in WT-CD cells (Figure 4C, arrowheads). Thus, there seems to be no defect in the ability of mutant tubulin to be targeted to the tips of preformed cilia.
At 72 h (12 generations), few WT cells showed only extremely weakly HA-positive cilia within the anterior or posterior invariant zone, with a trace of HA-tagged tubulin uniformly distributed along the axoneme (Figure 4D, inset). The observed frequency of such cells roughly matched the expected frequency based on exponential growth and dilution of the starting cells. Thus, in WT cells, old cilia persist for many generations, but the relative content of old tubulin decreases along the entire length of cilia.
The mutant cells showed a similar pattern of exchange in old cilia. At 80 h in the mutant cells composed of three to four subcells, HA signal of WT tubulin in the old cilia was barely detectable but clearly above the background seen in newly assembled cilia located outside of the invariant zones. This reduction in HA signal also occurred uniformly along the length of the mutant axoneme (Figure 3C, inset). Strikingly, with increased content of mutant tubulin we observed gradual shortening of old cilia, which eventually reached the size of cilia made de novo primarily of mutant tubulin (Figure 1C). Thus, cilia made primarily of mutant tubulin are short regardless of whether they were assembled de novo from mutant tubulin or obtained a high content of mutant tubulin by postassembly renewal. Because they are infrequent and short, we were unable to verify whether the shortened old cilia retained their CP but that seems unlikely because at 85 h the mutant chains were completely paralyzed and examination with differential interference contract showed no movement of cilia at the cell ends (unpublished data).
The TuG Domain of β-Tubulin Does Not Affect the Exchange of Tubulin in Cortical Organelles
Like cilia, cortical MT organelles are glycylated (Thazhath et al., 2002) and are relatively stable. The question arises whether, like cilia, the cortical organelles also undergo slow subunit exchange and whether lack of glycylation affects this process. To determine whether cortical tubulin exchange occurs, we partially deciliated cells, before immunofluorescence. The pattern of tubulin flux in the cortex of WT cells was similar to that in cilia, with a clear anterior and a less prominent posterior invariant zone (Figure 7A). Similar invariant zones were seen in the mutant strain (Figure 7, B and C). Furthermore, in both strains, old cortical MTs undergo slow exchange. Many old transverse MT bundles (TMs) showed tips with higher contents of mutant tubulin compared with the region of the same bundle more proximal to the BB (Figure 7, A-C, inset). Thus, subunit exchange in TMs is initiated distal to the BB and proceeds to the BB's proximal end, as in cilia.
Figure 7.
Tubulin exchange in the cortex of βDDDE440-CD and WT-CD cells. Cells were partly deciliated to enable visualization of cortical MTs. The WT-CD cell in A was labeled with anti-HA (red) and 12G10 anti-α-tubulin antibodies (green). The βDDDE440-CD cells in B and C were labeled with anti-HA (red) and 6-11-B-1 anti acetylated α-tubulin (green). (A) WT-CD cell 12.5 h after Cd removal. Note scattered old cortical units indicated by stronger anti-HA labeling. Inset, tubulin exchange in TMs occurs at the distal tips. (B and C) βDDDE440-CD cells labeled at 12 and 22 h, respectively. Note the cluster of older cortical units in the anterior (in B) and in the posterior region (in C). The inset in C shows TMs exchanging subunits at distal tips as in A.
The subunit exchange in LMs was more rapid compared with TMs in both types of cells, because LMs labeled only weakly with the HA antibody by 12 h (Figure 7, A and B). The contractile vacuole pore (CVP) also underwent rapid subunit exchange because no HA labeling was detectable at 12 h (2 generations) (Figure 4A, note that the cell shown has both an anterior and a posterior invariant zone, indicating that the posterior CVPs in this cell were formed before Cd removal). Thus, the rates of tubulin exchange in the cell cortex are organelle specific and the subunit targeting, exchange pattern, and rates seems unaffected in the glycylation-deficient β-tubulin.
Multiple Types of Cortical Microtubular Organelles in the DDDE440 Mutants Are Hypertrophic
At late times after Cd removal, the density of cilia in mutants increased dramatically compared with WT-CD cells (Figure 3, B and C). This correlated with an increased density of BBs within cortical rows (Figure 5A). Although the majority of BBs were ultrastructurally normal, some showed apparent additional MTs associated with the triplets (Figure 5, C and D, arrows). Additional basal body MTs were not seen in WT cells.
The organization of LMs in the mutants was of special interest because we found previously that these MTs fail to sever during cytokinesis and therefore could obstruct the contractile ring and lead to cytokinesis arrest. Indeed, by ultrastructural analysis, we found that LM bundles in the mutants at the two-subcell stage contained almost twice as many (11.7 ± 3.7 MTs, n = 37) MTs as WT (6.3 ± 1.8 MTs, n = 12). Figure 5B shows an LM of the βDDDE440 mutant with a profile of 14 MTs (arrows). This hypertrophy of LMs could be directly related to their impaired severing during cytokinesis.
Tetrahymena has a complex OA comprised of four compound ciliary structures with ∼120 cilia. The undulating membrane (UM) is made of a double file of BBs, of which only one is ciliated. Three membranelles, each consisting of three files of BBs (adoral zone of membranelles, AZM), are located to the cell's left of the UM (Figure 1A) (Frankel, 1999). In a WT AZM, among the three rows of BBs, only the most posterior one has well developed postciliary (PC) MTs, whereas in the two more anterior rows PC is limited to a single MT (Jerka-Dziadosz, 1981). Strikingly, PCs in some mutant OAs, the anterior and middle AZM rows had PCs with multiple MTs (Figure 5E). Thus, several types of microtubular organelles, including LMs, BBs, and PCs, show hypertrophic features in the mutant cortex. This is in striking contrast to cilia, which either lack or have abnormally short MTs. Thus, the consequences of lack of proper glycylation sites in the cell cortex are opposite in nature to what is observed in cilia in the same cells.
DISCUSSION
This article describes the dynamics of MTs in Tetrahymena at the whole cell level and evaluates the potential of glycylation-deficient β-tubulin in assembly and long-term maintenance of MT organelles. We showed that the ciliary and cortical cytoskeleton of normal Tetrahymena is a complex mosaic of organelles that contain varying ratios of tubulins of different age. The older ciliary and cortical units are present in two clusters: at the anterior end and posterior end of the cell. These invariant zones represent territories with a low probability of insertion of new BBs and therefore with higher retention of old BBs and associated cilia (Figure 1). Our results agree with the previously observed preferential insertion of new BBs in the equatorial region of Tetrahymena (Nanney, 1975; Kaczanowski, 1978; Frankel et al., 1981). The posterior and anterior invariant zones have been extensively documented in another ciliate Paramecium tetraurelia (Iftode et al., 1989).
We showed that cilia and cortical units of invariant zones undergo relatively slow tubulin subunit exchange. The observed retention of tubulin in assembled organelles over many generations is in agreement with an autoradiographic profile of total protein studied in the ciliate Oxytricha fallax (Grimes and Gavin, 1987). We observed that in Tetrahymena cilia, nearly complete replacement of tubulins required ∼80 h, which is equivalent to ∼13 generations in WT cells under the conditions used here. Thus, assuming that the rate of the process was constant, we observed an exchange of ∼1%/h. Earlier pulse-chase studies in Tetrahymena pyriformis showed a similar low turnover rate of 2.25%/h (Nelsen, 1975). Studies on flagella also indicated a slow rate of exchange in Chlamydomonas (Gorovsky et al., 1970). A more recent study in Chlamydomonas showed a higher turnover rate of at least 20% of total flagellar proteins within 6 h (Song and Dentler, 2001).
A study of tubulin exchange in Chlamydomonas flagella by using tagged tubulin in dikaryons showed that preformed flagella exchange tubulin subunits within the distal one-third of the structure (Marshall and Rosenbaum, 2001). In that study, tubulin subunit exchange beyond the distal one-third could not be examined, owing to complete resorption of flagella in Chlamydomonas dikaryons in preparation for meiosis. Although we observed exchange at the distal tips of axonemes, over longer times we demonstrated exchange occurred along the entire axonemal length. A similar pattern of exchange along the axoneme was observed for a component of the paraflagellar rod in preformed flagella of Trypanosoma (Bastin et al., 1999).
We detected major differences between the rates of subunit exchange among subtypes of stable ciliary and cortical MTs. More rapid subunit exchange was found in the cortical LMs. Interestingly, in Paramecium, the homologous structure (Sundaraman and Hanson, 1976), subsequently named the “cytospindle” (Cohen et al., 1982) is seen only transiently during cell division (Iftode et al., 1989). Although in Tetrahymena LMs persist during the entire cell cycle, they exchange subunits more rapidly than other cortical structures, which is in agreement with the dynamic character of cytospindle in Paramecium. Furthermore, CVP MTs also exchange tubulin more rapidly compared with other cortical MTs and cilia. Although the differences in the exchange rates of specific types of cortical structures likely reflect different functions of these organelles, its molecular basis is not known.
We observed a strong effect of the TuG domain on ciliary assembly. This result is surprising because PTMs are widely considered as postassembly events that lead to gradual accumulation of modified tubulins as the organelle matures. Because the levels of preexisting WT tubulin were depleted in the βDDDE440-CD strain, assembly of the entire axoneme was inhibited. With a delay, however, new cilia formed from mutant tubulin but they were very short and lacked the CP. Because the Cd-dependent mutants had residual content of WT β-tubulin due to basal expression of the MTT1 promoter, it is possible that the axonemes would not form if WT β-tubulin was eliminated. This is unlikely because constitutive mutants (progenies of heterokaryons) after seven to eight generations since the complete shutoff of WT-tubulin, are covered with densely packed short cilia (Figure 1G in Thazhath et al., 2002). The fact that both the CP and the outer doublets were affected to some extent is consistent with the presence of TuG in both structures (Pechart et al., 1999). The shortness of axonemes deficient in TuG is probably a consequence of low TuG content rather than the absence of CP, because Chlamydomonas mutants that lack most of the CP components (pf-18) have normal length flagella (Warr et al., 1966; McVittie, 1972). However, flagella of the pf-18 mutant have a residual non-MT material in the center of the axoneme (Adams et al., 1981), opening a possibility that non-MT components of CP are required for axoneme size regulation.
The lack of CP is the most striking defect in the newly formed axonemes deficient in TuG. We can propose three explanations for why deficiency in TuG leads to a failure to form CP and not doublets. One possibility is that TuG affects intraflagellar transport (IFT), the motility system operating inside cilia and flagella that delivers components from the cell body (Rosenbaum and Witman, 2002). Short doublet MTs (but not CP) can be assembled in Tetrahymena IFT mutants (Brown et al., 1999b, 2003) and in sea urchin eggs in which IFT was inhibited by antibodies (Morris and Scholey, 1997). Thus, CP assembly could be more dependent on IFT than assembly of the doublets. Furthermore, it has been suggested that the relative rates of anterograde and retrograde IFT pathways regulate the steady-state length of flagella (Marshall and Rosenbaum, 2001). Thus, defective anterograde IFT could lead to slow assembly and short size of doublets.
A second explanation reflects the unique mode of CP nucleation. The CP MTs are nucleated from the transitional plate, whereas the peripheral MTs are extensions of the triplets of the BB. A deficiency in γ-tubulin led to assembly of 9 + 0 axonemes in Trypanosoma (McKean et al., 2003), indicating CP MTs are more sensitive to lack of γ-tubulin than doublets. TuG could be involved in the nucleation of CP MTs within the transitional region in conjunction with γ-tubulin.
Finally, it is possible that reduction in TuG affects the ends of growing axonemal MTs, making the CP less stable. CP formation involves attachment of the plus end of growing MTs to the ciliary membrane via capping complexes (Dentler and Rosenbaum, 1977; Sale and Satir, 1977). In agreement with this hypothesis, some mutant cilia had highly disorganized doublets near the tips (Figure 6C). The tip of the axoneme has a unique biochemical composition, including the presence of an MT end-associated protein, EB1 (Pedersen et al., 2003). TuG could participate in association of the cap-specific proteins with the ends of axonemal MTs. Capping complexes also connect the A tubules of doublets with the ciliary membrane (Dentler, 1980). However, doublets may still polymerize to some extent without a proper attachment due to their higher intrinsic stability.
It was striking that even the youngest newly formed mutant axonemes lacked CP, suggesting a function for TuG during an early stage of assembly. Previous studies showed that longer chains of polyglycine accumulate with a delay after assembly of cilia (Adoutte et al., 1991; Bressac et al., 1995). Thus, it is possible that the observed TuG function during the early stage of axonemal assembly involves relatively short chains of polyglycine or even monoglycylation. In agreement with this hypothesis, antibodies specific to monoglycylated tubulins (Bre et al., 1996) reacted more strongly with the assembling cilia, compared with longer mature cilia of the same Tetrahymena (Brown et al., 1999b). Tubulins with a relatively low content of glycylation may be optimal for IFT and early assembly events, whereas long chain glycylation may have distinct functions after assembly. One possible function of long chain glycylation is in the regulation of ciliary beating, based on the inhibitory effect of a monoclonal antibody specific to polyglycylation on axoneme bending in vitro (Bre et al., 1996). Thus, the gradual accumulation and lengthening of lateral chains of polyglycine during axonemal assembly could make MTs competent for a series of distinct functions that occur successively during and after assembly.
Although the TuG domain mutation led to reduced polymerization or stability of ciliary MTs, it had a seemingly opposite effect on cortical MTs. We observed an increased density of BBs as well as supernumerary MTs near BB triplets. The effect of the βDDDE440 mutation on BBs is unique because other mutations of the tubulin superfamily proteins led to the lack of specific MTs of the triplets (Dutcher, 2001). Furthermore, the cortical LMs as well as the PC MTs of oral membranelles in mutant cells contained an abnormally high number of MTs.
We should first consider that the cortical hypertrophy is secondary to ciliary defects. Lack of proper assembly of axonemal MTs could lead to an increased concentration of unpolymerized tubulin in the cell body, which could drive hyperpolymerization of MTs. However, at least in the case of LMs, this seems unlikely. Mutants that fail to assemble cilia due to the absence of IFT proteins, are able to assemble and disassemble LMs and divide normally, as long as external physical force is provided to substitute for the motile process (rotokinesis) used by Tetrahymena for abscission of the cytoplasmic bridge (Brown et al., 1999a, 2003). Thus, it is more likely that TuG has a direct function in the biogenesis of cortical MTs.
Previously, we showed that the cytokinesis defect in the βDDDE440 mutants correlates with the lack of severing of LM bundles (Thazhath et al., 2002). We show here that the mutant LM bundles are composed of an abnormally large number of MTs. In normal cells, LM bundles consists of multiple shorter MTs, which partially overlap (Pitelka, 1961). The size of individual LM MTs is regulated so that there are on average six MTs on a given LM cross section. The LMs of the βDDDE440 mutants showed roughly twice the normal number of MTs. We could not determine whether the observed LM hypertrophy is a result of increased total number of MTs or solely derived from the increased length of overlapping MTs. The cause-effect relationship between the hypertrophy of LMs and lack of their severing during cytokinesis is unclear. It is possible the observed lack of severing is the result of excessive number of MTs in LM bundles. This model is in agreement with hypertrophy of additional types of MTs in the cortex (BBs and PCs) and suggests a general cortical function for glycylation in limiting polymerization of MTs. Alternatively, glycylation may be required for active severing of MTs in the equatorial region at the time of cytokinesis, and lack of proper severing could cause the excessive growth of LMs.
The fundamentally different influence of the same TuG domain mutation on cortical and ciliary MTs suggests that either TuG domain changes the intrinsic properties of different MTs in distinct ways or that the same intrinsic modification functions as a signal that can be interpreted differently by localized trans-acting mechanisms. The first hypothesis would require that the TuG content, site utilization, or lateral chain length be different between the cortical and ciliary MTs. We first should consider that among the five major sites of TuG on β-tubulin, nonidentical sites are used in the cell cortex and in cilia. However, this is extremely unlikely because cells can have a nearly normal phenotype when four of these sites are inactivated, and none of the five sites is essential, either individually or in pairs (Xia et al., 2000). Also, virtually all sites of TuG (and of tubulin glutamylation) can be eliminated on β-tubulin in cells containing an α-tubulin whose COOH-terminal tail of has been replaced with the COOH-terminal tail of β-tubulin (Duan and Gorovsky, 2002). It is still possible that the glycine side chain length differs between the cortex and cilia. Mass spectrometry revealed that the cortical and cell body tubulins of Paramecium had much shorter chains compared with axonemal tubulins (Bre et al., 1998). The second hypothesis (not mutually exclusive with the first one) postulates that TuG acts as a signal for binding distinct MAPs in distinct locations. In cilia, such organelle-targeted MAPs could have a polymerization-promoting and stabilizing effect, whereas in the cell cortex different MAPs may be required for limiting the size and number of assembled MTs.
Acknowledgments
We thank a University of Georgia-Athens undergraduate, Henry Heffler, for assistance in the construction of WT-CD strains. We are grateful to the staff of the Center for Ultrastructural Research of the University of Georgia-Athens for continuing technical support. We thank David Mitchell (SUNY Upstate Medical University, Syracuse, NY), Joel Rosenbaum (Yale University, New Haven, CT), and Elizabeth Smith (Dartmouth College, Hanover, NH) for helpful comments. The 12G10 monoclonal antibodies were raised by E. Marlo Nelsen and are available from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa. This work was supported by the National Science Foundation grant number 235826 to J.G. and a National Institutes of Health award GM-026973 to M.A.G.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-03-0247. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0247.
Abbreviations used: AZM, adoral zone of membranelles; BB, basal body; Cd, cadmium; CP, central pair; CVP, contractile vacuole pore; HA hemagglutinin; IFT, intraflagellar transport; LM, longitudinal microtubule; MAP, microtubule-associated protein; MT, microtubule; PC, postciliary microtubule; pm, paromomycin; TuG, tubulin glycylation; TM, transverse microtubule; UM, undulating membrane; WT, wild-type.
References
- Adams, G.M.W., Huang, B., Piperno, G., and Luck, D.J.L. (1981). Central pair microtubular complex polypeptide composition as revealed by analysis of mutants. J. Cell Biol. 91, 69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adoutte, A., Claisse, M., Maunoury, R., and Beisson, J. (1985). Tubulin evolution: ciliate-specific epitopes are conserved in the ciliary tubulin in metazoa. J. Mol. Evol. 22, 220-229. [DOI] [PubMed] [Google Scholar]
- Adoutte, A., Delgado, P., Fleury, A., Levilliers, N., Lainé, M.-C., Marty, M.-C., Boisvieux-Ulrich, E., and Sandoz, D. (1991). Microtubule diversity in ciliated cells: evidence for its generation by post-translational modification in the axonemes of Paramecium and quail oviduct cells. Biol. Cell 71, 227-245. [DOI] [PubMed] [Google Scholar]
- Allen, R.D. (1969). The morphogenesis of basal bodies and accessory structures of the cortex of the ciliate protozoan Tetrahymena pyriformis. J. Cell Biol. 40, 716-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, A. (2002). Coordination of posttranslational modifications of bovine brain alpha-tubulin. Polyglycylation of delta2 tubulin. J. Biol. Chem. 277, 46140-46144. [DOI] [PubMed] [Google Scholar]
- Bastin, P., McRae, T.H., Francis, S.B., Matthews, K.R., and Gull, K. (1999). Flagellar morphogenesis: protein targeting and assembly in the paraflagellar rod of trypanosomes. Mol. Cell. Biol. 19, 8191-8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bré, M.H., et al. (1996). Axonemal tubulin polyglycylation probed with two monoclonal antibodies: widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J. Cell Sci. 109, 727-738. [DOI] [PubMed] [Google Scholar]
- Bré, M.H., Redeker, V., Vinh, J., Rossier, J., and Levilliers, N. (1998). Tubulin polyglycylation: differential posttranslational modification of dynamic cytoplasmic and stable axonemal microtubules in Paramecium. Mol. Biol. Cell 9, 2655-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressac, C., Bré, M.H., Darmanaden Delorme, J., Laurent, M., Levilliers, N., and Fleury, A. (1995). A massive new posttranslational modification occurs on axonemal tubulin at the final step of spermatogenesis in Drosophila. Eur. J. Cell Biol. 67, 346-355. [PubMed] [Google Scholar]
- Brown, J.M., Fine, N.A., Pandiyan, G., Thazhath, R., and Gaertig, J. (2003). Hypoxia regulates assembly of cilia in suppressors of Tetrahymena lacking an intraflagellar transport subunit gene. Mol. Biol. Cell 14, 3192-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.M., Hardin, C., and Gaertig, J. (1999a). Rotokinesis, a novel phenomenon of cell locomotion-assisted cytokinesis in the ciliate Tetrahymena thermophila. Int. Cell Biol. Rep. 23, 841-848. [DOI] [PubMed] [Google Scholar]
- Brown, J.M., Marsala, C., Kosoy, R., and Gaertig, J. (1999b). Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Mol. Biol. Cell 10, 3081-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone, F.J., and Gorovsky, M.A. (1982). Cilia regeneration in Tetrahymena. Exp. Cell Res. 140, 474-476. [DOI] [PubMed] [Google Scholar]
- Cohen, J., Adoutte, A., Grandchamp, S., Houdebine, L.M., and Beisson, J. (1982). Immunochemical study of microtubular structures throughout the cell cycle of Paramecium. Biol. Cell 44, 35-44. [Google Scholar]
- Dentler, W.L. (1980). Structures linking the tips of ciliary and flagellar microtubules to the membrane. J. Cell Sci. 42, 207-220. [DOI] [PubMed] [Google Scholar]
- Dentler, W.L., and Rosenbaum, J.L. (1977). Flagellar elongation and shortening in Chlamydomonas. III. structures attached to the tips of flagellar microtubules and their relationship to the directionality of flagellar microtubule assembly. J. Cell Biol. 74, 747-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J., and Gorovsky, M.A. (2002). Both carboxy terminal tails of alpha and beta tubulin are essential, but either one will suffice. Curr. Biol. 12, 313-316. [DOI] [PubMed] [Google Scholar]
- Dutcher, S.K. (2001). The tubulin fraternity: alpha to eta. Curr. Opin. Cell Biol. 13, 49-54. [DOI] [PubMed] [Google Scholar]
- Frankel, J. (1999). Cell Biology of Tetrahymena. Methods Cell Biol. 62, 27-125. [DOI] [PubMed] [Google Scholar]
- Frankel, J., Nelsen, E.M., and Jenkins, L.M. (1981). Development of the ciliature of Tetrahymena thermophila. II. Spatial subdivision prior to cytokinesis. Dev. Biol. 88, 39-54. [DOI] [PubMed] [Google Scholar]
- Gorovsky, M.A., Carlson, K., and Rosenbaum, J.L. (1970). Simple method for quantitative densitometry of polyacrylamide gels using fast green. Anal. Biochem. 35, 359-370. [DOI] [PubMed] [Google Scholar]
- Grimes, G.W., and Gavin, R.H. (1987). Ciliary protein conservation during development in the ciliated protozoan, Oxytricha. J. Cell Biol. 105, 2855-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftode, F., Cohen, J., Ruiz, F., Rueda, A.T., Chen-Shan, L., Adoutte, A., and Beisson, J. (1989). Development of surface pattern during division in Paramecium. 1. Mapping of duplication and reorganization of cortical cytoskeletal structures in the wild type. Development 105, 191-211. [Google Scholar]
- Jerka-Dziadosz, M. (1981). Patterning of ciliary structures in janus mutant of Tetrahymena with mirror image cortical duplications. An ultrastructural study. Acta Protozool. 20, 337-356. [Google Scholar]
- Jerka-Dziadosz, M., Strzyzewska-Jowko, I., Wojsa-Lugowska, U., Krawczynska, W., and Krzywicka, A. (2001). The dynamics of filamentous structures in the apical band, oral crescent, fission line and the postoral meridional filament in Tetrahymena thermophila revealed by the monoclonal antibody 12G9. Protist 152, 53-67. [DOI] [PubMed] [Google Scholar]
- Johnson, K.A. (1998). The axonemal microtubules of the Chlamydomonas flagellum differ in tubulin isoform content. J. Cell Sci. 111, 313-320. [DOI] [PubMed] [Google Scholar]
- Kaczanowski, A. (1978). Gradients of proliferation of ciliary basal bodies and the determination of the position of the oral primordium in Tetrahymena. J. Exp. Zool. 204, 417-430. [DOI] [PubMed] [Google Scholar]
- Marshall, W.F., and Rosenbaum, J.L. (2001). Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155, 405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean, P.G., Baines, A., Vaughan, S., and Gull, K. (2003). Gamma-tubulin functions in the nucleation of a discrete subset of microtubules in the eukaryotic flagellum. Curr. Biol. 13, 598-602. [DOI] [PubMed] [Google Scholar]
- McVittie, A. (1972). Flagellum mutants of Chlamydomonas reinhardii. J. Gen. Microbiol. 71, 525-540. [DOI] [PubMed] [Google Scholar]
- Morris, R.L., and Scholey, J.M. (1997). Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J. Cell Biol. 138, 1009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney, D.L. (1975). Patterns of basal body addition in ciliary rows in Tetrahymena. J. Cell Biol. 65, 503-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen, E.M. (1975). Regulation of tubulin during ciliary regeneration of nongrowing Tetrahymena. Exp. Cell Res. 94, 152-158. [DOI] [PubMed] [Google Scholar]
- Orias, E., and Rasmussen, L. (1976). Dual capacity for nutrient uptake in Tetrahymena. IV. Growth without food vacuoles. Exp. Cell Res. 102, 127-137. [DOI] [PubMed] [Google Scholar]
- Pechart, I., Kann, M.L., Levilliers, N., Bre, M.H., and Fouquet, J.P. (1999). Composition and organization of tubulin isoforms reveals a variety of axonemal models. Biol. Cell 91, 685-697. [DOI] [PubMed] [Google Scholar]
- Pedersen, L.B., Geimer, S., Sloboda, R.D., and Rosenbaum, J.L. (2003). The microtubule plus end tracking protein EB1 is localized to the flagellar tip and basal bodies in Chlamydomonas reinhardtii. Curr. Biol. 13, 1969-1974. [DOI] [PubMed] [Google Scholar]
- Pitelka, D.R. (1961). Fine structure and silver line and fibrillar systems of three tetrahyminid species. J. Protozool. 8, 75-89. [Google Scholar]
- Redeker, V., Levilliers, N., Schmitter, J.-M., Le Caer, J.-P., Rossier, J., Adoutte, A., and Bre, M.H. (1994). Polyglycylation of tubulin: a post-translational modification in axonemal microtubules. Science 266, 1688-1691. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, J.L. (2000). Cytoskeleton: functions for tubulin modifications at last. Curr. Biol. 10, R801-R803. [DOI] [PubMed] [Google Scholar]
- Rosenbaum, J.L., and Child, F.M. (1967). Flagellar regeneration in protozoan flagellates. J. Cell Biol. 34, 345-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, J.L., and Witman, G.B. (2002). Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3, 813-825. [DOI] [PubMed] [Google Scholar]
- Sale, W.S., and Satir, P. (1977). The termination of the central microtubules from the cilia of Tetrahymena pyriformis. Cell Biol. Int. 1, 45-49. [DOI] [PubMed] [Google Scholar]
- Sasse, R., and Gull, K. (1988). Tubulin post-translational modifications and the construction of microtubular organelles in Trypanosoma brucei. J. Cell Sci. 90, 577-589. [DOI] [PubMed] [Google Scholar]
- Shang, Y., Song, X., Bowen, J., Corstanje, R., Gao, Y., Gaertig, J., and Gorovsky, M.A. (2002). A. robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 99, 3734-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L., and Dentler, W. (2001). Flagellar protein dynamics in Chlamydomonas. J. Biol. Chem. 276, 29754-29763. [DOI] [PubMed] [Google Scholar]
- Stephens, R.E. (1997). Synthesis and turnover of embryonic sea urchin ciliary proteins during selective inhibition of tubulin synthesis and assembly. Mol. Biol. Cell 8, 2187-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaraman, V., and Hanson, E.D. (1976). Longitudinal microtubules and their functions during asexual reproduction in Paramecium tetraurelia. Genet. Res. 27, 205-211. [DOI] [PubMed] [Google Scholar]
- Thazhath, R., Liu, C., and Gaertig, J. (2002). Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat. Cell Biol. 4, 256-259. [DOI] [PubMed] [Google Scholar]
- Vinh, J., Langridge, J.I., Bré, M.H., Levilliers, N., Redeker, V., Loyaux, D., and Rossier, J. (1999). Structural characterization by tandem spectroscopy of the posttranslational modifications of tubulin. Biochemistry 38, 3133-3139. [DOI] [PubMed] [Google Scholar]
- Warr, J.R., McVittie, A., Randall, J.T., and Hopkins, J.M. (1966). Genetic control of flagellar structure in Chlamydomonas. Genet. Res. 7, 335-351. [Google Scholar]
- Westermann, S., and Weber, K. (2003). Post-translational modifications regulate microtubule function. Nat. Rev. Mol. Cell. Biol. 4, 938-947. [DOI] [PubMed] [Google Scholar]
- Xia, L., Hai, B., Gao, Y., Burnette, D., Thazhath, R., Duan, J., Bré, M.H., Levilliers, N., Gorovsky, M.A., and Gaertig, J. (2000). Polyglycylation of tubulin is essential and affects cell motility and division in Tetrahymena thermophila. J. Cell Biol. 149, 1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]