Abstract
Proteins in the transforming growth factor-β (TGF-β) family recognize transmembrane serine/threonine kinases known as type I and type II receptors. Binding of TGF-β to receptors results in receptor down-regulation and signaling. Whereas previous work has focused on activities controlling TGF-β signaling, more recent studies have begun to address the trafficking properties of TGF-β receptors. In this report, it is shown that receptors undergo recycling both in the presence and absence of ligand activation, with the rates of internalization and recycling being unaffected by ligand binding. Recycling occurs as receptors are most likely internalized through clathrin-coated pits, and then returned to the plasma membrane via a rab11-dependent, rab4-independent mechanism. Together, the results suggest a mechanism wherein activated TGF-β receptors are directed to a distinct endocytic pathway for down-regulation and clathrin-dependent degradation after one or more rounds of recycling.
INTRODUCTION
TGF-β is a ubiquitous 25-kDa polypeptide that regulates a variety of cellular processes, including matrix deposition, mitosis, development, differentiation, and apoptosis (Roberts, 1992; ten Dijke et al., 1996). The response to TGF-β treatment usually depends on the cell type involved, with effects as diverse as growth and growth inhibition (Massagué, 1996; Moses and Serra, 1996). TGF-β binds to single-pass transmembrane serine/threonine kinases referred to as type I and II TGF-β receptors (Bassing et al., 1994; ten Dijke et al., 1994). On binding of TGF-β to the constitutively active type II receptor (T2R), the type I receptor (T1R) is recruited and phosphorylated by T2R. The activated T1R then phosphorylates downstream signaling intermediates such as the Smad proteins, which translocate to the nucleus and function as transcriptional comodulators (Franzén et al., 1993; Macias-Silva et al., 1996; Yingling et al., 1996).
Study of the endocytic response of TGF-β receptors to ligand has been difficult due to nonspecific TGF-β binding and the fact that different receptor complexes form on the cell surface (heteromers vs. homomers) and undergo distinct endocytic fates (Anders et al., 1997). To overcome these problems, our laboratory created a chimeric receptor system where the ligand binding extracellular domains of the granulocyte/macrophage-colony stimulating factor (GM-CSF) receptors were fused to the transmembrane and cytoplasmic domains of the type I and type II TGF-β receptors (Anders and Leof, 1996). Using a number of well-established assays for TGF-β action, it could be shown that TGF-β signaling is fully recapitulated by the chimeric system (Anders and Leof, 1996; Anders et al., 1997, 1998). In addition, use of the chimeric system (Anders et al., 1997) facilitated TGF-β endocytic assays, which showed, similar to other studies (Ehrlich et al., 2001; Yao et al., 2002), that ligand-induced internalization of TGF-β receptor complexes occurs through a clathrin-dependent process. Other reports, however, have proposed roles for both clathrin-dependent and -independent processes in TGF-β receptor endocytosis (Zwaagstra et al., 2001; Di Guglielmo et al., 2003).
Recent work also has shown that although T1R activation can occur at the plasma membrane, downstream signaling through the Smads requires receptor internalization (Hayes et al., 2002; Penheiter et al., 2002; Di Guglielmo et al., 2003), thus illustrating the central role played by endocytosis in TGF-β signaling. However, although the above-mentioned information shows that our laboratory and those of others have begun to address the topic of receptor trafficking, the recycling of TGF-β receptors has been left largely unexamined.
Recycling, defined as the return of a particular membrane molecule to the cell surface after at least one round of internalization, is a fundamental process used by a variety of receptor systems. For example, the transferrin and low-density lipoprotein receptors recycle for the purpose of transporting critical nutrients into the cell (Trowbridge, 1991; Gliemann, 1998; Moos and Morgan, 2000; Hussain, 2001). In addition, many G protein-coupled receptors as well as synaptic vesicles are recycled back to the cell surface after ligand-dependent activation or neurotransmitter release, respectively (Guatimosim and von Gersdorff, 2002; Morgan et al., 2002; Tyler et al., 2002). Although studies addressing the recycling of TGF-β receptors are scarce, early reports proposed that internalized receptors were either rapidly replaced or recycled back to the cell surface (Massagué and Like, 1985; Sathre et al., 1991). More recent studies have shown that the T2R is constitutively internalized, but the question of whether the internalized receptors are degraded or recycled back to the plasma membrane was not addressed (Ehrlich et al., 2001; Di Guglielmo et al., 2003). Additionally, our laboratory has demonstrated that in the absence of ligand, monensin treatment resulted in a dramatic loss of surface receptor binding (Doré et al., 2001). Monensin is an ionophoric drug that disrupts degradation and trafficking pathways, but it leaves endocytosis unaffected (Mollenhauer et al., 1990). Although the results with monensin indicate that TGF-β receptors are likely internalized constitutively, they do not rule out the possibility of rapid degradation and replacement, thus failing to firmly establish a role for recycling in TGF-β receptor homeostasis.
In the current study, we set out to determine the trafficking itinerary of TGF-β receptors in both the presence and absence of ligand activation. In addition, we desired to define the rates and cellular machinery involved in these processes. The results show that 1) TGF-β receptors recycle and are degraded in a clathrin-dependent manner; 2) raft-dependent endocytosis is likely of minor importance, although it cannot formally be ruled out; 3) recycling occurs through a rab11-dependent mechanism only (unlike transferrin, which recycles through both rab11 and rab4; Sheff et al., 1999); and 4) ligand stimulation has no effect on the initial rates of internalization or receptor recycling.
MATERIALS AND METHODS
Materials
Monensin, nystatin, chloramine T, and cycloheximide (CHX) were from Sigma-Aldrich (St. Louis, MO). Alexa fluor 594 transferrin, Alexa fluor 488 cholera toxin B subunit, Prolong mounting reagent, and all fluorescent secondary antibodies were from Molecular Probes (Eugene, OR). Antibodies against clathrin heavy chain and c-myc were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-GM-CSFR-β antibody was from eBioscience (San Diego, CA), and mouse anti-early endosome antigen 1 (EEA1) was from BD Biosciences (San Diego, CA). EEA1 primary antibody was used in conjunction with the Zenon fluorescent labeling system (Molecular Probes) to allow simultaneous labeling with two mouse antibodies for immunofluorescence studies. RNA oligonucleotides were synthesized by Dharmacon (Lafayette, CO). Immobilized Ficin, protein A-Sepharose, D-salt polyacrylamide 6000 desalting columns, and BS3 cross-linking reagent were from Pierce Chemical (Rockford, IL). 125I-TGF-β1, 125I-GM-CSF, and 35S-Easy Tag were from PerkinElmer Life and Analytical Sciences (Boston, MA). DNA transfections were performed using FuGENE 6 from Roche Diagnostics (Indianapolis, IN) according to manufacturer's instructions, by using 3 μl of transfection reagent per microgram of DNA, whereas RNA transfections were performed using Oligofectamine from Invitrogen (Carlsbad, CA). Rabbit anticaveolin-1 was a generous gift from Dr. Mark McNiven (Mayo Clinic, Rochester, MN). DN AP180-C (myc-tagged C-terminus) was generously provided by Dr. Harvey McMahon (MRC Laboratory of Molecular Biology Neurobiology Division Cambridge, England).
Cell Culture and Drug Treatment Assays
Mink lung epithelial cells (Mv1Lu) stably expressing either α1,β2 (Mb202α1-18) or α2,β1 (Mb102α2-9) chimeric receptors were maintained in 10% fetal bovine serum DMEM with 100 μg/ml geneticin and 50 μg/ml hygromycin as described previously (Anders et al., 1997, 1998). α and β designations refer to extracellular domains of the GM-CSFR-α and -β subunits, whereas 1 and 2 refer to the transmembrane and cytoplasmic domains of the TGF-β type 1 and 2 receptors, respectively. Thus, β2 refers to a chimeric receptor with a GM-CSFR-β extracellular domain and a TGF-β type II receptor cytoplasmic domain. For drug treatment studies, cells were seeded in six-well plates at 4 × 105 cells/well. After overnight incubation, the cultures were treated with 20 μg/ml CHX or 100 μM monensin at 37°C for the indicated times, after which cells were moved to 4°C and binding assays performed as described previously (Anders et al., 1997). CHX (20 μg/ml) resulted in >90% inhibition of protein synthesis by 35S-amino acid incorporation assay (unpublished data). After chemical treatments cells were moved to 4°C and processed for binding assays performed as described previously (Anders et al., 1997) or for cross-linking (below).
Immunofluorescence and Colocalization
To determine the colocalization of chimeric TGF-β receptors with markers of endocytosis, cells were seeded on coverslips and preincubated with ∼2 μg/ml Fab fragment mouse monoclonal anti-GM-CSFR-β antibody (see below) for 1 h at 4°C in antibody buffer (50 mM HEPES, DMEM, pH 7.2). Coverslips were washed three times with antibody buffer and then incubated for 4 min at 37°C in DMEM. For internalization assays, incubation at 4°C was eliminated and cells were allowed to bind and internalize antibody for 20 min at 37°C. Transferrin was visualized by incubation in serum-free medium for 30 min, followed by treatment with 20 μg/ml Alexa fluor 594 (red) transferrin at 37°C for 2 min. Cells were fixed in 4% formaldehyde and permeabilized for 2 min in 0.4% Triton X-100 phosphate-buffered saline (PBS). Goat anti-clathrin antibody (diluted 1:50 in blocking solution) or rabbit anticaveolin-1 (1:2000) were added for 30 min at 37°C, followed by washing and incubation for 30 min at room temperature with the appropriate fluorescent secondary antibodies, including anti-mouse to visualize receptors (all secondary fluorescent antibodies diluted 1:400).
For triple labeling studies, cells were incubated in the presence of receptor antibody at 12°C for 20 min, and then fixed and permeabilized. Primary goat anti-clathrin antibody, followed by Alexa fluor 488 (green) anti-goat and Alexa fluor 594 (red) antibodies were then incubated as described above. Another round of fixation minimized subsequent antibody cross-reaction by immobilizing all bound antibodies. Mouse anti-EEA1 antibody, precomplexed to the Zenon secondary antibody reagent according to the manufacturer's instructions, was incubated on coverslips for 30 min. After washing and a final fixation step, samples were mounted as described below.
For studies using fluorescent lactosylceramide (LacCer), cell membranes were labeled with BODIPY-LacCer as described previously (Singh et al., 2003). For colocalization of LacCer with caveolin-1, live cells transiently expressing a caveolin-1-mRed (cav1-mRed) construct (fluorescent tag at C terminus, transfected as described above) were used for colocalization studies as described previously (Sharma et al., 2004).
To determine the effects of inhibitors of endocytosis on internalization pathways, cells were pretreated with 25 or 50 μg/ml nystatin for 30 min or transfected with dominant negative myc-tagged AP180 (DN AP180) 24 h before staining. Transferrin was visualized as described above, except cells were exposed to fluorescent transferrin for 10 min, followed by brief acid treatment to remove surface fluorescence. Studies of BODIPY-LacCer internalization were performed as described previously (Singh et al., 2003) by using a 3-min incubation at 37°C for internalization. For assessment of the effects of DN AP180 on LacCer internalization, DN AP180 was transfected concomitant with a DsRed nuclear (DsRed-Nuc) construct to allow identification of transfected cells during live-cell imaging. For other experiments with fixed cells expressing DN AP180, transfected cells were identified by fluorescent labeling with a myc antibody (diluted 1:50). TGF-β receptor trafficking was assessed after internalization of prebound mouse anti-GM-CSFR-β antibody for 20 min at 37°C. For microscopy, coverslips were mounted with Prolong mounting medium (for experiments with fixed cells) and imaged by standard fluorescence microscopy at room temperature by using an oil immersion 60× objective with a numerical aperture of 1.40 on an Olympus AX70 fluorescence microscope equipped with a Hamamatzu C4742-95 digital camera. Image processing and quantitation of colocalization and total cell-associated fluorescence was performed using the MetaMorph imaging program (Universal Imaging, Downingtown, PA).
Clathrin RNA Interference (RNAi)
Mb202α1-18 cells were transfected with the small interfering RNA (siRNA) oligo chc-2, which specifically targets the clathrin heavy chain, as well as the nonfunctional control oligo μ2-1, as described previously (Motley et al., 2003). For adequate clathrin knockdown, we found it necessary to transfect four times at 24-h intervals, by using the transfection method described previously (Motley et al., 2003). Cells were trypsinized and replated as needed to maintain optimum confluence (∼50%). Clathrin levels were monitored using immunofluorescence labeling of clathrin (described above), whereas the effects of clathrin knockdown on TGF-β receptor and transferrin internalization were performed as outlined above.
Receptor Cross-linking
Mb202α1-18 cells expressing native and chimeric TGF-β receptors were seeded at 1 × 106 cells/p60 tissue culture dish and incubated overnight at 37°C. Dishes were treated with or without 100 μM monensin or 20 μg/ml CHX for various times at 37°C and then cross-linked with 125I-TGF-β as described previously (Penheiter et al., 2002), after which the cells were harvested and snap frozen for later protein normalization and characterization by SDS-PAGE and radiography. Alternatively, cross-linking was performed with 100 pM 125I-GM-CSF in GM-CSF binding buffer (200 mM HEPES, 2.5% bovine serum albumin, DMEM, pH 7.4). For receptor degradation studies, Mv1Lu or Mb202α1-18 cells were seeded at 5 × 106/p100 plate and incubated overnight. Cells were treated with or without potassium depletion as described previously (Anders et al., 1997) and then allowed to bind either 1 ng/ml 125I-TGF-β or 100 pM 125I-GM-CSF in buffer B (50 mM HEPES, 100 mM NaCl, 1 mM CaCl2, 2.5% bovine serum albumin, pH 7.4) with or without 10 mM KCl at 4°C for 1 h. Plates were then washed extensively in buffer A (50 mM HEPES, 100 mM NaCl, pH 7.4) with or without 10 mM KCl and cross-linked as described above in buffer A at 4°C for 1 h. The cross-linking reaction was quenched with 10 mM Tris, pH 7.0, after which cells were washed and incubated in buffer B with or without 10 mM KCl and with or without 25 μg/ml nystatin at 37°C for the incubated times, followed by harvesting and processing as described above.
Direct Recycling Assay
Direct recycling was assayed based on a protocol by Fraile-Ramos et al. (2001). Cells were seeded on coverslips as outlined above and incubated with anti-GM-CSFR-β antibody Fab fragment at 4 or 37°C for 1 h. Any remaining surface antibody was removed by washing in DMEM, pH 2.0. Cells were then incubated with Alexa fluor 488-conjugated anti-mouse antibody (diluted 1:200) at 4 or 37°C for 1 h followed by a second round of washing and acid stripping. Cultures were fixed and imaged with fluorescence microscopy as described above.
Fab anti-GM-CSFR-β fragments were generated using immobilized ficin and purified with protein A-Sepharose according to the manufacturer's instructions. Approximately 2 μg/ml Fab fragments (as estimated by SDS-PAGE) were used in the primary antibody incubation step of the direct recycling assay.
Radiolabeled Antibody Internalization and Recycling
Purified Fab antibody (see above) was labeled with 125I to a specific activity of 75 μCi/μg with chloramine T and purified using D-salt polyacrylamide 6000 size exclusion columns. Cells were incubated 2 h at 4°C with 5-8 ng/ml labeled antibody in antibody buffer. Buffer with labeled antibody was removed and warmed to 37°C and then readded to cells for the indicated times at 37°C. Incubation with fresh 37°C medium yielded similar results (unpublished data). After acid stripping with DMEM, pH 2.0, cell-associated radioactivity was assessed as described previously (Anders et al., 1997). For recycling assays, after the acid strip, cells were placed at 37°C in fresh medium for various times after which a second acid strip was performed and counts collected.
RESULTS
Loss of Surface TGF-β Receptors Is Not Due to Degradation and Replacement
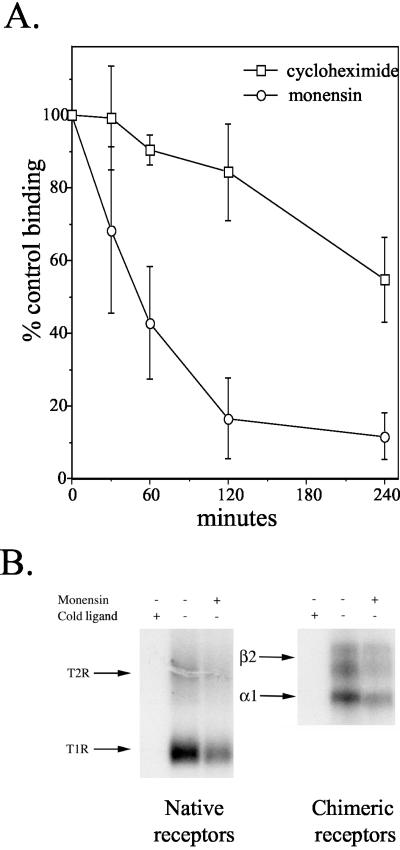
Previous work in our laboratory showed a loss of surface TGF-β receptors in the presence of monensin (Doré et al., 2001). Although this result suggests that TGF-β receptors recycle in the absence of ligand, an alternative explanation is that receptors are continually undergoing a rapid cycle of synthesis, export to the plasma membrane, internalization, and degradation. To address that possibility, the loss in TGF-β receptor binding after monensin treatment was compared with that observed when new protein synthesis was blocked with CHX. Addition of CHX resulted in an approximate 15% loss of surface receptors by 2 h, consistent with the receptors undergoing slow ligand-independent degradation (Figure 1A). This is in contrast to the 80% decrease in receptor number after 2-h monensin treatment. The distinct rates of receptor loss observed with monensin (half-life ∼53 min) and CHX (half-life ∼270 min) suggest that rapid degradation and replacement cannot fully explain the monensin-dependent receptor decrease.
Figure 1.
Effect of monensin is not due to rapid degradation and replacement. (A) Mb102α2-9 cells were incubated with either 20 μg/ml cycloheximide (□) or 100 μM monensin (○) for the indicated times at 37°C. After shifting to 4°C, 125I-GM-CSF binding was performed for both conditions as described in Materials and Methods. Data are expressed as percentage of ligand binding values of untreated cells and indicate the mean ± SD of three experiments done in duplicate. (B) Cells were treated (+) with cold ligand (10 ng/ml TGF-β, left; 10 ng/ml GM-CSF, right) or 100 μM monensin as in panel A for 2 h. 125I-TGF-β (left) or 125I-GM-CSF (right) binding was performed for 2 h at 4°C, after which the plasma membrane ligand/receptor complex was cross-linked at 4°C for 1 h with 1 μg/ml BS3 and visualized by SDS-PAGE and autoradiography. Results are representative of two experiments each.
To rule out the possibility that the above-mentioned results reflect properties of the chimeric receptor system only, ligand/receptor cross-linking was used to determine whether endogenous TGF-β receptors also recycle in the absence of ligand (Figure 1B). Cells were treated in the presence or absence of monensin for 2 h at 37°C, after which 125I-TGF-β was bound and cross-linked at 4°C by using a membrane-impermeable cross-linker. In agreement with the chimeric receptor results shown in Figure 1A, monensin treatment resulted in a loss of both T1R (63.8% loss) and T2R (85.4% loss) (Figure 1B, lanes 1-3). Analogous data were obtained when radiolabeled GM-CSF was cross-linked to the chimeric receptors (Figure 1B, lanes 4-6), further supporting the conclusion that the two receptor systems display similar trafficking behavior.
Direct Recycling Assay
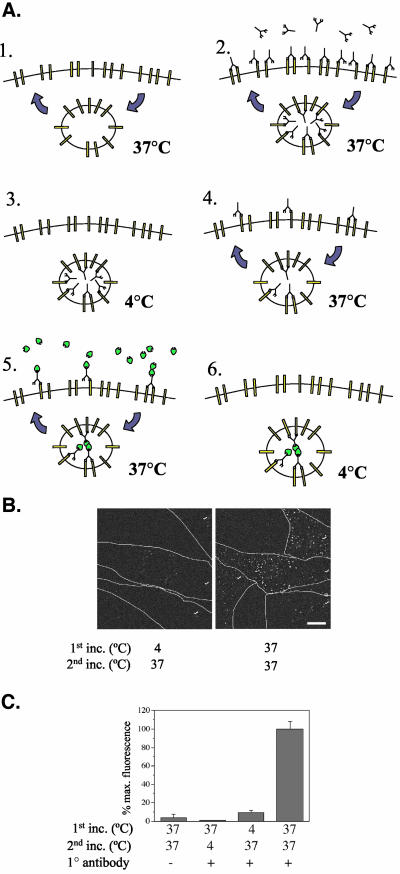
Whereas the previous results strongly suggest a mechanism of constitutive TGF-β receptor recycling, alternative conclusions are possible. To directly document recycling, a modification of a procedure by Fraile-Ramos et al. (2001) was used whereby an antibody recognizing the extracellular receptor domain is visualized through 1.5 cycles of recycling (illustrated in Figure 2A and discussed in the figure legend). To eliminate the possibility of abnormal receptor complex formation caused by the divalent monoclonal antibody, Fab fragments were generated and purified for use. Cells incubated at 4°C (to block vesicle trafficking) had similar background staining to those never treated with primary antibody (Figure 2, B and C), thus eliminating the possibility that incomplete stripping might allow for the detection of nonrecycling receptors. However, cells exposed to antibody at 37°C for both the first and second 1-h incubations showed vesicle labeling (Figure 2, B and C). Because intracellular labeling could only occur if cell membrane receptors underwent 1.5 rounds of recycling, the results directly document that antibody-bound TGF-β receptors constitutively recycle in a temperature-dependent manner. Similar findings were observed when whole antibody was used (unpublished data).
Figure 2.
Direct recycling assay. (A) Diagram of direct recycling assay. 1, recycling receptors move from the plasma membrane to an internal recycling compartment at 37°C. 2, antibody recognizes the receptor extracellular domain, resulting in antibody internalization. 3, acid treatment at 4°C removes surface antibodies. 4, cells are returned to 37°C (recycling continues) such that internalized antibody continues trafficking to the cell surface. 5, 37°C incubation with fluorescent secondary antibody (green objects) labels recycled receptors bound with primary antibody; continued recycling results in internalization of fluorescent antibody. 6, acid strip (4°C) removes any surface antibody, such that fluorescent label remains only on those receptors that have undergone 1.5 rounds of recycling. (B and C) Cells were incubated with or without Fab fragment mouse anti-GM-CSFR-β antibody for 1 h at 4 or 37°C (first incubation). After washing and acid stripping at 4°C to remove any noninternalized antibody, cells were exposed to Alexa fluor 488 anti-mouse antibody (1:200) for 1 h at 4 or 37°C (second incubation), washed, and acid stripped again. Coverslips were fixed, mounted, and quantitated (C) for cell-associated fluorescence as described in Materials and Methods. Outlines show individual cells indicated with (✓). Data shown in C are expressed as percentage of the cell-associated fluorescence of the “37 37” result and indicate the mean ± SEM of ∼100 cells. Bar, 10 μm.
Recycling/Internalization of Ligand-activated Receptors
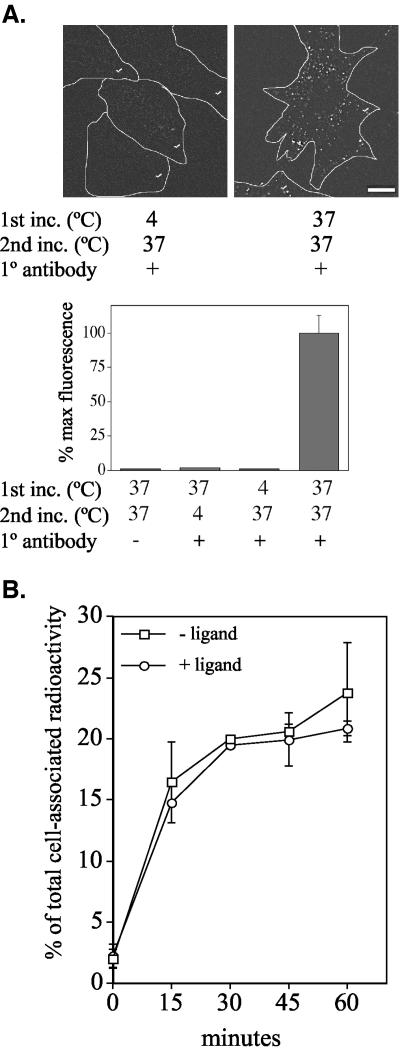
Although Figures 1 and 2 conclusively establish recycling of unstimulated TGF-β receptors, the trafficking itinerary of ligand-activated receptors is currently unknown. Because the antibody used in Figure 2 binds to the β subunit of the GM-CSF receptor in such a manner as to allow concomitant ligand binding (unpublished data), the above-mentioned direct recycling assay was performed in the presence of 10 ng/ml GM-CSF to determine whether ligand-bound receptors recycle. As shown in Figure 3A, recycling activity was observed after ligand addition in a similar manner to that of nonactivated receptors. Given that TGF-β receptor down-regulation has been well documented (Wakefield et al., 1987; Mercier et al., 1995; Zhao and Buick, 1995; Anders et al., 1997, 1998; Zwaagstra et al., 1999), it might be assumed that ligand-bound receptors would be targeted for degradation and recycle to a lesser extent (if at all) than unstimulated receptors. Although Figure 3A indicates that is not the case, a more readily quantitative assay was used to directly compare recycling under these two conditions. This was accomplished by radiolabeling Fab fragment anti-GM-CSFR-β antibody with 125I and following the cellular entrance and exit of antibody-labeled plasma membrane receptors. Surprisingly, ligand-dependent and -independent receptor recycling occurred at the same rate (Figure 3B), suggesting that TGF-β receptors may be directed through similar routes regardless of their activation state. No significant difference was observed if a 10-fold greater concentration of GM-CSF was used, or if GM-CSF ligand also was present during the 60-min chase at 37°C (unpublished data).
Figure 3.
TGF-β receptors recycle at the same rate in the presence and absence of ligand. (A) Mb202α1-18 cells were processed for imaging and fluorescence quantitation as in Figure 2, B and C, except 10 ng/ml GM-CSF was included in both incubations. Bar, 10 μm. (B) Cultures were labeled with 125I-Fab anti-GM-CSF receptor-β for 2 h at 4°C in the presence (○) or absence (□) of 10 ng/ml GM-CSF. After washing and incubation at 37°C for 30 min (in the presence or absence of 10 ng/ml GM-CSF), labeled receptor antibody was removed by acid wash and the cultures returned to 37°C. At the indicated times, trichloroacetic acid-precipitable radioactivity in the spent medium and that removed from the cell surface by a second DMEM, pH 2.0, wash were combined and designated recycled counts. Results are expressed as percentage of the total cell-associated radioactive counts after the first acid strip and before further incubation at 37°C, and indicate the mean ± SD of two experiments done in duplicate.
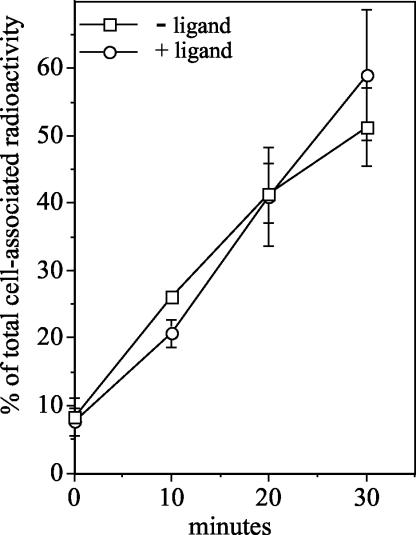
Whereas Figure 3 shows similar TGF-β receptor recycling independent of ligand binding, the majority of growth factor-activated receptors respond to ligand with an enhanced rate of internalization (Wang et al., 1983; Wiley et al., 1991; Knutson, 1992; Shapiro and Ahn, 1998). As such, to further assess the effect of ligand activation on TGF-β receptor trafficking, the internalization rates of ligand-bound and non-bound receptors were compared. No significant difference was observed by the addition of ligand (Figure 4), with an internalization rate of 1.7% of total labeled receptors internalized per minute in the presence or absence of ligand. This result further demonstrates that TGF-β receptors are trafficked in a similar manner regardless of their activation state.
Figure 4.
TGF-β receptors internalize at the same rate regardless of activation state. Mb202α1-18 cells were prebound with radiolabeled antibody in the presence (○) or absence (□) of 10 ng/ml GM-CSF as in Figure 3B and then incubated at 37°C for the indicated times. Surface antibody was removed by acid treatment at 4°C, after which cells were processed to determine internalized radioactivity (see Materials and Methods). Results are expressed as percentage of total cell-associated radioactive counts before incubation at 37°C and indicate the mean ± SD of two experiments done in duplicate.
Receptor Recycling Occurs through a Clathrin-dependent Mechanism
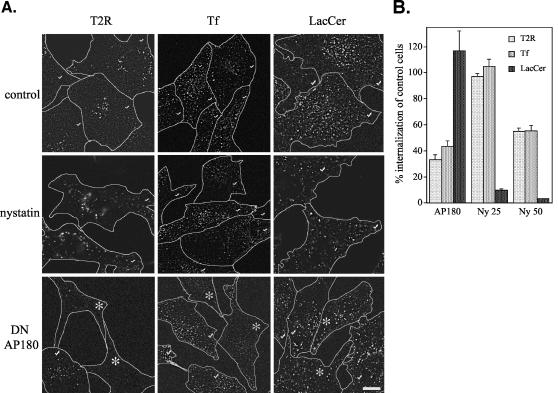
Previous work in our laboratory has shown that internalization of TGF-β receptors in response to ligand is a clathrin-dependent process (Anders et al., 1997, 1998). To determine whether the internalization step in ligand-independent receptor recycling occurred through a similar mechanism, immunofluorescence microscopy was used to visualize the colocalization of TGF-β receptors with either clathrin or caveolin-1. As shown in Figure 5A, the type II TGF-β receptor colocalized extensively with clathrin after a 4-min incubation at 37°C, whereas little association with caveolin-1 was detected. This finding was extended to include the type I receptor and quantified (Figure 5B). Both the type I and type II receptors colocalized 30-40% with clathrin. This level was shown to be similar to the colocalization of clathrin with transferrin, a well-established marker of clathrin-dependent internalization (Figure 5B). Although a previous publication has suggested that TGF-β receptors internalize through caveolae as well as clathrin pits (Di Guglielmo et al., 2003), and colocalization of cav1-mRed and BODIPY-LacCer was easily detected, colocalization of TGF-β receptors with caveolin-1 was shown to occur at a similar basal level to that of transferrin and caveolin-1 (Figure 5B). Furthermore, ligand stimulation resulted in little or no difference in the degree of receptor colocalization with either clathrin or caveolin-1.
Figure 5.
Colocalization of TGF-β receptors with clathrin. (A) MB202α1-18 cells were used for colocalization of the T2R with clathrin or caveolin-1. Live cells with prebound Fab anti-GM-CSFR-β antibody were allowed to internalize for 4 min at 37°C. After fixation, permeabilization, and blocking, coverslips were treated with either goat anti-clathrin (top row, shown as costain with T2R) or rabbit anti-caveolin-1 (middle row, costain with T2R). Cultures were washed and incubated with Texas Red donkey anti-mouse and either Alexa fluor 488 (green) donkey anti-goat (top row), or Alexa fluor 488 goat anti-rabbit (middle row). Alternatively, cells were transfected with cav1-mRed, surface labeled with BODIPY-LacCer (see Materials and Methods), and imaged without fixation (bottom row). Insets are enlargements of outlined regions in larger images. Images were collected as described in Materials and Methods. Bar, 10 μm for unenlarged images. (B) TGF-β T1R or T2R were visualized by fluorescence microscopy by using Mb102α2-9 or Mb202α1-18 cells, respectively, for colocalization studies with clathrin (black bars) or caveolin-1 (gray bars). Transferrin (Tf), caveolin-1 (cav), and (LacCer) were used as controls to colocalize with clathrin or caveolin-1 as indicated. For colocalization with LacCer, transfected cav1-mRed was used instead of endogenous caveolin-1. Treatment with fluorescent transferrin was performed as described in Materials and Methods. Each value in B represents the mean colocalization percentage of the marker indicated on the x-axis with either clathrin or caveolin-1 as indicated, and represents the mean ± SEM for 50-80 cells. (C) Cells were incubated with receptor antibody at 12°C for 20 min and then fixed, permeabilized, and labeled so as to visualize three distinct molecules: chimeric T2R (red), clathrin (green) and EEA1 (blue) (see Materials and Methods). Colocalization of red/green, yellow; blue/red, magenta; blue/green, cyan; and red/green/blue, white.
Whereas colocalization of TGF-β receptors with clathrin strongly suggests internalization through clathrin-coated pits, the fact that the above-mentioned experiments were performed with a 4-min incubation at 37°C allows for the possibility that receptors were internalized through a clathrin-independent pathway and then subsequently merged with clathrin-associated endosomes. Although no association of receptors with caveolin-1 could been seen at 4°C, TGF-β receptors also could not be found associated with clathrin at this temperature. Clathrin/receptor association was observable, however, when cells were incubated at 12°C (Figure 5C), at which temperature we have previously shown that TGF-β receptors are not internalized (Penheiter et al., 2002). To verify that colocalization under these conditions did not result from receptors associated with intracellular clathrin structures, cells were triple labeled for T2R, clathrin, and EEA1, a marker for early endosomes. As shown in Figure 5C, when internalization is prevented by 12°C incubation, TGF-β receptors colocalize with clathrin, but not EEA1. Although the reason for the necessity of 12°C incubation is not clear, it may reflect altered receptor antibody recognition or improper receptor conformation at 4°C.
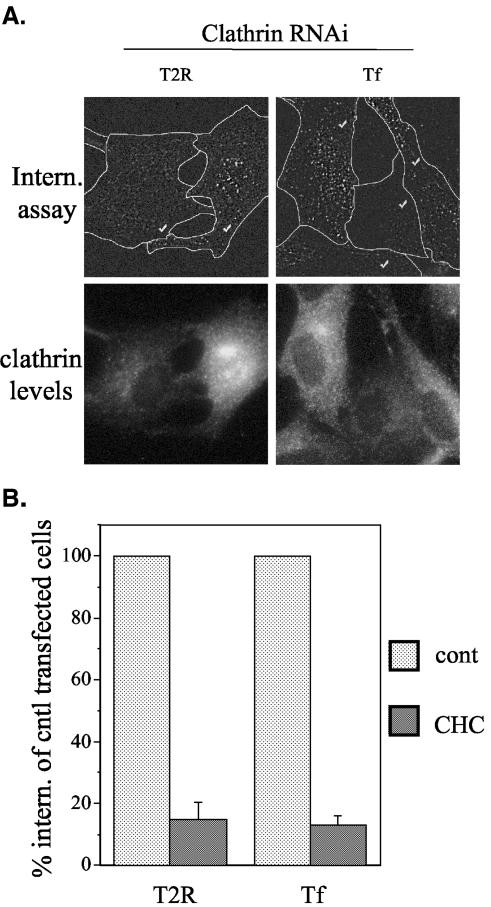
In light of the aforementioned study in which caveolae were shown to be involved in TGF-β receptor internalization (Di Guglielmo et al., 2003), the possible role of caveolae in TGF-β receptor internalization was further explored. As shown in Figure 5, A and B, LacCer colocalized well with cav1-mRed in Mv1Lu-derived cells and has been previously shown to be internalized through caveolae in human skin fibroblasts (Singh et al., 2003; Sharma et al., 2004; reviewed in Pelkmans and Helenius, 2002). Thus, LacCer was chosen as a likely legitimate marker for endocytosis through caveolae. As expected (Singh et al., 2003), LacCer internalization was specifically inhibited by 25 μg/ml cholesterol-binding drug nystatin, whereas transferrin uptake was greatly diminished by dominant negative (DN) AP180, which is known to inhibit clathrin-dependent endocytosis (Zhao et al., 2001) (Figure 6, A and B). Interestingly, a higher nystatin concentration (50 μg/ml) nonspecifically inhibited the uptake of transferrin (Figure 6B), in a manner analogous to results obtained with methyl-β-cyclodextrin, another cholesterol depleting drug, in which higher concentrations were shown to inhibit both clathrin- and caveolae-dependent internalization (Rodal et al., 1999; Subtil et al., 1999). Thus, 25 μg/ml was chosen as an appropriate nystatin concentration for inhibition of caveolar internalization. With the appropriate conditions established for specifically inhibiting caveolaeand clathrin-dependent internalization, the effects of nystatin and DN AP180 on TGF-β receptor internalization were examined. In agreement with the results of Figure 5, endocytosis of TGF-β receptors was significantly inhibited (67%) by DN AP180 transfection. In contrast, treatment with nystatin (at the appropriate concentration of 25 μg/ml) had no effect on receptor internalization, whereas 50 μg/ml nystatin, which nonspecifically inhibited transferrin, also inhibited TGF-β internalization by ∼45% (Figure 6B). As such, specific disruption of caveolar internalization, in contrast to inhibition of clathrin-dependent processes, does not effect TGF-β receptor endocytosis. However, to eliminate a possible (although unlikely) scenario in which DN AP180 (as well as other clathrin inhibitors used to study TGF-β receptor internalization; Anders et al., 1997; Ehrlich et al., 2001; Razani et al., 2001; Hayes et al., 2002; Penheiter et al., 2002; Yao et al., 2002; Di Guglielmo et al., 2003) act nonspecifically to inhibit both caveolar- and clathrin-dependent endocytosis, the effect of RNAi-mediated clathrin knockdown on internalization was examined. As shown in Figure 7, extensive reduction of cellular clathrin levels (∼70-100%) inhibited TGF-β receptor internalization by >85%, with transferrin uptake being impeded to a virtually identical extent. Thus, the results of Figures 5, 6, 7 reveal that TGF-β receptors in Mv1Lu cell clones are internalized through a clathrin-dependent mechanism(s), with caveolar internalization being unlikely to play a major role.
Figure 6.
TGF-β receptors are dependent on clathrin-associated mechanisms, but not caveolae, for internalization. Mb202α1-18 cells were transfected with myc-DN AP180 24 h before experiments or treated with 25 or 50 μg/ml nystatin as indicated. TGF-β receptors were visualized after internalization of prebound mouse anti-GM-CSFR-β antibody for 20 min at 37°C in the presence of 25 μg/ml nystatin or DN AP180, followed by processing with fluorescent secondary antibody as described in Figure 5. For transferrin and BODIPY-LacCer visualization, cells were treated as described in Materials and Methods in the presence or absence of the indicated inhibitors. Outlines show individual cells indicated with (✓). Transfected cells (*) were identified by immunofluorescence by using the myc epitope of DN AP180 for TGF-β receptor and transferrin internalization assays, whereas cotransfection of DsRed-Nuc allowed for identification of transfected cells during live cell imaging of LacCer internalization. Bar, 10 μm. (B) Cell-associated fluorescence of the experiments depicted in A were quantitated. Values for nystatin treatments represent the mean percentage of fluorescence of ∼300 cells ± SEM as compared with untreated controls. Values for transfected cells represent the mean percentage of fluorescence of 50-100 cells ± SEM compared with untransfected control cells.
Figure 7.
Effect of RNAi-mediated clathrin knockdown on TGF-β receptor internalization (A) Mb202α1-18 cells were transfected with either a siRNA oligonucleotide targeting clathrin heavy chain, or a nonfunctional control oligo as indicated. Outlines show individual cells indicated with (✓). After transfection, treated cell pools were split onto two coverslips for analysis of TGF-β receptor and transferrin internalization studies (top row), and were costained with clathrin antibody to monitor the extent of clathrin knockdown (bottom row). Bar, 10 μm. (B) Cells displaying >70% clathrin knockdown were selected and quantitated for TGF-β receptor and transferrin endocytosis. Values represent the cell-associated fluorescence of cells targeted for clathrin heavy chain knockdown (CHC) compared with those treated with nonfunctional oligonucleotide (cont) and are the mean ± SEM of 50-80 cells.
Lack of Caveolar Involvement in Receptor Degradation
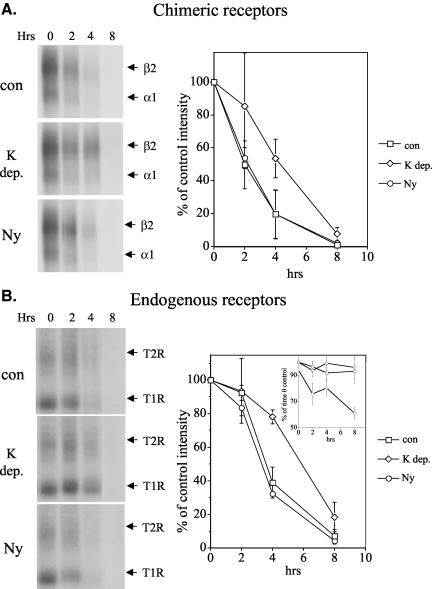
The above-mentioned results (Figures 5, 6, 7) suggest that caveolae are of minor importance in TGF-β receptor internalization. Because it has been proposed that TGF-β receptor degradation and signaling is mediated via caveolar- and clathrin-dependent internalization, respectively (Di Guglielmo et al., 2003), we examined whether inhibiting these pathways would affect the half-life of cell surface TGF-β receptors by performing similar degradation experiments. In contrast to results obtained elsewhere (Di Guglielmo et al., 2003), inhibiting clathrin-dependent internalization by potassium depletion increased TGF-β receptor half-life by ∼2-2.5 h (Figure 8), whereas nystatin treatment had no effect. Similar results were obtained by cross-linking chimeric receptors in MB202α1-18 cells with 125I-GM-CSF (Figure 8A) or by assaying endogenous TGF-β receptors in the parental Mv1Lu cells by using 125I-TGF-β (Figure 8B). These results suggest that degradation, like signaling, is a clathrin-dependent process that does not seem to involve caveolae to any major extent (see Discussion).
Figure 8.
TGF-β receptor degradation is clathrin-dependent. Confluent cells were treated with or without potassium depletion and then affinity labeled with radiolabeled ligand (see Materials and Methods). Cultures were then incubated at 37°C for the indicated times in the presence or absence of 25 μg/ml nystatin, after which cells were harvested and normalized protein samples processed by SDS-PAGE and autoradiography. Graphs represent the mean densitometric data ± SD from three separate experiments. Untreated control samples (con) are compared with samples undergoing potassium depletion (K depleted) and nystatin treatment (ny). (A) Mb202α1-18 cells were affinity labeled with 100 pM 125I-GM-CSF. (B) Mv1Lu cells were affinity labeled with 1 ng/ml 125I-TGF-β. Inset shows protein levels as assessed at treatment time points and represent the mean values ± SD for three separate experiments done in duplicate.
Rab11-dependent and rab4-independent TGF-β Receptor Recycling
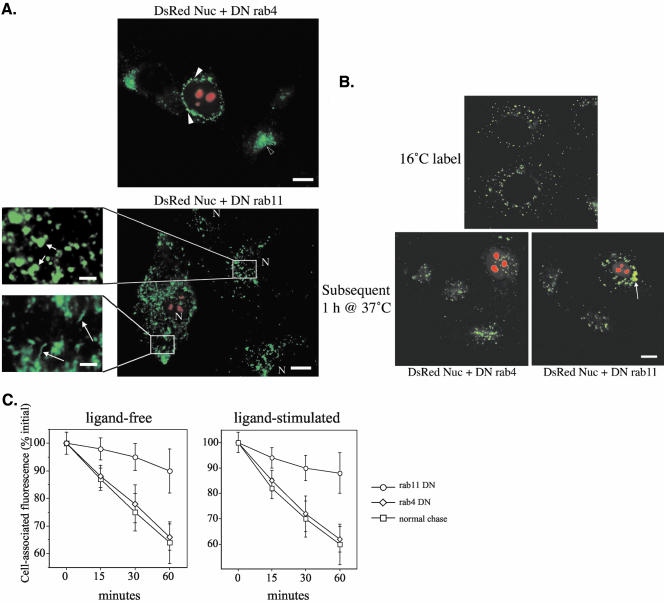
Given the well-established role of the small GTPase rab proteins in intracellular trafficking (Stenmark and Olkkonen, 2001; Zerial and McBride, 2001), the role(s) of rab4 and rab11 in TGF-β receptor recycling was examined. Rab4 is known to regulate recycling from sorting/early endosomes to the plasma membrane, whereas rab11 directs transport from the perinuclear recycling compartment back to the cell surface (Sonnichsen et al., 2000). To determine the function of rab4 and/or rab11 in TGF-β recycling, dominant negative forms were overexpressed (concomitant with a nuclear-localized DsRed construct to identify transfected cells) and the effect on transferrin and TGF-β receptor trafficking was assessed. Because transferrin is known to traffic both through a rapid rab4-dependent recycling pathway and a slower, rab11-dependent route (Sonnichsen et al., 2000), as expected, both mutants altered the morphology and trafficking of transferrin-associated compartments (Figure 9A). In accordance with previous studies, DN rab4 overexpression blocked recycling to the plasma membrane from early endosomes and caused a collapse of endosomal structures to the perinuclear region (McCaffrey et al., 2001), whereas DN rab11-expressing cells showed increased intracellular transferrin and disrupted recycling compartments and often demonstrated abnormal tubular structures (Wilcke et al., 2000; Choudhury et al., 2002) (Figure 9A). For assessment of the role of rab4 and 11 in TGF-β receptor trafficking, DN rab-transfected cells were first loaded with antibody at 16°C, at which temperature receptors were found to accumulate in endocytic vesicles (Figure 9B), corresponding to early endosomes (unpublished data). After stripping the antibody from the cell surface, cells were incubated at 37°C for the indicated times. As can be seen in Figure 9B, the receptor signal was similarly lost in untransfected and DN rab4-transfected cells. DN rab11 expression, however, caused a marked retention of cellular fluorescence (Figure 9B), suggesting that rab11, but not rab4, plays a significant role in intracellular TGF-β receptor recycling. Similar results were obtained when the same experiments were conducted on ligand-activated receptors (Figure 9C). These findings are in keeping with a recent report showing colocalization of TGF-β receptors with rab11 (Di Guglielmo et al., 2003). Thus, after clathrin-dependent internalization TGF-β receptors recycle (irrespective of their activation state) via a rab4-independent and rab11-dependent manner.
Figure 9.
TGF-β receptor recycling is rab11-dependent. Cells were grown on glass coverslips and transfected 48 h beforehand with DN rab4S22N or DN rab11S25N in conjunction with the DsRedNuc construct to identify transfected cells. (A) For transferrin labeling, cells were placed in serum-free medium for 30 min and then labeled with fluorescein isothiocyanate (FITC)-transferrin for 30 min at 37°C. Filled arrowheads show collapsed endosomal structures surrounding the nucleus in a DN rab4-transfected cell, whereas the unfilled arrowhead points out recycling endosomes in an untransfected cell (A, top). Long arrows indicate abnormal tubular structures (caused by inhibited budding and disrupted transport) in the DN rab11-transfected cell (A, bottom expanded box from lower panel) in comparison with the normal rounded structures in the untransfected cell (A, short arrows in top expanded box from lower panel). Bar, 10 μm for unenlarged images and 2 μm for enlargements. (B) For receptor assays, cells overexpressing DN rab4S22N or DN rab11S25N were labeled with GM-CSFR-β Fab antibody in the presence or absence of 10 ng/ml GM-CSF for 1 h at 16°C. After acid stripping of cell surface antibodies, cells were incubated at 37°C for the indicated times while replacing spent media with prewarmed fresh media every 10 min. After extensive washings, all cells were fixed, permeabilized, labeled with FITC-goat anti-mouse antibody, and mounted. Arrow shows retention of fluorescent signal in DN rab11-transfected cells (B, bottom right) compared with DN rab4-transfected cells (B, bottom left). Bar, 10 μm. (C) Fluorescence intensity remaining after various chase periods was quantified by image analysis and expressed as a percentage of the fluorescence present at 0-min chase. Values indicate the mean ± SD of the fluorescent values of 30 cells from three separate experiments.
DISCUSSION
Over the last few decades, the trafficking properties of many receptor systems have been elucidated, with several different regulatory mechanisms shown to be operative depending on the receptor(s) being studied (Stoorvogel et al., 1991; Amigorena and Bonnerot, 1999; Carpenter, 2000; Clague and Urbe, 2001; Kittler and Moss, 2001; Wiley and Burke, 2001; Clark et al., 2003). Here, we are now able to elucidate various aspects of TGF-β receptor trafficking, both in the presence and absence of receptor activation.
Previous indirect evidence indicated that TGF-β receptors undergo both constitutive and ligand-dependent recycling (Massagué and Like, 1985; Sathre et al., 1991; Doré et al., 2001). As such, to directly address these issues we developed a recycling assay that demonstrated that surface TGF-β receptors were first internalized and then subsequently returned to the plasma membrane (Figure 2). Similar results were obtained in the presence or absence of ligand (Figure 3). In addition, the rates of internalization were shown to be equivalent, with a value of 1.7% of total labeled receptors internalized per minute regardless of ligand treatment (Figure 4). These findings are somewhat surprising and unique in that 1) TGF-β receptors have been shown to be down-regulated in response to ligand and would be expected to remain intracellular (Anders et al., 1997, 1998); and 2) in contrast to the insulin-like growth factor II receptor (Oka and Czech, 1986; Braulke et al., 1987), most receptor systems studied (i.e., epidermal growth factor, insulin and thrombin receptors) are stimulated to internalize to a greater extent upon ligand activation (Wang et al., 1983; Wiley et al., 1991; Knutson, 1992; Shapiro and Coughlin, 1998). In that regard, a model (Figure 10) is summarized below that integrates these unique characteristics of TGF-β receptor trafficking.
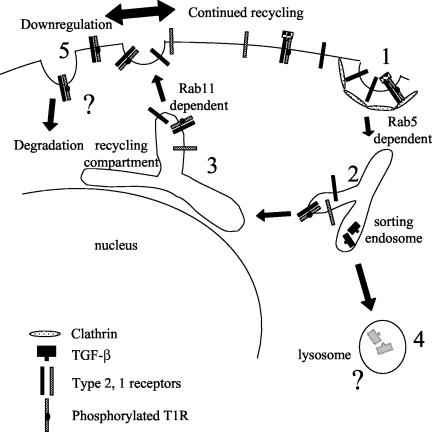
Figure 10.
Model of TGF-β receptor trafficking. Receptors are internalized in clathrin-coated pits at the same rate irrespective of ligand binding (1); thus, both activated and nonactivated receptors are shown in the same clathrin-associated structure. Smad activation likely occurs in a newly internalized nascent vesicle (2), after which receptors are trafficked to the sorting endosome (2) where they are sorted to recycling endosomes for rab 11-dependent recycling to the plasma membrane (3). TGF-β ligand dissociates and is transported to the lysosome (4) for degradation (see text). From the recycling compartment receptors return to the plasma membrane regardless of their initial activation state. Nonactivated receptors are subjected to further recycling, whereas activated receptors undergo down-regulation/degradation (5) (Mercier et al., 1995; Anders et al., 1997). The mechanism/structure(s) through which activated receptors are internalized for down-regulation or the compartment(s) where Smad signaling occurs is unknown.
Internalized molecules have been shown to enter cells via a number of different pathways. Previous studies showed conflicting evidence for TGF-β receptor association with clathrin coated pits and/or caveolae/rafts (Anders et al., 1997; Ehrlich et al., 2001; Razani et al., 2001; Hayes et al., 2002; Penheiter et al., 2002; Yao et al., 2002; Di Guglielmo et al., 2003). Although we and others (Hayes et al., 2002; Penheiter et al., 2002; Di Guglielmo et al., 2003) have shown that clathrin-dependent processes are required for TGF-β Smad signaling, the current study extends this analysis to investigating the pathway(s) for ligand-independent receptor trafficking.
Our results showed receptor colocalization with clathrin but not caveolin-1 (Figure 5). Functional significance of this association was assessed by demonstrating that transfection of DN AP180 and RNAi-mediated clathrin knockdown (both to inhibit clathrin-dependent internalization) inhibited receptor internalization, whereas nystatin treatment (to disrupt caveolae/lipid rafts) showed no effect (Figures 6 and 8). However, given the recent report showing clathrin- and caveolae/raft-dependent receptor pathways for TGF-β signaling and degradation, respectively (Di Guglielmo et al., 2003), we similarly examined the effects of endocytic inhibitors on receptor degradation. Whereas nystatin had no effect on receptor stability, the degradation of both chimeric and endogenous TGF-β receptors was diminished by preventing clathrin-dependent internalization (Figure 8). Although it is unknown why we and Di Guglielmo et al. (2003) show such distinct findings relating to caveolae function in TGF-β receptor trafficking, two technical issues may play important roles. First, in the same cell type we find that 50 μg/ml nystatin (the concentration used in the other study) similarly inhibits transferrin as well as uptake of caveolar markers (Figure 6, A and B); and, second, the long-term potassium depletion (2-8 h) required for these experiments results in significant cell loss (up to 40%; Figure 8B, inset), potentially biasing these samples toward lower values and obscuring the stabilizing effect of potassium depletion. In addition, a conceptual inconsistency arises from the fact that inhibiting clathrin-dependent processes would prevent the E3 ubiquitin ligase Smurf2 from targeting the TGF-β receptor for degradation (Kavsak et al., 2000; Piek et al., 2001). This latter point is of considerable interest because the induction of Smad7 is known to be dependent upon Smad3 (Piek et al., 2001) and, as mentioned above, both we and others have shown Smad activation to be dependent upon clathrin-dependent internalization (Hayes et al., 2002; Penheiter et al., 2002; Di Guglielmo et al., 2003). Thus, regardless of any role (or not) for caveolae, potassium depletion should result in TGF-β receptor stabilization simply by preventing Smad7 induction and subsequent Smurf2 ubiquitination of the receptor complex (Kavsak et al., 2000).
Treatment with DN rab constructs allowed us to compare the involvement of rab4 and rab11 in TGF-β receptor trafficking in the absence and presence of ligand. Whereas transferrin, a well-studied constitutively recycling protein, recycles through both rab4- and rab11-dependent processes (Sheff et al., 1999), we found that TGF-β receptor recycling involves only rab11 (Figure 9, B and C). Because ligand activation causes drastic changes in receptor function, we reasoned that activated receptors might recycle through distinct cellular compartments from quiescent receptors and thus might be shown to be dependent on a different profile of rab proteins. Interestingly, we found no effect of ligand activation; that being, receptors recycled through a rab11-dependent process irrespective of the receptors' activation state. Although this result is informative in elucidating the involvement of specific rab proteins, the modification(s) that distinguishes activated receptors from those that are constitutively recycling is unknown. Two likely possibilities include receptor phosphorylation and/or ubiquitination because both have been reported to regulate aspects of TGF-β receptor internalization/down-regulation and degradation, respectively (Anders et al., 1998; Kavsak et al., 2000).
The current results suggest a model in which both activated and quiescent TGF-β receptors are internalized and degraded via a clathrin-dependent pathway and recycle to the cell surface through a rab11-dependent process (Figure 10). While chimeric TGF-β receptor internalization through caveolae/rafts is likely to be of minor importance, it cannot be formally ruled out for native receptors. Despite the fact that activated TGF-β receptors stimulate Smad phosphorylation in an intracellular compartment downstream of dynamin function (Hayes et al., 2002; Penheiter et al., 2002) (most likely in a newly internalized rab5-associated vesicle; Panopoulou et al., 2002; Figure 10, 2), receptors reappear at the plasma membrane irrespective of activation state. At this time, activated receptors are evidently directed for eventual down-regulation/degradation, whereas unstimulated receptors continue to recycle (Figure 10). Although our results do not directly address the immediate itinerary of ligand, TGF-β receptors are most likely targeted to the early/sorting endosome where ligand dissociates and undergoes lysosomal degradation. This is the most probable outcome because the half-life of internalized TGF-β is significantly shorter than that of its receptors (Frolik et al., 1984; Koli and Arteaga, 1997; Massagué and Kelly, 1986; Wells et al., 1997).
A central question might be why a cell would shuttle receptors to and from the cell surface in the absence of ligand activation. Although the question remains unanswered by this study, at least two possible explanations exist. First, recycling may facilitate a receptors' ability to rapidly respond to ligand stimulation. Recycling promotes immediate cellular entry without the need for recruitment of the necessary internalization machinery. The receptors can then be more quickly transported to the appropriate intracellular locale where Smad signaling occurs (Hayes et al., 2002; Penheiter et al., 2002). Consistent with such a model is our recent demonstration that TGF-β receptors constitutively associate with the β2 subunit of AP2 (Yao et al., 2002). Second, constitutive recycling may provide a convenient method for the cell to regulate surface receptor number (Royle and Murrell-Lagnado, 2003), thereby regulating sensitivity to TGF-β ligand. For example, if a rapid decrease in surface receptors is needed, the internalization rate could be increased and/or recycling decreased. Conversely, modulating trafficking rates in the opposite manner would increase the number of plasma membrane receptors. Conclusively addressing this question, however, will await our ability to specifically inhibit constitutive and ligand-activated TGF-β receptor internalization.
Acknowledgments
This work was supported by funds from National Institutes of Health grants GM-54200 and GM-55816 to E.B.L. and GM-22942 to R.E.P., a National Niemann-Pick Disease Foundation fellowship to A.C. and the Mayo Foundation.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-03-0245. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0245.
References
- Amigorena, S., and Bonnerot, C. (1999). Fc receptor signaling and trafficking: a connection for antigen processing. Immunol. Rev. 172, 279-284. [DOI] [PubMed] [Google Scholar]
- Anders, R.A., Arline, S.L., Doré, J.J., and Leof, E.B. (1997). Distinct endocytic responses of heteromeric and homomeric transforming growth factor beta receptors. Mol. Biol. Cell 8, 2133-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders, R.A., Doré, J.J., Jr., Arline, S.L., Garamszegi, N., and Leof, E.B. (1998). Differential requirement for type I and type II transforming growth factor beta receptor kinase activity in ligand-mediated receptor endocytosis. J. Biol. Chem. 273, 23118-23125. [DOI] [PubMed] [Google Scholar]
- Anders, R.A., and Leof, E.B. (1996). Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-beta (TGF-beta) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-beta signaling. J. Biol. Chem. 271, 21758-21766. [DOI] [PubMed] [Google Scholar]
- Bassing, C.H., Yingling, J.M., Howe, D.J., Wang, T., He, W.W., Gustafson, M.L., Shah, P., Donahoe, P.K., and Wang, X.-F. (1994). A transforming growth factor β type I receptor that signals to activate gene expression. Science 263, 87-89. [DOI] [PubMed] [Google Scholar]
- Braulke, T., Gartung, C., Hasilik, A., and von Figura, K. (1987). Is movement of mannose 6-phosphate-specific receptor triggered by binding of lysosomal enzymes? J. Cell Biol. 104, 1735-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, G. (2000). The EGF receptor: a nexus for trafficking and signaling. Bioessays 22, 697-707. [DOI] [PubMed] [Google Scholar]
- Choudhury, A., Dominguez, M., Puri, V., Sharma, D.K., Narita, K., Wheatley, C.L., Marks, D.L., and Pagano, R.E. (2002). Rab proteins mediate Golgi transport of caveola-internalized glycosphingolipids and correct lipid trafficking in Niemann-Pick C cells. J. Clin. Investig. 109, 1541-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague, M.J., and Urbe, S. (2001). The interface of receptor trafficking and signalling. J. Cell Sci. 114, 3075-3081. [DOI] [PubMed] [Google Scholar]
- Clark, M.R., Massenburg, D., Zhang, M., and Siemasko, K. (2003). Molecular mechanisms of B cell antigen receptor trafficking. Ann. NY Acad. Sci. 987, 26-37. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo, G.M., Le Roy, C., Goodfellow, A.F., and Wrana, J.L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 5, 410-421. [DOI] [PubMed] [Google Scholar]
- Doré, J.J., Jr., Yao, D., Edens, M., Garamszegi, N., Sholl, E.L., and Leof, E.B. (2001). Mechanisms of transforming growth factor-beta receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol. Biol. Cell 12, 675-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich, M., Shmuely, A., and Henis, Y.I. (2001). A single internalization signal from the di-leucine family is critical for constitutive endocytosis of the type II TGF-beta receptor. J. Cell Sci. 114, 1777-1786. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos, A., Kledal, T.N., Pelchen-Matthews, A., Bowers, K., Schwartz, T.W., and Marsh, M. (2001). The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol. Biol. Cell 12, 1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén, P., ten Dijke, P., Ichijo, H., Yamashita, H., Schultz, P., Heldin, C.-H., and Miyazono, K. (1993). Cloning of a TGFβ type I receptor that forms a heteromeric complex with the TGFβ type II receptor. Cell 75, 681-692. [DOI] [PubMed] [Google Scholar]
- Frolik, C.A., Wakefield, L.M., Smith, D.M., and Sporn, M.B. (1984). Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J. Biol. Chem. 259, 10995-11000. [PubMed] [Google Scholar]
- Gliemann, J. (1998). Receptors of the low density lipoprotein (LDL) receptor family in man. Multiple functions of the large family members via interaction with complex ligands. Biol. Chem. 379, 951-964. [PubMed] [Google Scholar]
- Guatimosim, C., and von Gersdorff, H. (2002). Optical monitoring of synaptic vesicle trafficking in ribbon synapses. Neurochem. Int. 41, 307-312. [DOI] [PubMed] [Google Scholar]
- Hayes, S., Chawla, A., and Corvera, S. (2002). TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 158, 1239-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain, M.M. (2001). Structural, biochemical and signaling properties of the low-density lipoprotein receptor gene family. Front. Biosci. 6, D417-D428. [DOI] [PubMed] [Google Scholar]
- Kavsak, P., Rasmussen, R.K., Causing, C.G., Bonni, S., Zhu, H., Thomsen, G.H., and Wrana, J.L. (2000). Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol. Cell 6, 1365-1375. [DOI] [PubMed] [Google Scholar]
- Kittler, J.T., and Moss, S.J. (2001). Neurotransmitter receptor trafficking and the regulation of synaptic strength. Traffic 2, 437-448. [DOI] [PubMed] [Google Scholar]
- Knutson, V.P. (1992). Ligand-independent internalization and recycling of the insulin receptor. Effects of chronic treatment of 3T3-C2 fibroblasts with insulin and dexamethasone. J. Biol. Chem. 267, 931-937. [PubMed] [Google Scholar]
- Koli, K.M., and Arteaga, C.L. (1997). Processing of the transforming growth factor beta type I and II receptors. Biosynthesis and ligand-induced regulation. J. Biol. Chem. 272, 6423-6427. [DOI] [PubMed] [Google Scholar]
- Macias-Silva, M., Abdollah, S., Hoodless, P., Pirone, R., Attisano, L., and Wrana, J.L. (1996). MADR2 is a substrate of the TGFβ receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell 87, 1215-1224. [DOI] [PubMed] [Google Scholar]
- Massagué, J. (1996). TGFβ signaling: receptors, transducers, and Mad proteins. Cell 85, 947-950. [DOI] [PubMed] [Google Scholar]
- Massagué, J., and Kelly, B. (1986). Internalization of transforming growth factor-β and its receptor in BALB/c 3T3 fibroblasts. J. Cell. Physiol. 128, 216-222. [DOI] [PubMed] [Google Scholar]
- Massagué, J., and Like, B. (1985). Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J. Biol. Chem. 260, 2636-2645. [PubMed] [Google Scholar]
- McCaffrey, M.W., Bielli, A., Cantalupo, G., Mora, S., Roberti, V., Santillo, M., Drummond, F., and Bucci, C. (2001). Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 495, 21-30. [DOI] [PubMed] [Google Scholar]
- Mercier, T., Gaillard-Sanchez, I., Martel, P., and Seillan-Heberden, C. (1995). Constitutive overexpression of c-fos protein in rat liver epithelial cells decreases TGF-beta synthesis and increases TGF-beta 1 receptors. Biochim. Biophys. Acta 1266, 64-72. [DOI] [PubMed] [Google Scholar]
- Mollenhauer, H.H., Morre, D.J., and Rowe, L.D. (1990). Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim. Biophys. Acta 1031, 225-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos, T., and Morgan, E.H. (2000). Transferrin and transferrin receptor function in brain barrier systems. Cell. Mol. Neurobiol. 20, 77-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, J.R., Augustine, G.J., and Lafer, E.M. (2002). Synaptic vesicle endocytosis: the races, places, and molecular faces. Neuromol. Med. 2, 101-114. [DOI] [PubMed] [Google Scholar]
- Moses, H.L., and Serra, R. (1996). Regulation of differentiation by TGF-β. Curr. Opin. Genet. Dev. 6, 581-586. [DOI] [PubMed] [Google Scholar]
- Motley, A., Bright, N.A., Seaman, M.N., and Robinson, M.S. (2003). Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, 909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka, Y., and Czech, M.P. (1986). The type II insulin-like growth factor receptor is internalized and recycles in the absence of ligand. J. Biol. Chem. 261, 9090-9093. [PubMed] [Google Scholar]
- Panopoulou, E., Gillooly, D.J., Wrana, J.L., Zerial, M., Stenmark, H., Murphy, C., and Fotsis, T. (2002). Early endosomal regulation of Smad-dependent signaling in endothelial cells. J. Biol. Chem. 277, 18046-18052. [DOI] [PubMed] [Google Scholar]
- Pelkmans, L., and Helenius, A. (2002). Endocytosis via caveolae. Traffic 3, 311-320. [DOI] [PubMed] [Google Scholar]
- Penheiter, S.G., Mitchell, H., Garamszegi, N., Edens, M., Doré, J.J., Jr., and Leof, E.B. (2002). Internalization-dependent and -independent requirements for transforming growth factor beta receptor signaling via the Smad pathway. Mol. Cell. Biol. 22, 4750-4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek, E., Ju, W.J., Heyer, J., Escalante-Alcalde, D., Stewart, C.L., Weinstein, M., Deng, C., Kucherlapati, R., Böttinger, E.P., and Roberts, A.B. (2001). Functional characterization of transforming growth factor β signaling in Smad2- and Smad3-deficient fibroblasts. J. Biol. Chem. 276, 19945-19953. [DOI] [PubMed] [Google Scholar]
- Razani, B., Zhang, X.L., Bitzer, M., von Gersdorff, G., Bottinger, E.P., and Lisanti, M.P. (2001). Caveolin-1 regulates transforming growth factor (TGF)beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J. Biol. Chem. 276, 6727-6738. [DOI] [PubMed] [Google Scholar]
- Roberts, A.B. (1992). Differential Expression of the TGFβ isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol. Reprod. Dev. 32, 91-98. [DOI] [PubMed] [Google Scholar]
- Rodal, S.K., Skretting, G., Garred, O., Vilhardt, F., van Deurs, B., and Sandvig, K. (1999). Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10, 961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle, S.J., and Murrell-Lagnado, R.D. (2003). Constitutive cycling: a general mechanism to regulate cell surface proteins. Bioessays 25, 39-46. [DOI] [PubMed] [Google Scholar]
- Sathre, K.A., Tsang, M.L.-S., Weatherbee, J.A., and Steer, C.J. (1991). Binding and internalization of transforming growth factor-β1 by human hepatoma cells: evidence for receptor recycling. Hepatology 14, 287-295. [PubMed] [Google Scholar]
- Shapiro, M.J., and Coughlin, S.R. (1998). Separate signals for agonist-independent and agonist-triggered trafficking of protease-activated receptor 1. J. Biol. Chem. 273, 29009-29014. [DOI] [PubMed] [Google Scholar]
- Shapiro, P.S., and Ahn, N.G. (1998). Feedback regulation of Raf-1 and mitogen-activated protein kinase (MAP) kinase kinases 1 and 2 by MAP kinase phosphatase-1 (MKP-1). J. Biol. Chem. 273, 1788-1793. [DOI] [PubMed] [Google Scholar]
- Sharma, D.K., Brown, J.C., Choudhury, A., Peterson, T.E., Holicky, E., Marks, D.L., Simari, R., Parton, R.G., and Pagano, R.E. (2004). Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol. Biol. Cell 15, 3114-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff, D.R., Daro, E.A., Hull, M., and Mellman, I. (1999). The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R.D., Puri, V., Valiyaveettil, J.T., Marks, D.L., Bittman, R., and Pagano, R.E. (2003). Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol. Biol. Cell 14, 3254-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen, B., De Renzis, S., Nielsen, E., Rietdorf, J., and Zerial, M. (2000). Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark, H., and Olkkonen, V.M. (2001). The Rab GTPase family. Genome Biol. 2, REVIEWS3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel, W., Strous, G.J., Ciechanover, A., and Schwartz, A.L. (1991). Trafficking of the transferrin receptor. Targeted Diagn. Ther. 4, 267-304. [PubMed] [Google Scholar]
- Subtil, A., Gaidarov, I., Kobylarz, K., Lampson, M.A., Keen, J.H., and McGraw, T.E. (1999). Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96, 6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke, P., Franzen, P., Yamashita, H., Ichijo, H., Heldin, C.H., and Miyazono, K. (1994). Serine/threonine kinase receptors. Prog. Growth Factor Res. 5, 55-72. [DOI] [PubMed] [Google Scholar]
- ten Dijke, P., Miyazono, K., and Heldin, C.-H. (1996). Signaling via heterooligomeric complexes of type I and type II serine/threonine kinase receptors. Curr. Opin. Cell Biol. 8, 139-145. [DOI] [PubMed] [Google Scholar]
- Trowbridge, I.S. (1991). Endocytosis and signals for internalization. Curr. Opin. Cell Biol. 3, 634-641. [DOI] [PubMed] [Google Scholar]
- Tyler, W.J., Perrett, S.P., and Pozzo-Miller, L.D. (2002). The role of neurotrophins in neurotransmitter release. Neuroscientist 8, 524-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield, L.M., Smith, D.M., Masui, T., Harris, C.C., and Sporn, M.B. (1987). Distribution and modulation of the cellular receptor for transforming growth factor-β. J. Cell Biol. 105, 965-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C.C., Sonne, O., Hedo, J.A., Cushman, S.W., and Simpson, I.A. (1983). Insulin-induced internalization of the insulin receptor in the isolated rat adipose cell. Detection of the internalized 138-kilodalton receptor subunit using a photoaffinity 125I-insulin. J. Biol. Chem. 258, 5129-5134. [PubMed] [Google Scholar]
- Wells, R.G., Yankelev, H., Lin, H.Y., and Lodish, H.F. (1997). Biosynthesis of the type I and type II TGF-β receptors. J. Biol. Chem. 272, 11444-11451. [DOI] [PubMed] [Google Scholar]
- Wilcke, M., Johannes, L., Galli, T., Mayau, V., Goud, B., and Salamero, J. (2000). Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-Golgi network. J. Cell Biol. 151, 1207-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley, H.S., and Burke, P.M. (2001). Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic 2, 12-18. [DOI] [PubMed] [Google Scholar]
- Wiley, H.S., Herbst, J.J., Walsh, B.J., Lauffenburger, D.A., Rosenfeld, M.G., and Gill, G.N. (1991). The role of tyrosine kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 266, 11083-11094. [PubMed] [Google Scholar]
- Yao, D., Ehrlich, M., Henis, Y.I., and Leof, E.B. (2002). Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol. Biol. Cell 13, 4001-4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling, J.M., Das, P., Savage, C., Zhang, M., Padgett, R.W., and Wang, X.-F. (1996). Mammalian dwarfins are phosphorylated in response to transforming growth factor β and are implicated in control of cell growth. Proc. Natl. Acad. Sci. USA 93, 8940-8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial, M., and McBride, H. (2001). Rab proteins as membrane organizers. Nat Rev Mol. Cell. Biol. 2, 107-117. [DOI] [PubMed] [Google Scholar]
- Zhao, J., and Buick, R.N. (1995). Regulation of transforming growth factor β receptors in H-ras oncogene-transformed rat intestinal epithelial cells. Cancer Res. 55, 6181-6188. [PubMed] [Google Scholar]
- Zhao, X., Greener, T., Al-Hasani, H., Cushman, S.W., Eisenberg, E., and Greene, L.E. (2001). Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J. Cell Sci. 114, 353-365. [DOI] [PubMed] [Google Scholar]
- Zwaagstra, J.C., El-Alfy, M., and O'Connor-McCourt, M.D. (2001). Transforming growth factor (TGF)-beta 1 internalization: modulation by ligand interaction with TGF-beta receptors types I and II and a mechanism that is distinct from clathrin-mediated endocytosis. J. Biol. Chem. 276, 27237-27245. [DOI] [PubMed] [Google Scholar]
- Zwaagstra, J.C., Kassam, Z., and O'Connor-Mccourt, M.D. (1999). Down-regulation of transforming growth factor-beta receptors: cooperativity between the types I, II, and III receptors and modulation at the cell surface. Exp. Cell Res. 252, 352-362. [DOI] [PubMed] [Google Scholar]