Abstract
Previous work has implicated the Hog1 stress-activated protein kinase (SAPK) in osmotic and oxidative stress responses in the human pathogen Candida albicans. In this study, we have characterized the role of Hog1 in mediating these and other stress responses in C. albicans. We provide evidence that a SAPK-dependent core stress response exists in this pathogen. The Hog1 SAPK is phosphorylated and it accumulates in the nucleus in response to diverse stress conditions. In addition, we have identified Hog1-regulated genes that are induced in response to stress conditions that activate Hog1. These analyses reveal both activator and repressor functions for the Hog1 SAPK. Our results also demonstrate that stress cross-protection, a classical hallmark of the core stress response, occurs in C. albicans between stresses that activate the Hog1 SAPK. Importantly, we find that the core stress response in C. albicans has adapted to the environmental niche of this human pathogen. This niche specificity is reflected by the specific environmental conditions that drive the Hog1-regulated core stress response in C. albicans and by differences in the molecular circuitry that control this response.
INTRODUCTION
The ability of cells to sense and respond rapidly to changes in their environment is critical for survival. Pathogenic microbes adapt to changing microenvironments during disease progression and have the added challenge of contending with host defenses. Candida albicans is the major systemic fungal pathogen of humans (Odds, 1988). This yeast frequently causes superficial infections in relatively immunocompetent individuals, and life-threatening systemic infections in immunocompromised patients. Its success as a pathogen is based partly upon its resistance to oxidative stresses and other environmental insults. For example, C. albicans can evade oxidative killing by macrophages (Lo et al., 1997), and inactivating stress responses attenuates virulence (Wysong et al., 1998; Alonso-Monge et al., 1999). Although these observations suggest that C. albicans has evolved specific responses to protect it in the diverse microenvironments it encounters during disease progression in its human host, the molecular mechanisms underlying such responses are poorly understood.
Global approaches have been employed to elucidate the molecular responses of yeast cells to a diverse range of environmental stresses. For example, transcript profiling of the budding yeast Saccharomyces cerevisiae (Gasch et al., 2000; Causton et al., 2001) and the distantly related fission yeast Schizosaccharomyces pombe (Chen et al., 2003) revealed that a large fraction of their genomes respond in a stereotypical manner to a range of diverse stress conditions. These stereotypical responses have been termed the Environmental Stress Response (ESR) or the Common Environmental Response (CER) in budding yeast (Gasch et al., 2000; Causton et al., 2001), and the Core Environmental Stress Response (CESR) in fission yeast (Chen et al., 2003). These responses are thought to underlie the previously identified general stress response and the phenomenon of stress cross-protection (reviewed in Siderius and Mager, 1997), in which exposure to one type of stress can protect the cell against subsequent exposure to an apparently unrelated stress.
S. pombe and S. cerevisiae use different strategies to regulate their core stress response genes and this partly involves differential regulation of their stress-activated protein kinase (SAPK) pathways. In S. pombe, the CESR is controlled predominantly by the Sty1 SAPK (Chen et al., 2003), which is activated in response to diverse stress conditions such as oxidative stress, osmotic stress, temperature upshift, nutrient limitation, DNA damaging agents, and heavy metals (Millar et al., 1995; Shiozaki and Russell, 1995, 1996; Degols et al., 1996; Degols and Russell, 1997; Shieh et al., 1997, 1998; Shiozaki et al., 1998; Buck et al., 2001). In contrast, the ESR/CER in S. cerevisiae is not governed by a single regulatory pathway. Instead, different stresses control a common set of genes via different signaling pathways and transcription factors (Gasch et al., 2000; Causton et al., 2001). For example, the S. cerevisiae Hog1 SAPK, unlike the homologous fission yeast Sty1 SAPK, responds mainly to changes in osmolarity. Hence, in budding yeast, Hog1 plays a role in the regulation of core stress genes in response to this stress (Posas et al., 2000; Rep et al., 2000; O'Rourke et al., 2002; O'Rourke and Herskowitz, 2004), but not other stresses (Gasch et al., 2001).
Although less is known about stress responses in C. albicans, these responses are clearly important for the survival of this pathogen in its mammalian host, because deletion of the C. albicans Hog1 SAPK results in impaired virulence (Alonso-Monge et al., 1999). Recent studies have shown that there are clear differences between the stress responses of this yeast and those of S. cerevisiae and S. pombe. For example, transcript profiling revealed that C. albicans does not mount a common transcriptional response after exposure to environmental conditions that stimulate general stress responses in S. cerevisiae and S. pombe (Enjalbert et al., 2003). Furthermore, homologues of the S. cerevisiae Msn2/4 transcription factors, which play a key role in regulating the ESR/CER in S. cerevisiae, have no obvious roles in stress responses in C. albicans (Nicholls et al., 2004).
In this study, we demonstrate for the first time, that C. albicans mounts a specialized core stress response that has evolved to fit the environmental niches occupied by this fungal pathogen. Significantly, we find that the C. albicans Hog1 SAPK plays a pivotal role in the regulation of this core stress response.
MATERIALS AND METHODS
Strains and Growth Conditions
The strains used in this study are given in Table 1. C. albicans strains were grown in either rich YPD medium or SD minimal medium (Sherman, 1991), and S. pombe strains were grown in either rich YE5S medium or EMM synthetic minimal medium as described previously (Moreno et al., 1991; Alfa et al., 1993). All strains were grown at 30°C unless indicated otherwise.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| RM1000 | ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG | Negredo et al., 1997 |
| WT | ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG CIp20 (URA3, HIS1) | This study |
| JC4 | ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG, HOG1-HM:URA3 | This study |
| ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG, hog1 :: LoxP-URA3-LoxP, hog1 :: LoxP-HIS1-LoxP | ||
| JC10 | ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG, hog1 :: LoxP-URA3-LoxP, hog1 :: LoxP-HIS1-LoxP | This study |
| JC50 | ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG, hog1 :: LoxP-ura3-LoxP, hog1 :: LoxP-HIS1-LoxP CIp20 (URA3, HIS1) | This study |
| JC52 | ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG, hog1 :: LoxP-ura3-LoxP, hog1 :: LoxP-HIS1-LoxP CIp20-HOG1 (URA3, HIS1) | This study |
| JC63 | ura3 :: λimm434/ura3 :: λimm434, his1 :: hisG/his1 :: hisG, HOG1-YFP:URA3 HOG1-YFP:HIS1 | This study |
| CHP428 | h+ his7-366 ade6-210 leu1-32 ura4-D18 | Gift from Charlie Hoffman |
| JM1160 | h− sty1 :: ura4+ leu1-32 ura4-D18 | Millar et al., 1995 |
Strain Construction
Oligonucleotide primers used in this study are listed in Table 2. To delete HOG1, disruption cassettes comprising of either the URA3 or HIS1 gene flanked by loxP sites and 80 base pairs of DNA sequence corresponding to regions of the HOG1 open reading frame (ORF) were generated by polymerase chain reaction (PCR) by using the oligonucleotide primers HOGdelF and HOGdelR and the plasmid templates pLUL2 or pLHL2, respectively (Dennison, Ramsdale, Manson, and Brown, unpublished data). Disruption cassettes were transformed into C. albicans RM1000 to sequentially disrupt both alleles of HOG1 and generate strain JC10. Accurate gene disruption was confirmed by PCR and DNA sequencing. Introduction of these cassettes resulted in the deletion of codons 45-359 of the 411 codon HOG1 ORF. To reintroduce HOG1 into JC10, the HOG1 locus was PCR amplified using the oligonucleotide primers HOG1PromF and HOG1TermR and cloned into the URA3- and HIS1-containing integrating plasmid CIp20, a derivative of CIp10 (Murad et al., 2000). CIp20 and CIp20-HOG1 were integrated at the RPS10 locus in a 5-fluoroorotic acid-resistant derivative of JC10, to create strains JC50 and JC52, respectively. To tag Hog1 at the C terminus with 6-His residues and two copies of the myc-epitope, the HOG1 gene was amplified by PCR by using the oligonucleotide primers HOG1PstF and HOG1HMR and ligated into CIp-C-ZZ (Blackwell et al., 2003), which had been digested with PstI and NheI to remove the TEV-protein A sequence. The resulting CIp-C-HOG1HM plasmid was linearized by digestion with HpaI to target chromosomal integration at the HOG1 locus in C. albicans RM1000 to generate strain JC4. Chromosomal insertion of the C-terminal His6-myc tag was confirmed by PCR and DNA sequencing. To chromosomally tag Hog1 with YFP, HOG1-specific sequences were added to the universal primer sequences described previously (Gerami-Nejad et al., 2001) to generate the oligonucleotide primers HOG1YFPF and HOG1YFPR. These primers were used in combination with the plasmid templates pYFP-URA3 and pYFP-HIS1 to generate HOG1-YFP cassettes by PCR (Gerami-Nejad et al., 2001). The HOG1-YFP cassettes were sequentially transformed into C. albicans RM1000, to create strain JC63, and correct integration at both HOG1 loci was confirmed by PCR and DNA sequencing.
Table 2.
Oligonucleotide primers used in this study
| Oligonucleotide | Sequence 5′-3′ |
|---|---|
| HOGdelF | caccaatagatacactgagctaaatcccgtgggaatgggagcatttggtttggtgtgctcagccgttgatagattaactgccagggttttcccagtcacg |
| HOGdelR | gccacaccaacagtttgatgaaagtctaaaatttcactgtacatcataactctccaagtatccactggcaagtctgcgtcctcactaaagggaacaaaagc |
| HOG1PstF | aatgtctgcagatggagaatttacaagaacc |
| HOG1PromF | gcgcggatccgacatttccgttaaagtgtccac |
| HOG1TermR | gcgcggatccggggaaacagtgaatatgtaaaatgtg |
| HOG1HMR | gaattcgctagcttaatgatggtgatgatggtgtaagtcctcctcgctgatcaaatttttgttcttcagccatggacaaatcttcttcagaaattaacttttgctcctcagctccgttggcggaatccaag |
| HOG1YFPF | aatgaaactgagggttccgaacaaccagacctgcaagtggagcaaaacaacttggattccgccaacggagctggtggtggttctaaaggtgaagaattatt |
| HOG1YFPR | gttaatagtaatagtaatacatatttcacttttaaatttatttctataattgctagcttgtatttttgaagctctagaaggaccacctttgattg |
| HOG1BamF | gcgcggatcccatgtctgcagatggagaatttacaagaacc |
| HOG1BamR | gcgcggatccttaagctccgttggcggaatcc |
| HSP12-F | atgtctgacgccggaag |
| HSP12-R | agtactatcagcgccgg |
| RHR2-F | gacaaagactcaacaaccag |
| PHR2-R | ccttgaattcgtcagtttcc |
| ACT1-F | gatgaagcccaatccaaaag |
| ACT1-R | ggagttgaaagtggtttggt |
Plasmid Construction
To create pREP41MH-HOG1, the C. albicans HOG1 gene was amplified by PCR from genomic DNA by using the oligonucleotide primers HOG1BamF and HOG1BamR and ligated into the BamHI site of pREP41MH (Craven et al., 1998).
Stress Sensitivity Tests
For stress sensitivity assays, strains to be tested were grown at 30°C to mid-exponential phase. Cells were diluted in YPD media, and 103 cells were spotted in 5 μl onto YPD agar containing the specific compound at the indicated concentration. Plates were incubated at 30°C for 24 h, unless indicated otherwise.
For stress cross-protection analysis, exponential cultures of wild-type (WT), Δhog1 (JC10), and Δhog1+HOG1 (JC52) cells were pretreated for 1 h with the following stress conditions: untreated control, oxidative stress (0.4 mM H2O2), osmotic stress (0.3 M NaCl), or heat stress (temperature shift from 23 to 37°C). Cells were collected by centrifugation and washed once in prewarmed YPD before resuspension in an equal volume of YPD. Aliquots (10 ml) were then incubated with increasing concentrations of H2O2. Cells were taken after 1 h, diluted, and plated onto YPD plates to determine surviving cell numbers. Plates were incubated for 24 h at 30°C, and survival was expressed as a percentage of viable cells at time = 0.
Hog1 Phosphorylation Assays
Strains containing chromosomally tagged Hog1-His6-myc (JC4) were grown to mid-exponential phase and exposed to a range of stress conditions for the indicated times. Hog1-His6-myc was partially purified from cell extracts using Ni2+-NTA agarose (QIAGEN, Valencia, CA), and phosphorylated Hog1 was detected by Western blot with an anti-phospho-p38 antibody (New England Biolabs, Beverly, MA) as described previously (Millar et al., 1995). Blots were stripped and total levels of Hog1 were then determined by probing with an anti-myc 9E10 antibody (Sigma-Aldrich, St. Louis, MO).
Hog1 Phosphorylation of Sty1 Substrates
To assay Hog1 activity in S. pombe, lysates were prepared from wild-type cells (CHP429) or sty1- cells (JM1160), transformed with the empty vector pREP41MH, or with pREP41MH-HOG1, before and after treatment with 0.6 M KCl for 10 min. Lysates (300 μg) were incubated with 5 μg of recombinant GST-Atf1 (Wilkinson et al., 1996) or His6-Srk1K153A (Smith et al., 2002) (prebound to glutathione [GSH]-agarose or Ni2+-NTA agarose, respectively) for 1 h at 4°C. The beads were washed three times with lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM imidazole, 0.1% NP-40, 50 mM NaF, 2 mM Na2VO3, 1 mM phenylmethylsulfonyl fluoride, 0.07 trypsin inhibitor units/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin) and once with kinase buffer (20 mM HEPES, pH 7.5, 20 mM MgCl2, 2 mM diothiothreitol, 20 μM ATP). Beads were resuspended in 20 μl of kinase buffer with 5 μCi of [γ32-P]ATP, and kinase reactions were allowed to proceed for 30 min at 30°C. Samples were analyzed by SDS-PAGE on 10% gels, transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), and autoradiographed.
Fluorescence Microscopy
Localization of chromosomally yellow fluorescent protein (YFP)-tagged Hog1 was determined by fluorescence microscopy. Samples of exponentially growing C. albicans cells (OD595 = 0.5) expressing chromosomally tagged Hog1-YFP (JC63), untreated or treated with a range of stress conditions for the indicated times were collected and fixed in 3.7% paraformaldehyde. Cells were spread onto poly-l-lysine-coated slides and coverslips mounted onto slides by using Vectashield mounting medium containing 1.5 mg/ml 4′-6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA). DAPI and YFP fluorescence were captured by exciting cells with 365- and 450- to 490-nm wavelengths, respectively, by using an Axiovert microscope (Carl Zeiss, Jena, Germany), with a 100× oil immersion objective, and Axiovision imaging system.
Northern Analysis
C. albicans RNA preparation and Northern analysis were performed as described previously (Brown et al., 2001). Gene-specific probes were amplified by PCR from genomic DNA by using the oligonucleotide primers listed in Table 2.
RESULTS
C. albicans Hog1 Is Activated in Response to Diverse Stimuli
Previous studies have shown that the S. pombe Sty1 SAPK responds to a diverse range of stress conditions (reviewed in Toone and Jones, 1998). In contrast, the Hog1 SAPK, in the evolutionarily distant yeast S. cerevisiae, responds mainly to osmotic stress (Brewster et al., 1993) and to a lesser extent to heat stress (Winkler et al., 2002). We were interested in whether the related SAPK in C. albicans, Hog1, responds to a range of stress conditions (like the fission yeast Sty1 SAPK), or is primarily an osmosensing kinase (like the S. cerevisiae Hog1 SAPK). Therefore, we determined the levels of active phosphorylated Hog1 in C. albicans after exposure to a range of different environmental stresses. To facilitate this analysis the strain JC4 was created in which one copy of Hog1 is C-terminally tagged with six histidine residues and two copies of the myc epitope (Hog1-His6-myc). This fusion was functional, because hog1/HOG1-His6-myc cells displayed no increased sensitivity to various stress treatments compared with heterozygous hog1/HOG1 cells (our unpublished data). JC4 cells were treated with a range of stress conditions for the indicated times, and Hog1 phosphorylation was monitored by Western blotting with an antibody that recognizes only the active, phosphorylated form of SAPKs (Millar et al., 1995). The Hog1 fusion protein is tagged with the myc epitope, and hence blots were reprobed with an anti-myc antibody to confirm even loading.
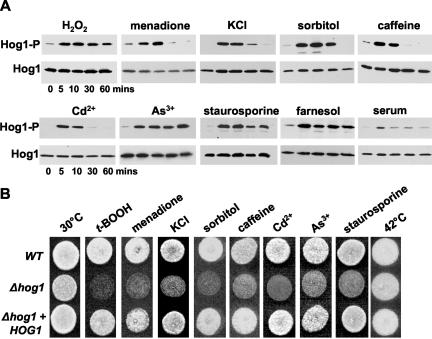
Hog1 was originally described as regulating osmotic stress responses in C. albicans (San-Jose et al., 1996). However, during the course of this study, it was reported that C. albicans Hog1 also responds to oxidative stress conditions (Alonso-Monge et al., 2003). Here, we have confirmed and extended this analysis by showing that significant levels of Hog1 phosphorylation are observed after exposure to a wide range of conditions (Figure 1A). Moreover, the kinetics and magnitude of Hog1 activation depended upon the particular stress condition imposed on the cell. For example, exposure to osmotic stresses such as KCl and sorbitol, the superoxide generator menadione, the heavy metal Cd2+, or the purine analogue caffeine resulted in rapid but transient activation of Hog1. In contrast, treatment with H2O2, the heavy metal As+, or the drug staurosporine resulted in a rapid but prolonged activation of Hog1. Intriguingly, we also found that Hog1 exhibits a rapid and sustained activation upon exposure to the recently identified quorum-sensing molecule in C. albicans, the isoprenoid farnesol (Hornby et al., 2001; Ramage et al., 2002). Significantly, Δhog1 cells displayed increased sensitivity compared with wild-type cells when challenged with many of the same stresses, and this sensitivity was reversed upon introduction of the wild-type HOG1 gene into the Δhog1 strain (Figure 1B). This emphasizes the importance of Hog1 signaling in the cellular responses to these stresses. However, we did note that Δhog1 cells and wild-type cells grew equally well in the presence of the quorum sensing molecule farnesol (our unpublished data).
Figure 1.
C. albicans Hog1 is phosphorylated in response to diverse stresses and Δhog1 cells are sensitive to these stresses. (A) Western blot analysis of Ni2+-NTA agarose-purified Hog1-His6-myc isolated from C. albicans cells after treatment with the following compounds: 5 mM H2O2, 0.3 mM menadione, 0.6 M KCl, 1.2 M sorbitol, 10 mM caffeine, 0.5 mM CdSO4, 2 mM Na2As3, 2.0 μM staurosporine, 0.1 mM farnesol, or 20% serum. Western blots were probed with an anti-phospho p38 antibody, which only recognizes the phosphorylated, active form of C. albicans Hog1 (Hog1-P). Total levels of Hog1 protein was determined by stripping and reprobing the blot with an anti-myc antibody that recognizes both phosphorylated and unphosphorylated forms of Hog1 (Hog1). (B) Approximately 103 cells from exponentially growing wild-type (WT), Δhog1 (JC50), or Δhog1+HOG1 (JC52) strains were spotted onto YPD plates containing the compounds listed above, with the exception that t-BOOH (1 mM) was used instead of H2O2. Plates were incubated at 30°C (or 42°C) for 24 h.
Previous work has suggested that the Hog1 SAPK pathway also is involved in the regulation of yeast-hypha morphogenesis in C. albicans, because serum-induced hyphal development is derepressed in hog1 cells (Alonso-Monge et al., 1999). Hence, we also examined the response of Hog1 to serum. As illustrated in Figure 1A, we observed a slight increase in Hog1-phosphorylation after serum treatment, which is consistent with a role for Hog1 in regulating morphogenesis.
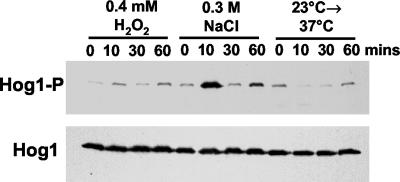
Clearly, the C. albicans Hog1 SAPK responds to a diverse range of stimuli (Figure 1). Based on studies in S. pombe (Chen et al., 2003), we reasoned that conditions that activate C. albicans Hog1 might result in the induction of a common set of genes that are regulated by this SAPK. However, previous experiments in C. albicans suggested that a common set of genes were not induced in response to osmotic stress, oxidative stress, and temperature upshift (Enjalbert et al., 2003). In this transcript-profiling study, the authors chose experimental conditions that had previously been shown to stimulate the ESR/CER in S. cerevisiae (0.3 M NaCl, 0.4 mM H2O2, and temperature shift from 23 to 37°C; Gasch et al., 2000; Causton et al., 2001). However, they did not examine activation of C. albicans Hog1 in response to these treatments. Therefore, we tested whether the Hog1 SAPK is activated by the osmotic stress, oxidative stress, and temperature upshift conditions used by Enjalbert et al. (2003). Significantly, only one condition strongly activated the Hog1 SAPK (treatment with 0.3 M NaCl; Figure 2). Indeed, the temperature shift appeared to cause a decrease in the basal level of phosphorylated Hog1 (Figure 2; see below). These findings probably account for the absence of a common transcriptional response to the three stress conditions used in the transcript-profiling study (Enjalbert et al., 2003).
Figure 2.
Activation profile of the Hog1 SAPK in response to the transcript-profiling conditions. Western blot analysis of Ni2+-NTA agarose purified Hog1-His6-myc from wild-type C. albicans cells after treatment with the same conditions used in the transcript-profiling study (Enjalbert et al., 2003), namely; 0.3 M NaCl, 0.4 mM H2O2, or temperature shift from 23 to 37°C, for the times indicated. Phosphorylated Hog1 (Hog1-P) and total Hog1 (Hog1) levels were detected as described in Figure 1.
The C. albicans-specific Activation Profile of the Hog1 SAPK
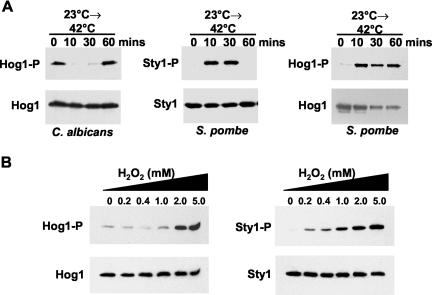
Our results indicate that the C. albicans SAPK pathway has a unique activation profile that differs from both the S. pombe Sty1 and S. cerevisiae Hog1 SAPKs. For example, both the S. pombe and S. cerevisiae SAPKs are activated in response to temperature upshift (Shieh et al., 1998; Shiozaki et al., 1998; Nguyen and Shiozaki, 1999; Winkler et al., 2002). However, the C. albicans Hog1 SAPK is not activated upon temperature upshift. In stark contrast, a significant and reproducible decrease in the basal level of Hog1 activation was observed upon shifting cells from 23°C to either 37 or 42°C (Figures 2 and 3A). Also, C. albicans Δ hog1 cells did not display impaired growth compared with wild-type cells in response to temperature upshift (Figure 1B). This is in stark contrast with S. pombe cells lacking sty1+, which struggle to grow at 36°C (Figure 4A). Furthermore, although the Sty1 SAPK is activated in response to low concentrations of H2O2 (Figure 3B; Quinn et al., 2002), C. albicans Hog1 activation is only seen at much higher levels of H2O2 (>2 mM) (Figure 3B). This unique activation profile of the C. albicans Hog1 SAPK pathway may have evolved to protect this human pathogen against host defenses and promote its survival in the human host (see Discussion).
Figure 3.
C. albicans Hog1 and the S. pombe Sty1 SAPKs respond differently to oxidative stress and temperature upshift. (A) Phosphorylation of S. pombe Sty1 and C. albicans Hog1 in response to temperature shift from 23 to 42°C. C. albicans HOG1 also was expressed heterologously in sty1- cells and phosphorylation of Hog1 was analyzed under the same conditions. (B) Phosphorylation of C. albicans Hog1 and S. pombe Sty1 was compared after treatment with a range of H2O2 concentrations. Phosphorylated Hog1 (Hog1-P) and total Hog1 (Hog1) levels were detected as described in Figure 1. Phosphorylated Sty1 (Sty1-P) and total Sty1 (Sty1) levels were detected as described previously (Millar et al., 1995).
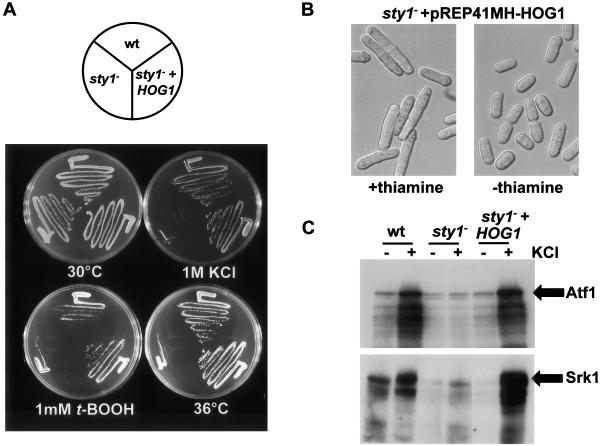
Figure 4.
C. albicans Hog1 is a functional homologue of S. pombe Sty1. sty1- cells were transformed with pREP41MH-HOG1, in which the expression of C. albicans HOG1 is under the control of the fission yeast thiamine-repressible nmt41 promoter. (A) Expression of HOG1 in sty1- cells complements sty1--associated stress phenotypes. The growth of wild-type cells, sty1- cells, and sty1- cells transformed with pREP41MH-HOG1 was compared upon exposure to osmotic stress (0.6 M KCl), oxidative stress (1 mM t-BOOH), temperature upshift (36°C), or no stress (30°C). (B) Expression of HOG1 in sty1- cells rescues the sty1--associated cell cycle defect. sty1- cells, transformed with pREP41MH-HOG1, were grown in the presence or absence of thiamine and differential interference contrast images captured. (C) Active Hog1 phosphorylates Sty1 substrates. Extracts prepared from wild-type (wt), sty1-, and sty1- cells transformed with pREP41MH-HOG1, that had been harvested before (-) or after (+) treatment with 0.6 M KCl for 10 min, were incubated with recombinant GST-Atf1 prebound to GSH-agarose or His6-Srk1K153A prebound to Ni2+-NTA agarose beads for 1 h at 4°C. A kinase assay was performed and phosphorylation of GST-Atf1 and His6-Srk1K153A was assessed by SDS-PAGE and autoradiography.
Heat shock-induced activation of the Sty1 SAPK is regulated via inhibition of Pyp1, the major tyrosine phosphatase that dephosphorylates and inactivates Sty1 (Nguyen and Shiozaki, 1999). The contrasting response of the C. albicans Hog1 SAPK to temperature upshift suggested that Hog1, or regulators of this SAPK pathway, respond in a different manner to this treatment. To explore this further, we examined the phosphorylation of the C. albicans Hog1 SAPK in S. pombe sty1- cells in response to temperature upshift (Figure 3A). In direct contrast to the effects of heat observed in C. albicans, Hog1 was rapidly phosphorylated when the host fission yeast cells were exposed to a temperature upshift from 23 to 42°C. These data strongly suggest that the contrasting responses of these fungi to temperature are mediated by divergence in the regulators that relay temperature upshift signals to the SAPK.
C. albicans HOG1 Complements Phenotypes Associated with Deletion of the S. pombe sty1+ Gene
The C. albicans and S. pombe SAPKs are both activated in response to several stresses. Therefore, we investigated whether expression of C. albicans HOG1 in S. pombe could complement phenotypes associated with deletion of the sty1+ gene. In addition to key roles in stress signaling (reviewed in Toone and Jones, 1998), Sty1 also regulates cell cycle progression in fission yeast. For example, sty1- cells are considerably elongated due to a delay in G2 (Millar et al., 1995; Shiozaki and Russell, 1995). C. albicans HOG1 was expressed in sty1- cells under the control of a medium strength nmt41+ thiamine-repressible promoter (Basi et al., 1993). In the absence of thiamine, ectopic expression of HOG1 rescued both the sensitivity of sty1- cells to osmotic, oxidative and heat stresses, and the sty1--associated cell cycle defect (Figure 4, A and B).
Because HOG1 can complement phenotypes associated with loss of sty1+, it was likely that Hog1 can phosphorylate many, if not all, key Sty1 substrates. Hence, we tested whether the C. albicans SAPK phosphorylates two previously identified substrates of Sty1; Atf1 and Srk1. Atf1 is a transcription factor (Shiozaki and Russell, 1996; Wilkinson et al., 1996), and Srk1 is a serine/threonine protein kinase (Smith et al., 2002). Extracts were prepared from wild-type S. pombe cells (CHP429), sty1- cells (JM1160), and sty1- cells expressing HOG1, both before and after treatment with 0.6 M KCl for 10 min. Extracts were incubated with either recombinant GST-Atf1 or His6-tagged Srk1, and the phosphorylation of the fusion proteins was assayed (Figure 4C). In agreement with previous studies, no stress-induced phosphorylation of Atf1 and Srk1 was observed using sty1- extracts. However, significant Atf1 and Srk1 phosphorylation was seen with extracts prepared from wild-type cells or from sty1- cells expressing HOG1 (Figure 4C, lanes 2 and 6). Hence, C. albicans Hog1 can phosphorylate targets of S. pombe Sty1. Therefore, C. albicans Hog1 is a functional homologue of S. pombe Sty1.
Cellular Localization of Hog1
Previous studies of the S. pombe Sty1 SAPK (Gaits et al., 1998) and the S. cerevisiae Hog1 SAPK (Ferrigno et al., 1998; Reiser et al., 1999) revealed that these kinases are regulated at the level of cellular localization. In particular, both were found to translocate to the nucleus after activation. For example, the Sty1 SAPK accumulates in the nucleus after exposure to oxidative and osmotic stresses (Gaits et al., 1998; Smith et al., 2002), whereas the S. cerevisiae Hog1 SAPK accumulates in the nucleus after osmotic stress (Ferrigno et al., 1998; Reiser et al., 1999). Therefore, the cellular localization of the C. albicans Hog1 SAPK was examined in response to a range of stress conditions. To facilitate these studies, both copies of the HOG1 gene were chromosomally tagged with YFP, and the cellular localization of Hog1-YFP was examined by fluorescence microscopy. HOG1-YFP/HOG1-YFP cells displayed no increased sensitivity to various stress treatments compared with HOG1/HOG1 cells, illustrating that the Hog1-YFP fusion protein is functional in C. albicans (our unpublished data).
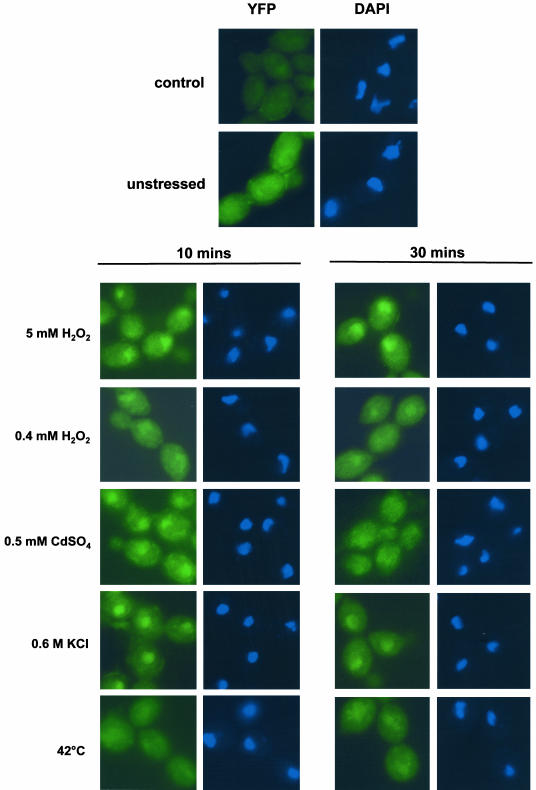
As illustrated in Figure 5, Hog1 did not accumulate in the nuclei of cells treated with stresses that do not activate Hog1, such as low levels of H2O2 (0.4 mM) or temperature shift from 23 to 42°C. In contrast, Hog1 rapidly accumulated in the nucleus after exposure to the stresses that do activate this SAPK. For example, exposure to high doses of H2O2 (5 mM), osmotic stress (0.6 M KCl), or heavy metal stress (0.5 mM CdSO4) resulted in rapid nuclear accumulation of Hog1. Moreover, the kinetics of nuclear accumulation of Hog1 depended on the stress-condition imposed on the cell and also correlated with the kinetics of phosphorylation (compare Figure 1A with Figure 5). For example, the rapid but transient phosphorylation of Hog1 in response to heavy metal and osmotic stress was mirrored by a rapid but transient nuclear accumulation of Hog1, which peaked at 10 min after exposure to stress (Figure 5). However, after exposure to high doses of H2O2, significant amounts of Hog1 remained in the nucleus after 30 min (Figure 5), which paralleled the sustained phosphorylation of Hog1 under this condition (Figure 1A).
Figure 5.
C. albicans Hog1-YFP accumulates in the nucleus in response to specific stresses. The localization of YFP-tagged Hog1 was determined by fluorescence microscopy in cells treated with the indicated stresses at 10-and 30-min time points (YFP). The position of the nuclei in these cells was visualized with DAPI. An autofluorescence control also is shown (control).
Identification of Hog1-regulated Genes
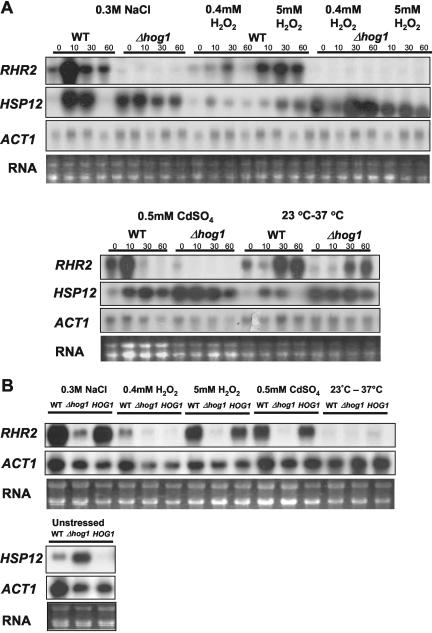
Our data show that the C. albicans Hog1 SAPK is phosphorylated and accumulates in the nucleus in response to a range of stress conditions. We predict that this will lead to the regulation of common gene targets of this SAPK pathway. However, to date, genes regulated by the Hog1 SAPK in C. albicans have not been identified. To identify candidate Hog1 gene targets, we looked for C. albicans genes that were induced in response to the osmotic stress conditions used in the transcript-profiling experiments (Enjalbert et al., 2003) and that seem to be homologues of known Hog1 targets in S. cerevisiae (Posas et al., 2000; Rep et al., 2000). Two genes were selected for analysis, HSP12 (IPF16067), which encodes a putative heat shock protein; and RHR2, which encodes a putative glycerol phosphate phosphatase (http://genolist.pasteur.fr/CandidaDB). Northern analyses were performed to examine the expression of these genes in wild-type and Δhog1 cells after exposure to low or high doses of oxidative stress (0.4 and 5 mM H2O2), osmotic/saline stress (0.3 M NaCl), heavy metal stress (0.5 mM CdSO4), or temperature upshift (from 23 to 37°C).
The RHR2 mRNA was induced in response to osmotic stress, which is in agreement with the previously published microarray data (Enjalbert et al., 2003). We found that high levels of oxidative stress or heavy metal stress also stimulated induction of RHR2. Moreover, RHR2 induction is dependent on the Hog1 SAPK because no detectable expression of RHR2 mRNA was observed in Δhog1 cells treated with the same stresses (Figure 6A). This lack of induction of RHR2 in Δhog1 cells is reversed upon integration of the wild-type HOG1 gene in the hog1 mutant (Figure 6B). Furthermore, the kinetics of RHR2 induction in wild-type cells paralleled the kinetics of Hog1 activation and nuclear localization (compare Figure 1A with Figures 5 and 6). These data indicate that Hog1 positively regulates RHR2 expression in response to osmotic, oxidative, and heavy metal stresses. We also found that RHR2 is slightly induced in response to temperature upshift, but this induction was not dependent on the Hog1 SAPK. This suggests that a separate, Hog1-independent signaling pathway regulates RHR2 expression in response to temperature upshift.
Figure 6.
Expression of Hog1-regulated genes in C. albicans. (A) Northern blot analysis of RNA isolated from mid-log cultures of wild-type (WT) and Δhog1 (JC10) cells treated with the indicated stresses for 0, 10, 30, and 60 min by using probes specific for RHR2, HSP12, and ACT1 (loading control). rRNA was stained with ethidium bromide (RNA). (B) Northern blot analysis comparing RHR2 and HSP12 expression in Δhog1+HOG1 (JC52), wild-type (WT), and Δ hog1 (JC50) cells. Cells were treated with the indicated stresses for 10 min.
Like RHR2, HSP12 was induced in response to osmotic stress (again in agreement with Enjalbert et al., 2003), and in response to high levels of oxidative stress, or heavy metal stress in wild-type cells. We noted that HSP12 also was slightly induced in response to temperature upshift. However, in contrast to RHR2, Hog1 seems to repress HSP12 because high basal levels of HSP12 expression were observed consistently in Δhog1 cells, even under nonstressed conditions (Figure 6A). The high basal level of HSP12 expression in the Δhog1 strain was reversed upon integration of the wild-type HOG1 gene in the hog1 mutant (Figure 6B). It is not yet clear whether the role of Hog1 in the regulation of HSP12 expression is direct. However, as HSP12 is only strongly induced in response to stress conditions that activate Hog1, it is possible that stress-induced phosphorylation of Hog1 functions to alleviate Hog1-mediated repression.
Collectively, this Northern analysis indicates that Hog1 plays different roles in the regulation of specific stress-activated genes in C. albicans.
Stress Cross-Protection in C. albicans Mediated by the Hog1 SAPK
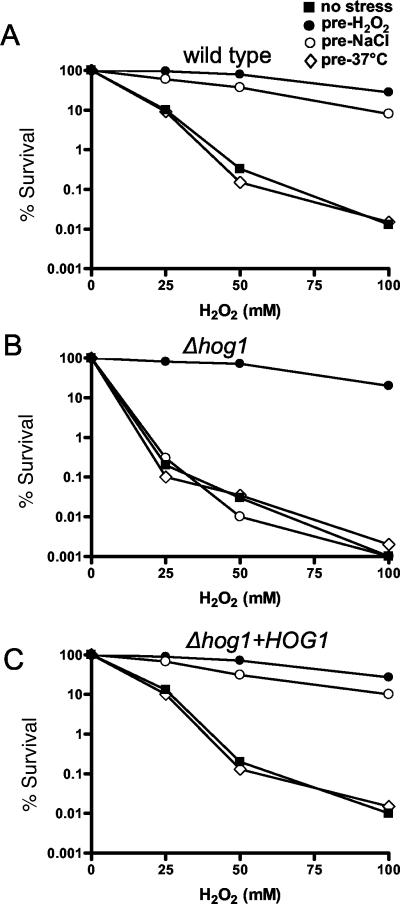
The existence of a general stress response has been proposed to explain the phenomenon of cross-protection, wherein exposure to a non-lethal dose of one stress can protect against a potentially lethal dose of a seemingly unrelated stress. Importantly, cross-protection has been observed in S. cerevisiae (Lewis et al., 1995) and S. pombe (Chen et al., 2003; M. Toone, personal communication), both of which have been shown to mount general stress responses. Previous work in C. albicans demonstrated that pretreatment of cells with a mild stress, such as oxidative or temperature upshift, stimulates adaptation that protects against subsequent exposure to higher levels of the same stress (Jamieson et al., 1996; Arguelles, 1997). However, Enjalbert et al. (2003) could detect no stress cross-protection in C. albicans under the conditions used in their transcript-profiling study, reinforcing their suggestion that this fungus might not mount a general stress response. However, we have shown that only one of the stress conditions used in their transcript-profiling study activates Hog1 (0.3 M NaCl; Figure 2). We predicted that stress cross-protection would be seen in C. albicans, but only upon exposure to stress conditions that activate the Hog1 SAPK. To test this prediction, cells were pretreated with the range of stress conditions used in the transcript-profiling study (Enjalbert et al., 2003) and then subjected to acute doses of H2O2. As demonstrated previously (Jamieson et al., 1996), cell survival to acute doses of H2O2 was notably improved by pretreatment of cells with a low dose of H2O2 (0.4 mM) (Figure 7A). Significantly, however, we also found that pretreatment of cells with an osmotic stress which activates Hog1 (0.3 M NaCl) provided substantial protection against exposure to 100 mM H2O2. However, pretreatment of cells with a temperature upshift (which does not activate Hog1) conferred no cross-protection against oxidative stress (Figure 7A).
Figure 7.
Stress cross-protection in C. albicans. Wild-type (WT), Δhog1 (JC10), or Δhog1+HOG1 cells (JC52) were either unstressed or pretreated for 1 h with osmotic stress (0.3 M NaCl), low-dose oxidative stress (0.4 mM H2O2), or temperature upshift (23-37°C), and cell survival was calculated after a subsequent 1-h exposure to increasing doses of H2O2. The experiments were each repeated at least three times, and the data from one representative experiment are shown.
To investigate the role of Hog1 in stress-cross protection, cell survival experiments were repeated in Δhog1 cells. The protection afforded by pretreatment of cells with a low dose of H2O2 (0.4 mM) against acute doses of the same agent was retained in Δhog1 cells (Figure 7B). Hence, adaptation to an oxidative stress is not dependent upon Hog1. This correlates with our observation that Hog1 is not activated in response to low doses of H2O2. However, the protection against an acute dose of H2O2, conferred by an osmotic stress of 0.3 M NaCl, was lost in Δhog1 cells (Figure 7B). Importantly, integration of the wild-type HOG1 gene into the hog1 mutant restored wild-type levels of stress cross-protection (Figure 7C). Therefore, stress cross-protection does exist in C. albicans, and this phenomenon is mediated by the Hog1 SAPK.
DISCUSSION
In this article, we demonstrate that the human pathogen C. albicans mounts a core environmental stress response. We show that the C. albicans Hog1 SAPK is activated and translocates to the nucleus in response to a diverse range of stress conditions (Figure 5). These observations confirm and considerably extend a recent study that found that C. albicans Hog1 is phosphorylated in response to oxidative as well as osmotic stress stimuli (Alonso-Monge et al., 2003). We also have shown that the C. albicans Hog1 SAPK is a functional homologue of the S. pombe Sty1 SAPK, which is responsible for activating the core environmental response in this yeast. In addition, we have identified examples of Hog1-regulated genes that are stereotypically induced in response to the same diverse stress conditions that activate Hog1 (Figure 6). Furthermore, we find that stress cross-protection, a classical hallmark of a general stress response, does occur in C. albicans with stresses that activate the Hog1 SAPK (Figure 7). Hence, collectively our data indicate that C. albicans mounts a core stress response and that the Hog1 SAPK is pivotal to this response.
A previous transcript-profiling study suggested that C. albicans “lacks the strong general stress response exhibited by S. cerevisiae” (Enjalbert et al., 2003). These authors observed that no genes were commonly induced by oxidative stress (0.4 mM H2O2), osmotic stress (0.3 M NaCl), and temperature upshift (23-37°C) treatments, although these conditions had previously been found to stimulate strong core stress responses in S. cerevisiae. However, we have established that only one of the three conditions (0.3 M NaCl) used in their study activates the Hog1 SAPK in C. albicans (Figure 2), which presumably explains why they did not observe a general stress response under these conditions. We have shown that other conditions do stimulate the activation of the Hog1 SAPK (Figure 1), and under such conditions C. albicans displays phenotypes associated with a general stress response (Figures 6 and 7).
The identification of target genes for C. albicans Hog1 has uncovered both activator and repressor functions for this SAPK. We show that both RHR2 (encoding a putative glycerol phosphate phosphatase) and HSP12 (encoding a putative heat shock protein) are Hog1 targets (Figure 6). However, Hog1 represses HSP12 under nonstress conditions, whereas RHR2 is induced in a Hog1-dependent manner in response to conditions that activate this SAPK. We note that the repression of HSP12 by Hog1 in C. albicans is in striking contrast to the situation in S. cerevisiae, where Hog1 is required to activate HSP12 (Varela et al., 1995). The fact that C. albicans Hog1 has both activator and repressor functions suggests that this kinase has multiple cellular targets. We predict that Hog1-regulated genes such as RHR2 and HSP12 form the basis of the core stress response in C. albicans, and genomic-based approaches are currently underway in our laboratories to identify further Hog1 target genes.
Our work indicates that the core stress response in C. albicans has evolved, in a niche-specific manner, to promote survival of this pathogen in the mammalian host. This conclusion is supported by two main findings. First, the conditions that stimulate the core stress response in C. albicans are different to those that induce similar responses in S. cerevisiae and S. pombe. Second, the molecular circuitry that senses and transduces stress signals has evolved to respond to those conditions that are important for the growth and survival of C. albicans.
C. albicans, S. cerevisiae, and S. pombe occupy contrasting niches, and hence one would expect the conditions which these yeast perceive as a stress to have diverged in a niche-specific manner. This is highlighted by our analysis of the response of the C. albicans Hog1 SAPK pathway to oxidative stress and heat treatments. For example, Hog1 activation was only observed at relatively high levels of H2O2 (>2 mM; Figure 3). This may be related to the fact that C. albicans is relatively resistant to oxidative stress compared with S. cerevisiae (Jamieson et al., 1996) and S. pombe (J.Q., unpublished observation). Low doses of H2O2 (≤0.5 mM) stimulate core stress responses in both S. cerevisiae and S. pombe (Gasch et al., 2000; Causton et al., 2001; Chen et al., 2003), but not in C. albicans (Enjalbert et al., 2003). We predict that the relative resistance of C. albicans to oxidative stress, and corresponding activation of the Hog1 SAPK only in response to high doses of H2O2, reflects the ability of this fungal pathogen to survive the relatively high levels of reactive oxygen species that are generated by the host immune system, for example, during phagocytic attack.
Regarding the effects of temperature upshift upon C. albicans, it is relevant that this pathogen has evolved to thrive in warm-blooded mammalian hosts, where temperature homeostasis is maintained, for example, at 37°C in humans. Consequently, the SAPK pathway in C. albicans is not activated in response to a temperature upshift; in fact, we see a consistent reduction in the basal levels of Hog1 phosphorylation after temperature increase (Figures 2 and 4A). Presumably this adaptation prevents the continuous stimulation of the Hog1 SAPK in response to temperatures of 37°C, which might desensitize the pathway to other significant stresses in the mammalian host. In contrast to the environmental niche occupied by C. albicans, the growth environments shared by S. cerevisiae and S. pombe are subject to significant temperature fluctuations. Not surprisingly, therefore, temperature upshift stimulates strong core stress responses in S. pombe and S. cerevisiae (Gasch et al., 2000; Causton et al., 2001; Chen et al., 2003), and the SAPK pathways in both of these organisms respond to temperature upshift (Shieh et al., 1998; Shiozaki et al., 1998; Nguyen and Shiozaki, 1999; Winkler et al., 2002). Therefore, it seems that the C. albicans Hog1 SAPK pathway has evolved not to respond to temperature upshift as a result of the selective pressures in warm-blooded mammalian hosts. To investigate this further, we examined the response of the C. albicans Hog1 SAPK to temperature upshift when it is expressed in S. pombe. Similar to the Sty1 SAPK, C. albicans Hog1 becomes rapidly phosphorylated upon temperature upshift in S. pombe. This result suggests that there has been a divergence in the circuitry that relays temperature upshift signals to the SAPK module in C. albicans and S. pombe. However, we cannot exclude the possibility that the C. albicans SAPK interacts differently with the regulators that relay temperature upshift signals in C. albicans and S. pombe.
In addition to stress responses, the C. albicans Hog1 SAPK pathway also has been implicated in the regulation of serum-induced dimorphism, because hog1 mutants display derepressed serum-induced hyphal formation (Alonso-Monge et al., 1999). Consistent with this we see an increase in the levels of active, phosphorylated Hog1 after exposure to serum (Figure 1). However, it should be noted that serum-induced levels of phosphorylated Hog1 are relatively low compared with other stress conditions (Figure 1), and a direct role for Hog1 in the regulation of morphogenic gene targets has not been established. Importantly, the deletion of HOG1 in S. cerevisiae results in inappropriate activation of the mating and filamentation mitogen-activated protein kinase pathways (O'Rourke and Herskowitz, 1998; Davenport et al., 1999). Hence, derepression of morphogenesis seen in C. albicans hog1 mutants may arise from a similar indirect phenomenon. We did, however, detect a significant response of the C. albicans Hog1 SAPK pathway to the quorum-sensing molecule farnesol. Quorum sensing is predicted to form the basis of cell density-dependent morphogenesis seen in C. albicans. The mechanism(s) underlying farnesol-mediated quorum sensing is not known (Hornby et al., 2001; Ramage et al., 2002), but our data suggest that the Hog1 SAPK pathway might play a key role in cell-to-cell signaling in C. albicans. Further experimentation is needed to investigate this intriguing possibility.
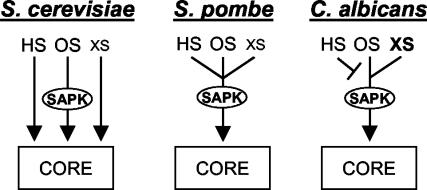
Together, our results suggest that a niche-specific Hog1-mediated core stress response has evolved in C. albicans to protect this human pathogen against host defenses and to promote its survival in the host. The evolution of this niche-specific response is reflected in the specific environmental conditions that trigger the Hog1-regulated core stress response in C. albicans and by differences in the molecular circuitry that drive this response (Figure 8). Many conserved regulatory modules have evolved to execute different cellular roles in C. albicans. Examples include Gcn4 (Tripathi et al., 2002), Rfg1 (Kadosh and Johnson, 2001) and Tup1 (Braun and Johnson, 2000; Murad et al., 2001). Because the Hog1 SAPK is required for virulence in C. albicans (Alonso-Monge et al., 1999), this pathway may represent a further example of a regulatory module that has been redirected toward virulence in this pathogenic fungus.
Figure 8.
Regulation of core stress responses in S. cerevisiae, S. pombe, and C. albicans. The SAPK pathways in S. pombe and C. albicans play a central role in regulating core stress responses. However, these pathways have adapted to respond in a niche-specific manner, reflecting the different growth environments of these two yeasts. In contrast, core stress responses in S. cerevisiae and S. pombe are stimulated by similar conditions, presumably as these organisms inhabit very similar environments. In S. cerevisiae, however, core stress responses are regulated by stress-specific pathways. HS, heat stress; OS, osmotic stress; XS, high doses of oxidative stress; xs, low doses of oxidative stress.
Acknowledgments
We thank Jeremy Brown, Simon Whitehall, and Mark Toone for discussions and comments on the manuscript. We also thank Cheryl Gale and Judith Berman for the kind gift of cassettes to allow us to construct the YFP-Hog1 fusion. J.Q. was funded by the Medical Research Council (Career Development Award G120/581). A.B. was funded by the British Council (CRP004), the Biotechnology and Biological Sciences Research Council (1/P11585), the Wellcome Trust (055015, 063204) and the European Commission (QLRT-2000-00795, MCRTN-504148).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-03-0181. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-03-0181.
References
- Alfa, C.E., Gallagher, I.M., and Hyams, J.S. (1993). Antigen localization in fission yeast. Methods Cell Biol. 37, 201-222. [DOI] [PubMed] [Google Scholar]
- Alonso-Monge, R., Navarro-Garcia, F., Molero, G., Diez-Orejas, R., Gustin, M., Pla, J., Sanchez, M., and Nombela, C. (1999). Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 181, 3058-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Monge, R., Navarro-Garcia, F., Roman, E., Negredo, A.I., Eisman, B., Nombela, C., and Pla, J. (2003). The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot. Cell 2, 351-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguelles, J.C. (1997). Theromotolerance and trehalose accumulation induced by heat shock in yeast cells of Candida albicans. FEMS Microbiol. Lett. 146, 65-71. [DOI] [PubMed] [Google Scholar]
- Basi, G., Schmid, E., and Maundrell, K. (1993). TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123, 131-136. [DOI] [PubMed] [Google Scholar]
- Blackwell, C., Russell, C.L., Argimon, S., Brown, A.J., and Brown, J.D. (2003). Protein A-tagging for purification of native macromolecular complexes from Candida albicans. Yeast 20, 1235-1241. [DOI] [PubMed] [Google Scholar]
- Braun, B.R., and Johnson, A.D. (2000). TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 155, 57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster, J.L., de Valoir, T., Dwyer, N.D., Winter, E., and Gustin, M.C. (1993). An osmosensing signal transduction pathway in yeast. Science 259, 1760-1763. [DOI] [PubMed] [Google Scholar]
- Brown, A.J.P., et al. (2001). Transcript analysis of 1003 novel yeast genes using high-throughput northern hybridisations. EMBO J. 20, 3177-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, V., Quinn, J., Soto Pino, T., Martin, H., Saldanha, J., Makino, K., Morgan, B.A., and Millar, J.B.A. (2001). Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol. Biol. Cell 12, 407-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causton, H.C., Ren, B., Koh, S.S., Harbison, C.T., Kanin, E., Jennings, E.G., Lee, T.I., True, H.L., Lander, E.S., and Young, R.A. (2001). Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell 12, 323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D., Toone, W.M., Mata, J., Lyne, R., Burns, G., Kivinen, K., Brazma, A., Jones, N., and Bahler, J. (2003). Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14, 214-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven, R.A., Griffiths, D.J., Sheldrick, K.S., Randall, R.E., Hagan, I.M., and Carr, A.M. (1998). Vectors for the expression of tagged proteins in Schizosaccharomyces pombe. Gene 221, 59-68. [DOI] [PubMed] [Google Scholar]
- Davenport, K.D., Williams, K.E., Ullmann, B.D., and Gustin, M.C. (1999) Activation of the Saccharomyces cerevisiae filamentation/invasion pathway by osmotic stress in high-osmolarity glycogen pathway mutants. Genetics 153, 1091-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols, G., and Russell, P. (1997). Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol. Cell. Biol. 17, 3356-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols, G., Shiozaki, K., and Russell, P. (1996). Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol. Cell. Biol. 16, 2870-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert, B., Nantel, A., and Whiteway, M. (2003). Stress-induced Gene Expression in Candida albicans: absence of a general stress response. Mol. Biol. Cell 14, 1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrigno, P., Posas, F., Koepp, D., Saito, H., and Silver, P.A. (1998). Regulated nucleo/cytoplasmic exchange of HOG1 MAPK requires the importin beta homologs NMD5 and XPO1. EMBO J. 17, 5606-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits, F., Degols, G., Shiozaki, K., and Russell, P. (1998). Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 12, 1464-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch, A.P., Spellman, P.T., Kao, C.M., Carmel-Harel, O., Eisen, M.B., Storz, G., Botstein, D., and Brown, P.O. (2000). Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch, A.P., Huang, M., Metzner, S., Botstein, D., Elledge, S.J., and Brown, P.O. (2001). Genomic expression responses to DNA-damaging agents and the regulatory role of the yeast ATR homolog Mec1p. Mol. Biol. Cell 12, 2987-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerami-Nejad, M., Berman, J., and Gale, C.A. (2001). Cassettes for PCR-mediated construction of green, yellow, and cyan fluorescent protein fusions in Candida albicans. Yeast 18, 859-864. [DOI] [PubMed] [Google Scholar]
- Hornby, J.M., Jensen, E.C., Lisec, A.D., Tasto, J.J., Jahnke, B., Shoemaker, R., Dussault, P., and Nickerson, K.W. (2001). Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67, 2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson, D.J., Stephen, D.W., and Terriere, E.C. (1996). Analysis of the adaptive oxidative stress response of Candida albicans. FEMS Microbiol. Lett. 138, 83-88. [DOI] [PubMed] [Google Scholar]
- Kadosh, D., and Johnson, A.D. (2001). Rfg1, a protein related to the Saccharomyces cerevisiae hypoxic regulator Rox1, controls filamentous growth and virulence in Candida albicans. Mol. Cell. Biol. 21, 2496-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, J.G., Learmonth, R.P., and Watson, K. (1995). Induction of heat, freezing and salt tolerance by heat and salt shock in Saccharomyces cerevisiae. Microbiology 141, 687-694. [DOI] [PubMed] [Google Scholar]
- Lo, H.J., Kohler, J.R., DiDomenico, B., Loebenberg, D., Cacciapuoti, A., and Fink, G.R. (1997). Nonfilamentous C. albicans mutants are avirulent. Cell 90, 939-949. [DOI] [PubMed] [Google Scholar]
- Millar, J.B., Buck, V., and Wilkinson, M.G. (1995). Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 9, 2117-2130. [DOI] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795-823. [DOI] [PubMed] [Google Scholar]
- Murad, A.M.A., Lee, P.R., Broadbent, I.D., Barelle, C.J., and Brown, A.J.P. (2000). CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16, 325-327. [DOI] [PubMed] [Google Scholar]
- Murad, A.M.A., d'Enfert, C., Gaillardin, C., Tournu, H., Tekaia, F., Talibi, D., Marechal, D., Marchais, V., Cottin, J., and Brown, A.J.P. (2001). Transcript profiling in Candida albicans reveals new cellular functions for the transcriptional repressors CaTup1, CaMig1 and CaNrg1. Mol. Microbiol. 42, 981-993. [DOI] [PubMed] [Google Scholar]
- Nicholls, S., Straffon, M., Enjalbert, B., Nantel, A., Macaskill, S., Whiteway, M., and Brown, A.J.P. (2004). Msn2/4-like transcription factors play no obvious roles in the stress responses of the fungal pathogen, Candida albicans. Eukaryot. Cell (in press). [DOI] [PMC free article] [PubMed]
- Nguyen, A.N., and Shiozaki, K. (1999). Heat-shock-induced activation of stress MAP kinase is regulated by threonine- and tyrosine-specific phosphatases. Genes Dev. 13, 1653-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds, F.C. (1988). Candida and candidosis, 2nd ed. London: Bailliere Tindall.
- O'Rourke, S.M., and Herskowitz, I. (1998). The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev. 12, 2874-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke, S.M., and Herskowitz, I. (2004). Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell 15, 532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke, S.M., Herskowitz, I., and O'Shea, E.K. (2002). Yeast go the whole HOG for the hyperosmotic response. Trends Genet. 18, 405-412. [DOI] [PubMed] [Google Scholar]
- Posas, F., Chambers, J.R., Heyman, J.A., Hoeffler, J.P., de Nadal, E., and Arino, J. (2000). The transcriptional response of yeast to saline stress. J. Biol. Chem. 275, 17249-17255. [DOI] [PubMed] [Google Scholar]
- Quinn, J., Findlay, V.J., Dawson, K., Millar, J.B., Jones, N., Morgan, B.A., and Toone, W.M. (2002). Distinct regulatory proteins control the graded transcriptional response to increasing H2O2 levels in fission yeast Schizosaccharomyces pombe. Mol. Biol. Cell 13, 805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage, G., Saville, S.P., Wickes, B.L., and Lopez-Ribot, J.L. (2002). Inhibition of C. albicans biofilm formation by farnesol, a quorum sensing molecule. Appl. Environ. Microbiol. 68, 5459-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser, V., Ruis, H., and Ammerer, G. (1999). Kinase activity-dependent nuclear export opposes stress-induced nuclear accumulation and retention of Hog1 mitogen-activated protein kinase in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell 10, 1147-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rep, M., Krantz, M., Thevelein, J.M., and Hohmann, S. (2000). The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 275, 8290-8300. [DOI] [PubMed] [Google Scholar]
- San-Jose, C., Monge, R.A., Perez-Diaz, R., Pla, J., and Nombela, C. (1996). The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 178, 5850-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, F. (1991). Getting started with yeast. Methods Enzymol. 194, 3-21. [DOI] [PubMed] [Google Scholar]
- Shieh, J.C., Wilkinson, M.G., Buck, V., Morgan, B.A., Makino, K., and Millar, J.B. (1997). The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 11, 1008-1022. [DOI] [PubMed] [Google Scholar]
- Shieh, J.C., Martin, H., and Millar, J.B. (1998). Evidence for a novel MAPKKK-independent pathway controlling the stress activated Sty1/Spc1 MAP kinase in fission yeast. J. Cell Sci. 111, 2799-2807. [DOI] [PubMed] [Google Scholar]
- Shiozaki, K., and Russell, P. (1995). Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature 378, 739-743. [DOI] [PubMed] [Google Scholar]
- Shiozaki, K., and Russell, P. (1996). Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 10, 2276-2288. [DOI] [PubMed] [Google Scholar]
- Shiozaki, K., Shiozaki, M., and Russell, P. (1998). Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol. Biol. Cell 9, 1339-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siderius, M., and Mager, W.H. (1997). General stress response. New York: Springer.
- Smith, D.A., Toone, W.M., Chen, D., Bahler, J., Jones, N., Morgan, B.A., and Quinn, J. (2002). The Srk1 protein kinase is a target for the Sty1 stress-activated MAPK in fission yeast. J. Biol. Chem. 277, 33411-33421. [DOI] [PubMed] [Google Scholar]
- Toone, W.M., and Jones, N. (1998). Stress-activated signalling pathways in yeast. Genes Cells 3, 485-498. [DOI] [PubMed] [Google Scholar]
- Tripathi, B.J., Wiltshire, C., Macaskill, S., Tournu, H., Budge, S., and Brown, A.J. (2002). Gcn4 co-ordinates morphogenetic and metabolic responses to amino acid starvation in Candida albicans. EMBO J. 21, 5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela, J.C., Praekelt, U.M., Meacock, P.A., Planta, R.J., and Mager, W.H. (1995). The Saccharomyces cerevisiae HSP12 gene is activated by the high-osmolarity glycerol pathway and negatively regulated by protein kinase A. Mol. Cell. Biol. 15, 6232-6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, M.G., Samuels, M., Takeda, T., Toone, W.M., Shieh, J.C., Toda, T., Millar, J.B., and Jones, N. (1996). The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 10, 2289-2301. [DOI] [PubMed] [Google Scholar]
- Winkler, A., Arkind, C., Mattison, C.P., Burkholder, A., Knoche, K., and Ota, I. (2002). Heat stress activates the yeast high-osmolarity glycerol mitogen-activated protein kinase pathway, and protein tyrosine phosphatases are essential under heat stress. Eukaryot. Cell 1, 163-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysong, D.R., Christin, L., Sugar, A.M., Robbins, P.W., and Diamond, R.D. (1998). Cloning and sequencing of a Candida albicans catalase gene and effects of disruption of this gene. Infect. Immun. 66, 1953-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]