Abstract
In Saccharomyces cerevisiae, deficiencies in the ESCRT machinery trigger the mistargeting of endocytic and biosynthetic ubiquitinated cargoes to the limiting membrane of the vacuole. Surprisingly, impairment of this machinery also leads to the accumulation of various receptors and transporters at the plasma membrane in both yeast and higher eukaryotes. Using the well-characterized yeast endocytic cargo uracil permease (Fur4p), we show here that the apparent stabilization of the permease at the plasma membrane in ESCRT mutants results from an efficient recycling of the protein. Whereas several proteins as well as internalized dyes are known to be recycled in yeast, little is known about the machinery and molecular mechanisms involved. The SNARE protein Snc1p is the only cargo for which the recycling pathway is well characterized. Unlike Snc1p, endocytosed Fur4p did not pass through the Golgi apparatus en route to the plasma membrane. Although ubiquitination of Fur4p is required for its internalization, deubiquitination is not required for its recycling. In an attempt to identify actors in this new recycling pathway, we found an unexpected phenotype associated with loss of function of the Vps class C complex: cells defective for this complex are impaired for recycling of Fur4p, Snc1p, and the lipophilic dye FM4-64. Genetic analyses indicated that these phenotypes were due to the functioning of the Vps class C complex in trafficking both to and from the late endosomal compartment.
INTRODUCTION
The endocytic pathway defines membrane traffic from the plasma membrane to the degradative compartment: the lysosome in animal cells and the vacuole in fungi and plants. Endocytosis is involved in many key cellular processes, such as receptor down-regulation, signal transduction, and cell polarity. Internalized proteins travel through two morphologically and biochemically distinct organelles, the early (EE) and late endosomes (LE), before undergoing degradation in the lysosome/vacuole (Gruenberg and Maxfield, 1995).
Not all internalized proteins undergo lysosomal degradation. In animal cells, many receptors cycle back to the plasma membrane after endocytosis (Maxfield and McGraw, 2004). This process fulfills several functions, including the reuse and/or relocalization of receptors in subdomains of the plasma membrane. Multiple routes for protein recycling have been identified (directly from the early endosome, through the trans-Golgi network, from the LE, etc.), illustrating the versatility of trafficking pathways. In the yeast Saccharomyces cerevisiae, the recycling of few endocytosed proteins also has been evidenced (Shaw et al., 2001). For example, the pheromone-induced internalization of Ste3p is followed by its recycling back to the plasma membrane (Chen and Davis, 2000, 2002), whereas the chitin synthase Chs3p cycles between sites of chitin deposition on the cell surface and an internal structure called the chitosome (Valdivia et al., 2002). The machinery, route(s), and molecular mechanisms underlying the sorting and recycling of these proteins are unknown. The recycling of the exocytic SNARE Snc1p, facilitating its reuse in several rounds of secretion, is better documented. Snc1p cycles between the plasma membrane, early endosome, and Golgi compartments (Lewis et al., 2000). Several factors, including the F-box protein Rcy1p and members of the aminophospholipid translocase protein family, are required for the recycling of Snc1p, but the molecular mechanism of their action is not yet understood (Galan et al., 2001; Hua et al., 2002). The fusion to the Golgi of endosomal vesicles containing Snc1p requires the RAB Ypt6p, the SNAREs Tlg1p and Tlg2p and a multisubunit tethering factor known as the VFT/GARP complex (Bensen et al., 2001; Siniossoglou and Pelham, 2001, 2002). The VFT/GARP complex belongs to a family of multisubunit tethering complexes, including the Exocyst, TRAPP, and Vps class C/HOPS complexes involved in fusion and docking at the plasma membrane, at early Golgi compartment, and at the vacuolar membrane, respectively (Whyte and Munro, 2002).
Work on yeast receptors and transporters has indicated that ubiquitination serves as the key signal for the internalization of these proteins (Rotin et al., 2000). Ubiquitin also has been shown to play a role at the endosomal membrane, directing endocytic and biosynthetic cargoes into internal vesicles of the late endosomal compartment (also known as the multivesicular body; MVB) (Katzmann et al., 2001; Urbanowski and Piper, 2001; Reggiori and Pelham, 2002). Three heteroligomeric complexes—ESCRT-I, -II, and -III—act sequentially at the MVB membrane in the recruitment and incorporation of ubiquitinated cargoes into internal vesicles. Dysfunction of the ESCRT machinery contributes to defects in growth control and development, budding of HIV1 viral particles, and immune response (Katzmann et al., 2002). Understanding the physiology of cells deficient in the MVB pathway is therefore of critical importance. S. cerevisiae cells lacking functional ESCRT complexes accumulate an exacerbated late endosomal compartment known as the class E (ClE) compartment. In ClE mutant cells, endocytic and biosynthetic cargoes accumulate in both the ClE compartment and the vacuolar membrane rather than being targeted to the vacuolar lumen. Surprisingly, several studies in yeast and mammalian cells have shown that impairment of the ESCRT machinery also leads to the accumulation of various receptors and transporters at the plasma membrane, including the epidermal growth factor receptor (EGFR) and the pheromone receptors in yeast (Davis et al., 1993; Li et al., 1999; Babst et al., 2000; Luo and Chang, 2000). The basis of this phenotype is not yet understood, but two alternative models have been proposed: 1) ClE mutant cells are also deficient for the internalization step of endocytosis and 2) constitutive recycling of internalized cargoes occurs in these cells. In addition, two recent articles showed that a subset of exocytic cargoes pass through the LE compartment before reaching the plasma membrane (Gurunathan et al., 2002; Harsay and Schekman, 2002). These results raise a third possibility that could account for the accumulation of receptors at the plasma membrane of ClE mutant cells: 3) secretion via the LE is enhanced if the ESCRT machinery is impaired.
We tried to distinguish between these possibilities by investigating the fate of the uracil permease Fur4p in ClE mutant cells. Fur4p is one of the main cargo proteins used as a model for following endocytosis in yeast (Rotin et al., 2000). Stress conditions (e.g., inhibition of protein synthesis, lack of a nutrient, and heat shock) trigger the rapid ubiquitination and endocytosis of Fur4p. On its way to the vacuole, Fur4p is incorporated into the internal vesicles of the MVB before degradation by vacuolar proteases.
MATERIALS AND METHODS
Strain Construction and Genetic Manipulations
Standard yeast growth conditions and genetic manipulations were used (Sherman et al., 1986). The yeast strains and plasmids used are described in Tables 1 and 2, respectively. Strains deleted for VPS37 (yJMG510), RCY1 (yJMG398), and VPS51 (yJMG453) were constructed as described by Longtine et al. (1998). Double mutant strains were constructed by crossing single mutant strains. Standard procedures were used for recombinant DNA manipulation (Sambrook et al., 1997). Polymerase chain reaction (PCR) was performed with the Expand polymerase kit as recommended by the manufacturer (Roche Diagnostics, Mannheim, Germany). Oligonucleotides were synthesized by Proligo (Paris, France); sequences are available upon request.
Table 1.
Yeast strains
| Strain | Relevant genotype | Source/reference |
|---|---|---|
| BY4741 | WT, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| BY4742 | WT, Mat α leu2Δ0, lys2Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG400 | vps20 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG475 | vps22 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG401 | vps23 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG402 | vps24 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG403 | vps25 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG404 | vps28 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG407 | vps36 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG408 | vps37 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG503 | pep4 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG367 | ypt7 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG498 | pep12 ::kanMX, Mat α, leu2Δ0, lys2Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| RH144-3d | sec18-1, Mat a, ura3-52, his4, leu2-3, 112, bar1-1 | H. Riezman |
| MHY1251 | doa4 ::LEU2, vps24 ::HIS3, Mat a, ura3-52, trp1-1, lys2-801 | Amerik et al. 2000 |
| yJMG510 | vps37 ::HIS3, Mat α, leu2Δ0, lys2Δ0, ura3Δ0 | This study |
| yJMG453 | vps51 ::HIS3, Mat α, leu2Δ0, lys2Δ0, ura3Δ0 | This study |
| yJMG531 | vps37 ::kanMX, vps51 ::kanMX, Mat α, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | This study |
| yJMG398 | rcy1 ::natMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | This study |
| yJMG528 | vps37 ::HIS3, rcy1 ::natMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0 | This study |
| PC173-3A | sec14-1, Mat α, leu2-3,112, ura3-52, suc2Δ9 | S. Friant |
| yJMG524 | vps37 ::kanMX, sec14-1, Mat α, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | This study |
| yJMG412 | vps29 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG529 | vps37 ::HIS3, vps29 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | This study |
| yJMG476 | vps33 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG523 | vps37 ::HIS3, vps33 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | This study |
| yJMG530 | vps37 ::HIS3, ypt7 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | This study |
| yJMG473 | vps16 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG474 | vps18 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG506 | vam3 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | Euroscarf |
| yJMG527 | vam3 ::HIS3, pep12 ::kanMX, Mat a, leu2Δ0, met15Δ0, ura3Δ0, his3Δ0 | This study |
Table 2.
Plasmids
| Plasmid name | Relevant characteristics | Reference |
|---|---|---|
| Yep352fF | end-FUR4, URA3, 2μ | Volland et al., 1994 |
| p195gF | GAL-FUR4, URA3, 2μ | Volland et al., 1994 |
| pFL38-gFP | GAL-FUR4-GFP, URA3, CEN | Marchal et al., 2002 |
| pFL38-KR-gFP | GAL-FUR4-KR-GFP, URA3, CEN | Blondel et al., 2004 |
| pFL38-Ub-KR-gFP | GAL-Ub-FUR4-KR-GFP, URA3, CEN | Blondel et al., 2004 |
| pJMG118 | Tpi-GFP-SNC1, URA3, CEN | Lewis et al., 2000 |
Antibodies, Western Blots, and Microscopy
Standard procedures were used for yeast cell extract preparation and immunoblotting, as described previously (Volland et al., 1994). Monoclonal antibody against green fluorescent protein (GFP) (Roche Diagnostics) was used as recommended by the manufacturer. The polyclonal antibody raised against the C terminus of Fur4p has been described previously (Volland et al., 1994).
Living cells were observed with an Olympus BY61 fluorescence microscope with a Spot charge-coupled device camera. GFP-tagged proteins were visualized using a Chroma GFPII filter (excitation 440-470 nm). Images were processed and normalized with Photoshop 6 (Adobe Photoshop, Adobe Systems, Mountain View, CA).
Fur4p Assays
Exocytosis, endocytosis, and Fur4p recycling were assessed in cells transformed with various plasmids encoding Fur4p under the control of the GAL promoter, to allow the regulation of Fur4p synthesis. pFL38gFP (GAL-Fur4-GFP, URA3, CEN) and p195gF (GAL-Fur4, URA3, 2 μ) gave 20- and 100-fold overproduction of the permease, respectively (Galan and Haguenauer-Tsapis, 1997; Marchal et al., 2002). The Fur4-GFP chimera is functional (Dupré and Haguenauer-Tsapis, 2001), and the overproduction of Fur4p does not affect endocytosis of the permease (Galan et al., 1996; unpublished data).
Uracil uptake was measured as described previously (Volland et al., 1994). Yeast cultures (1 ml) were incubated with 5 μM [14C]uracil (MP Biomedicals, Irvine, CA) for 30 s at 30°C and quickly filtered through Whatman GF/C filters. Filters were washed twice with ice-cold water and then counted for radioactivity.
The kinetics of Fur4p targeting to the plasma membrane were followed as described previously (Moreau et al., 1997). Briefly, cells transformed with p195gF were grown in synthetic medium supplemented with raffinose (2%) until early exponential growth phase, and Fur4p synthesis was induced by adding galactose (2%). Uracil uptake was measured every 25 min for 2 h 30 min. Crude extracts were prepared and the amount of Fur4p was checked by Western immunoblotting.
The endocytosis of Fur4p was followed in cells transformed with pFL38gFP. Cells were grown at 30°C, and Fur4-GFP synthesis was induced as described above. Glucose was then added (2%) to block Fur4-GFP synthesis, and 20 min later (this time is necessary to allow all Fur4-GFP to reach the plasma membrane) we added rapamycin (RAP, 0.4 μg/ml) to trigger endocytosis of the permease. The cells were visualized by fluorescence microscopy, and uracil uptake was measured every 30 min over 3 h, after the addition of RAP. Thermosensitive strains were transferred to a restrictive temperature 15 min before the addition of rapamycin.
To uncouple Fur4p endocytosis and recycling, we used the same protocol but replacing the RAP addition step by carbon starvation. The experiment was done at 24°C to slow permease trafficking. Cells expressing Fur4-GFP at the plasma membrane were quickly filtered, washed, and resuspended in minimal medium without a carbon source. Measurements of uracil uptake are not relevant in carbon starvation conditions because Fur4p-mediated transport is energy dependent; endocytosis of the permease was therefore followed by fluorescence microscopy. We added glucose once the GFP signal at the plasma membrane ceased to be detectable (classically 75 min after the start of carbon starvation). Redistribution of Fur4-GFP at the plasma membrane was followed by both fluorescence microscopy and uracil uptake measurements. We measured uracil uptake from 3 min after the addition of glucose; this time is sufficient to allow reliable uracil uptake measurement after the carbon starvation phase.
FM4-64 and GFP-Snc1p Recycling Assays
Cells transformed with pJMG118 (tpi-GFP-Snc1, URA3, CEN) grown in selective media until the early exponential growth phase (classically 0.50 OD units/ml) were observed by fluorescence microscopy. Crude extracts were prepared, and Western immunoblotting was used to analyze GFP-Snc1p phosphorylation.
FM4-64 recycling assays were performed as described previously (Wiederkehr et al., 2000). Briefly, yeast cells were allowed to take up FM4-64 for 15 min at 30°C and were then washed three times with ice-cold SD medium. After the last wash, the cells were resuspended in 10 μl of SD medium and kept on ice. Prewarmed SD medium at 30°C was added to the cells, and fluorescence was recorded over a period of 600 s on a HITACHI F-2000 spectrofluorimeter.
RESULTS
Cells Lacking Functional ESCRT Complexes Accumulate Fur4p at the Plasma Membrane
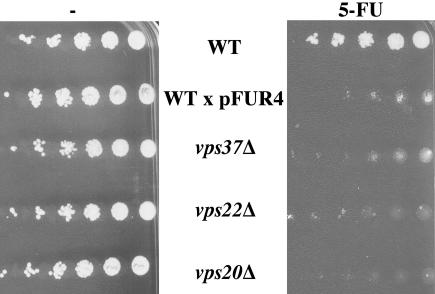
Fur4p transports 5-fluoro-uracil (5-FU), a toxic analog of uracil. We investigated whether ClE mutant cells accumulated Fur4p at the plasma membrane by checking the 5-FU sensitivity of ClE mutant strains (vps23Δ, vps37Δ, vps28Δ, vps22Δ, vps25Δ, vps36Δ, vps20Δ, and vps24Δ). As a control for cells hypersensitive to 5-FU, we plated wt cells overproducing Fur4p. All strains with defective ESCRT machinery tested were more sensitive to 5-FU than wt cells, suggesting that Vps ClE mutant cells accumulate active Fur4p at the plasma membrane (unpublished data). One representative strain deficient for either ESCRT I (vps37Δ), II (vps22Δ), or III (vps20Δ) is shown (Figure 1). In subsequent experiments, we used vps37Δ cells (ESCRT I) as a model for investigation of this phenotype.
Figure 1.
ESCRT mutant cells are hypersensitive to 5-FU. BY4741 (WT), yJMG408 (vps37Δ, ESCRT I), yJMG475 (vps22Δ, ESCRT II), yJMG400 (vps20Δ, ESCRT III) cells transformed with empty vector and BY4741 (WT) transformed with Yep352fF (end-FUR4), allowing overproduction of Fur4p (x pFUR4), were tested for sensitivity to 5-FU. Exponentially growing cells were serially diluted and spotted onto SD plates without (-, left) or with 2.5 μM 5-FU (5-FU, right).
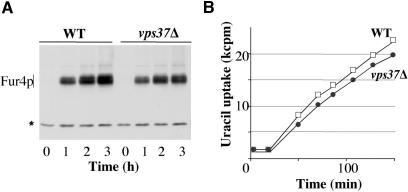
We first investigated whether this phenotype resulted from an increase in Fur4p secretion. We compared the kinetics of permease arrival at the plasma membrane in vps37Δ and wt cells, by using a plasmid encoding Fur4p, driven by the GAL promoter. Fur4p synthesis was induced in growing cells by adding galactose. The amount of Fur4p synthesized was roughly similar in the two strains, as shown by Western immunoblotting (Figure 2A). The kinetics of permease targeting to the plasma membrane, as assessed by uracil uptake measurements, were similar in vps37Δ and wt cells (Figure 2B).
Figure 2.
Fur4p secretion is not modified in ESCRT mutant cells. BY4741 (WT, white square) and yJMG408 (vps37Δ, black circle) cells transformed with p195GF (GAL-Fur4) were cultured in minimal media supplemented with raffinose until the early exponential growth phase. Fur4p synthesis was induced by adding galactose. (A) Crude extracts were prepared every hour after the addition of galactose, and Fur4p was detected by Western immunoblotting. The asterisk (*) indicates a nonspecific cross-reacting protein used as a loading control. (B) Uracil uptake was measured every 25 min over 150 min, results are expressed in kCPM.
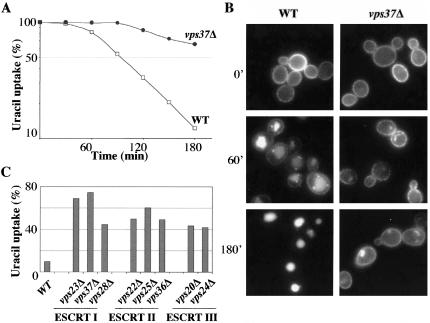
We then investigated whether impairment of the ESCRT machinery affected Fur4p endocytosis. We did this by expressing a functional Fur4-GFP fusion driven by the GAL promoter at the plasma membrane of vps37Δ and wt cells. Fur4-GFP synthesis was induced as described Figure 2, and glucose was added to stop Fur4-GFP synthesis. Twenty minutes after the addition of glucose (t = 0), cells were collected for microscopy fluorescence (Figure 3B) and uracil uptake measurement (Figure 3A). As expected, most of the Fur4p was at the plasma membrane at t = 0 in both wt and vps37Δ cells. At t = 0, we added rapamycin, which is known to mimic nitrogen starvation (Schmeizle and Hall, 2000) and therefore to trigger the endocytosis of Fur4p (Volland et al., 1994). In wt cells, Fur4p was rapidly internalized and targeted to the vacuolar lumen (Figure 3, A and B). In vps37Δ cells, Fur4p was significantly stabilized at the plasma membrane: 3 h after the addition of rapamycin, Fur4-GFP was located both at the plasma membrane and in a large intracellular structure (Figure 3B) that likely corresponds to the ClE compartment because endocytosed FM4-64 accumulated in the same structure (unpublished data). Consistent with the microscopy data, the decrease in uracil uptake was impaired in vps37Δ compared with wt cells (Figure 3A). We then investigated whether other ESCRT mutant cells displayed the same phenotype. We measured Fur4p endocytosis, as described in Figure 3, A and B, in eight other strains, each of which was defective for the ESCRT I, II, or III complex. We plotted the percentage of residual uracil uptake 3 h after the addition of rapamycin (Figure 3C). Fur4-GFP was significantly stabilized at the plasma membrane in all ClE mutant cells tested.
Figure 3.
Fur4p is stabilized at the plasma membrane of ESCRT mutant cells. The indicated strains transformed with pFL38gFP (GAL-Fur4-GFP) were cultured and Fur4-GFP synthesis was induced as described Figure 2. Glucose was added to block Fur4-GFP synthesis. The cells were incubated for 20 min, and rapamycin was then added to trigger endocytosis of the permease (t = 0). (A) Uracil uptake was measured every 30 min after the addition of rapamycin for 3 h in BY4741 (WT, white square) and yJMG408 (vps37Δ, black circle) cells. Results are expressed as a percentage of initial uptake at t = 0 on a semilog scale. (B) Cells were collected at the times indicated after rapamycin addition and visualized by fluorescence microscopy. (C) BY4741 (WT); yJMG401 (vps23Δ), yJMG408 (vps37Δ), and yJMG404 (vps28Δ) (ESCRT I); yJMG475 (vps22Δ), yJMG403 (vps25Δ), and yJMG407 (vps36Δ) (ESCRT II); yJMG400 (vps20Δ) and yJMG402 (vps24Δ) (ESCRT III) cells were treated as described above. Residual uracil uptake 3 h after rapamycin addition is plotted. Results are expressed as a percentage of initial uracil uptake at t = 0.
In summary, Fur4p, like several other transporters and receptors, accumulated at the plasma membrane of Vps ClE mutant cells.
Fur4p Is Efficiently Recycled Back to the Plasma Membrane in ESCRT Mutant Cells
The stabilization of Fur4p at the plasma membrane of ESCRT mutant cells upon rapamycin treatment is surprising, because it seems to imply a defect in the internalization step of endocytosis, even though the ESCRT machinery is known to act further downstream in the endocytic pathway. However, an alternative model consistent with the known ESCRT function would involve an initial internalization of the permease upon rapamycin treatment, followed by a rapid recycling to the plasma membrane. The overall balance of these processes would then result in apparent stabilization of the permease at the plasma membrane. A rigorous test of this hypothesis thus requires an uncoupling of Fur4p endocytosis and recycling. We reasoned that the application of a brutal stress such as carbon starvation, which has been reported to trigger the sudden internalization of Fur4p (Volland et al., 1994), would render recycling activity insignificant while preserving endocytosis.
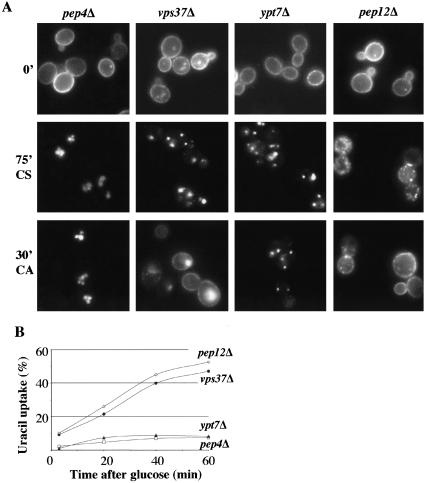
We therefore compared the fate of Fur4-GFP upon carbon starvation in pep4Δ (to avoid degradation of the permease) and vps37Δ cells. Cells cultured and induced for Fur4-GFP synthesis as described in Figure 3 were subjected to carbon starvation (CS). As expected, most of the Fur4-GFP was efficiently internalized and targeted to the vacuolar lumen after 75 min of carbon starvation in pep4Δ cells (Figure 4A, 75′CS). Importantly, the protein also was efficiently internalized in vps37Δ cells: after 75 min of carbon starvation most of the permease accumulated in intracellular structures. Thus, vps37Δ cells are not defective for the internalization step of endocytosis itself. We tested a putative recycling in these strains by adding glucose after the starvation period and following the pool of Fur4-GFP by both fluorescence microscopy and uracil uptake measurements. It should be noted that, in these experimental conditions, the de novo synthesis of Fur4-GFP is still blocked. As expected, no signal was recovered at the plasma membrane of pep4Δ cells, indicating that Fur4-GFP does not recycle from the vacuolar lumen (Figure 4A, 30′CA). In contrast, we observed rapid redistribution of Fur4-GFP to the plasma membrane in vps37Δ cells. Thirty minutes after the addition of glucose, Fur4-GFP was both at the plasma membrane and the ClE compartment (Figure 4A, 30′CA). Consistently, ∼50% of the initial Fur4p activity was recovered 1 h after addition of glucose in vps37Δ cells (Figure 4B). Does the relocation of Fur4p to the plasma membrane observed in ClE mutant cells correspond to classical vesicular transport? We addressed this question by combining the deletion of VPS37 with a thermosensitive loss of function allele of Sec18p known to block vesicular transport at 37°C. As expected, the recycling of Fur4p is blocked when Sec18p is inactivated, consistent with vesicular transport (unpublished data).
Figure 4.
Fur4p is efficiently recycled to the plasma membrane of ESCRT mutant cells. yJMG503 (pep4Δ, white square), yJMG408 (vps37Δ, black circle), yJMG367 (ypt7Δ, black triangle), and yJMG498 (pep12Δ, white diamond) cells transformed with pFL38gFP (GAL-Fur4-GFP) were cultured at 24°C, and Fur4-GFP synthesis was induced as described in Figure 3. At t = 0, cells were subjected to carbon starvation to trigger endocytosis of the permease, and glucose was added 75 min later. (A) Cells were visualized by fluorescence microscopy at t = 0 (0′), after 75 min of carbon starvation (75′CS) and 30 min after the addition of carbon (30′CA). Note that Fur4-GFP was efficiently internalized in all the strains and the protein was rapidly targeted back to the plasma membrane in vps37Δ and pep12Δ cells after the addition of glucose. (B) Uracil uptake was measured every 20 min after the addition of glucose. Results are expressed as a percentage of the initial uracil uptake measured immediately before carbon starvation.
In ESCRT-deficient cells, endocytosed Fur4p theoretically accumulates at the limiting membrane of the ClE compartment, resulting in efficient recycling. We asked whether Fur4p also was able to recycle from the internal vesicles of the normal MVB/LE compartment. We therefore assessed Fur4p recycling in cells defective for Ypt7p, the RAB protein required for fusion between the LE and vacuole. As expected, endocytosed Fur4p was blocked in punctate structures, presumably corresponding to LE in Δypt7 cells (Figure 4A, 75′CS). The addition of glucose did not trigger redistribution of the permease to the plasma membrane, as shown by both fluorescence microscopy and uracil uptake measurements (Figure 4, A and B). Once incorporated into the internal vesicles of the LE, Fur4p does not seem to be accessible for recycling, even though targeting to the vacuole and subsequent degradation (unpublished data) are blocked.
Given that Fur4p actively recycles if the endocytic pathway is blocked at MVB formation, we wished to determine whether blocking of the endocytic route earlier in the pathway also triggered recycling of the permease. Thus, we assessed Fur4p recycling in cells defective for the LE syntaxin Pep12p, in which trafficking between the EE and LE compartments is inhibited. After carbon starvation, endocytosed Fur4-GFP is located in small dots presumably corresponding to EE and/or vesicles (Figure 4A, 75′CS). After addition of glucose Fur4p recycles as efficiently in pep12Δ cells as in vps37Δ cells, as shown by both fluorescence microscopy and uracil uptake measurements (Figure 4, A and B)
Thus, Fur4-GFP can actively recycle to the plasma membrane if the endocytic pathway is blocked at the level of EE and MVB formation in cells defective for Pep12p and the ESCRT machinery, respectively. These data constitute the first formal demonstration of recycling of a permease in yeast. We then investigated the molecular mechanisms and machinery underlying recycling in Vps ClE mutant cells.
The Deubiquitination of Fur4p is Not Required for its Recycling
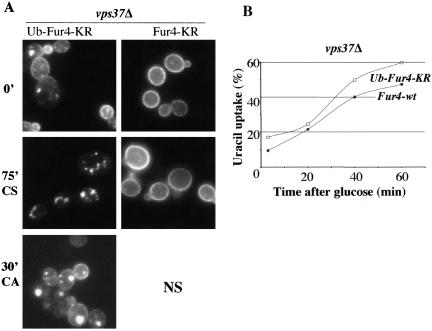
In wt cells, Fur4p ubiquitinated at the plasma membrane is deubiquitinated in a Doa4p-dependent manner presumably before MVB targeting (Dupré and Haguenauer-Tsapis, 2001). We asked whether impairment of the deubiquitination of Fur4p would affect the recycling of the permease. We assessed Fur4-GFP recycling in a vps24Δdoa4Δ strain described by Amerik et al. (2000)) lacking both the ESCRT III complex and Doa4p. Despite the loss of Doa4p function, we observed efficient recycling of Fur4-GFP in vps24Δdoa4Δ cells in the carbon starvation/addition protocol described above (unpublished data). Because isopeptidases other than Doa4p may deubiquitinate endocytic cargoes in ClE mutant cells (Amerik et al., 2000), we used an alternative strategy to investigate the role in recycling of a putative Fur4p deubiquitination. Whereas a construct in which the target lysines for ubiquitination are mutated to arginine (Fur4-KR) remains stable at the plasma membrane (Marchal et al., 2000) (Figure 5A), the in-frame fusion of ubiquitin to this construct (Ub-Fur4-KR) results in its constitutive internalization and incorporation into the internal vesicles of the MVB (Blondel et al., 2004). Because this kind of chimeric protein cannot be deubiquitinated by isopeptidases, we investigated its recycling in ClE mutant cells. Figure 5, A and B, shows that Ub-Fur4-KR was efficiently targeted back to the plasma membrane.
Figure 5.
Deubiquitination of Fur4p is not required for its recycling. yJMG408 (vps37Δ) cells transformed either with pFL38-KR-gFP (GAL-Fur4KR-GFP) or with pFL38gFPUb-KR (GAL-Ub-Fur4KR-GFP) were treated as described in Figure 4. (A) Cells were visualized by fluorescence microscopy at t = 0 (0′), after 75 min of carbon starvation (75′CS) and 30 min after the addition of carbon (30′CA) (left). Note that because Fur4-KR remained stable at the plasma membrane upon carbon starvation, the recycling of this mutant form cannot be followed (NS). (B) Uracil uptake was measured every 20 min after glucose addition in yJMG408 (vps37Δ) cells transformed with pFL38gFP-Ub-KR (GAL-Ub-Fur4KR-GFP, white square). To facilitate comparison, the curve from Figure 4B corresponding to yJMG408 (vps37Δ) transformed with pFL38gFP (GAL-Fur4-GFP, black circle) is replotted here. Results are expressed as a percentage of the initial uracil uptake measured immediately before carbon starvation.
Together, these results indicate that a complete deubiquitination of Fur4p is not required for its efficient recycling in Vps ClE mutant cells. Surprisingly, in cells expressing Ub-Fur4-KR no decrease of the uracil uptake was observed even 1 h after addition of glucose (Figure 5B), despite the in-frame fused ubiquitin molecule that is supposed to trigger a constitutive internalization of the chimera. We think that Ub-Fur4-KR is immediately recycled resulting in an apparent stability at the plasma membrane.
Fur4p Bypasses the Golgi Apparatus on its Way Back to the Plasma Membrane
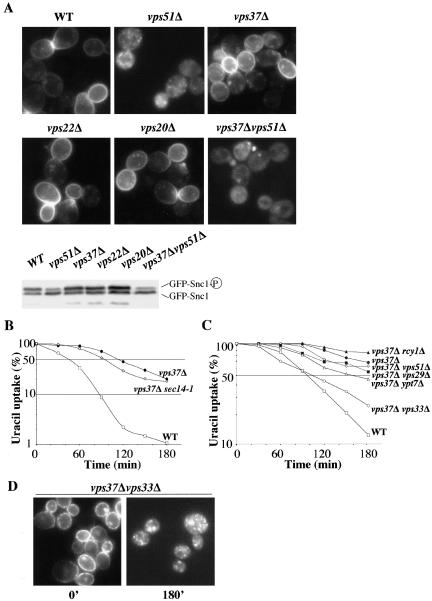
Snc1p travels from the EE to the Golgi on its way back to the plasma membrane. We investigated whether Fur4p and Snc1p followed the same recycling route in ClE mutant cells. We first investigated the recycling of GFP-Snc1p in ClE mutants. The steady-state distribution and phosphorylation status of GFP-Snc1p reflect the recycling efficiency of the SNARE protein (Lewis et al., 2000; Galan et al., 2001). In wild-type cells, GFP-Snc1p was mostly found in a hyperphosphorylated state at the plasma membrane. In strains deficient in the fusion of EE vesicles to the Golgi apparatus, such as vps51Δ cells (VFT complex), this protein accumulated in internal structures, mostly in an underphosphorylated state (Figure 6A). The ratio of internal to plasma membrane GFP-Snc1p was not affected in ClE mutant cells (Figure 6A), indicating that Snc1p recycling was not altered by ESCRT machinery dysfunction, in contrast to what was observed for Fur4p. This result is consistent with previously published data showing that the distribution of Snc1p is unaffected in Vps ClE cells lacking the ATPase Vps4p (Lewis et al., 2000).
Figure 6.
Fur4p and Snc1p follow different recycling roads. (A) BY4741 (WT), yJMG453 (vps51Δ, VFT), yJMG408 (vps37Δ, ESCRT I), yJMG475 (vps22Δ, ESCRT II), yJMG400 (vps20Δ, ESCRT III), and yJMG531 (vps37Δvps51Δ, ESCRT I, VFT) cells were transformed with pJMG118 (tpi-GFP-Snc1) and cultured until the early exponential growth phase. The subcellular distribution of GFPSnc1p was monitored by fluorescence microscopy (top). Crude extracts were prepared and the phosphorylation state of GFP-Snc1p was checked by Western immunoblotting in the indicated strains (bottom). (B) BY4741 (WT, white square), yJMG524 (vps37Δsec14-1, white diamond), and yJMG408 (vps37Δ, black circle) transformed with pFL38gFP (GAL-Fur4-GFP) were grown at 24°C and preshifted at 37° 15 min before addition of rapamycin. Uracil uptake was measured every 30 min after the addition of rapamycin over a 3-h period; results are expressed as a percentage of the initial uptake at t = 0 in a semilog scale. (C) yJMG528 (vps37Δrcy1Δ, black triangle), yJMG530 (vps37Δypt7Δ, white triangle), (yJMG531 (vps37Δvps51Δ, white diamond), yJMG529 (vps37Δvps29Δ, black square) and yJMG523 (vps37Δvps33Δ, white circle) cells were transformed, cultured, induced, and treated with rapamycin as described in Figure 3. The curves from Figure 3A corresponding to BY4741 (WT, white square) and yJMG408 (vps37Δ, black circle) transformed with pFL38gFP (GAL-Fur4-GFP) are replotted here. (D) yJMG523 (vps37Δvps33Δ) cells were collected at the time indicated after the addition of rapamycin and visualized by fluorescence microscopy. Note that Fur4-GFP accumulated in intracellular dots at t = 180′ (compare with Figure 3B).
We compared the recycling of the two proteins further by investigating whether the deletion of genes involved in the recycling of GFP-Snc1p also impaired Fur4-GFP recycling. We constructed a set of double mutants deficient in Vps37p and a protein required for Snc1p recycling—the F-Box protein Rcy1p, Vps51p (VFT complex) or Sec14p (exit from the Golgi apparatus). As expected, all the double mutants were deficient in Snc1p recycling (Figure 6A; unpublished data). We then investigated the ability of these strains to internalize and recycle Fur4p after the addition of rapamycin. Double deleted cells (vps37Δvps51Δ and vps37Δrcy1Δ) were grown at 30°C during the overall of the experiment (Figure 6C), whereas vps37Δsec14-1 ts cells were grown at 24°C and preshifted at 37°C 15 min before the addition of rapamycin (Figure 6B). Note that endocytosis of Fur4p in wt and vps37Δ cells is faster at 37°C than at 30°C (compare Figure 6, B and C). More importantly, none of the double mutants showed uracil uptake kinetics that significantly differed from those of vps37Δ cells (Figure 6, B and C). Similar results were obtained when these strains were tested directly for Fur4p recycling after carbon starvation followed by glucose addition, as described in Figure 4 (unpublished data). Thus, the machinery involved in Fur4p recycling in Vps ClE mutant cells clearly differs from the one used for the recycling of Snc1p, suggesting that the permease bypasses the Golgi on its way back to the plasma membrane.
The Fur4p recycling observed in vps37Δ cells may occur from the ClE compartment, which is thought to correspond to an exacerbated LE. We therefore investigated whether the machinery promoting exit from the LE was required for Fur4p recycling. Cargoes may leave the LE toward the TGN in a retromer-dependent manner, or toward the vacuole in a Ypt7p and Vps ClC complex-dependent manner (Whyte and Munro, 2002). In cells lacking both VPS37 and VPS29 (retromer subunit), Fur4p is efficiently recycled upon rapamycin addition (Figure 6C). This result confirms that internalized permease does not pass through the Golgi apparatus en route to the plasma membrane. Similarly, Fur4p recycling was not impaired in ypt7Δvps37Δ cells, suggesting that the permease does not recycle from the vacuolar membrane (Figure 6C). In contrast, the deletion of VPS33 in vps37Δ cells has a mild inhibitory effect on Fur4p recycling (Figure 6C). In Δvps37Δvps33, Fur4p was blocked in small intracellular structures 3 h after the addition of rapamycin (Figure 6D) from which it cannot recycle back to the plasma membrane. This raises the possibility that the VpsClC complex may be directly or indirectly involved in the recycling of plasma membrane proteins.
Multiple Recycling Defects in VpsClC Mutant Cells
The VpsClC complex was initially described as a central player in homotypic vacuolar fusion and heterotypic fusion between the LE and vacuole. Based on the similar phenotypes of VpsClC mutant cells and strains affected in both Golgi to LE transport (Vps class D mutants) and LE to vacuole transport (Vps class B mutants), it was suggested that the VpsClC complex also is involved in trafficking to the LE (Srivastava and Jones, 1998; Peterson and Emr, 2001; Subramanian et al., 2004). Given this dual function, we reasoned that the recycling defect of VpsClC mutants might result from the permease being blocked in early endosomal structures. However, Fur4p is efficiently recycled from the EE in pep12Δ cells (Figure 4). A possible explanation for that discrepancy is that the early endosomal structures in which Fur4p is blocked in VpsClC mutant cells are not competent for recycling. We tested this hypothesis by investigating in these cells the steady-state distribution of GFP-Snc1p, which is known to recycle from EE.
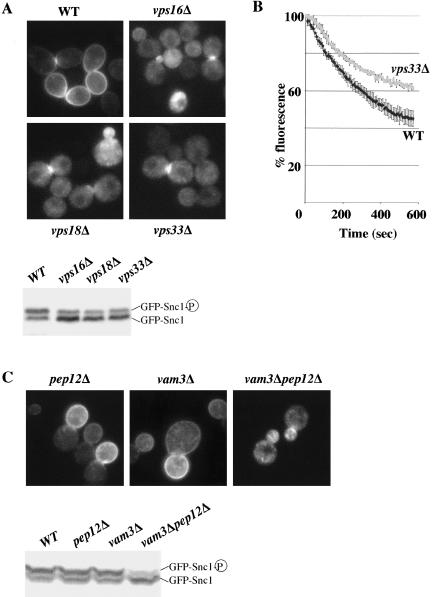
GFP-Snc1p displayed a predominantly intracellular distribution and accumulated in its underphosphorylated form in all strains defective for the VpsClC complex tested (vps33Δ, vps16Δ, vps18Δ), whereas it was found mainly at the plasma membrane in wild-type cells (Figure 7A). To confirm a general defect in the recycling pathway from the early endosomal compartment in VpsClC mutant cells, we quantified the secretion of an internalized fluorescent dye, FM4-64, in these cells, as described previously (Wiederkehr et al., 2000). The kinetics of FM4-64 secretion was slower in vps33Δ cells than in wt cells (Figure 7B), indicating that a deficiency in the VpsClC complex impairs the bulk recycling of membranes.
Figure 7.
Multiple recycling defects in Vps class C mutant cells. (A) BY4741 (WT) and yJMG473 (vps16Δ), yJMG474 (vps18Δ), yJMG476 (vps33Δ) cells defective for the VpsClC complex, transformed with pJMG118 (tpi-GFP-Snc1) were cultured and analyzed for GFP-Snc1p recycling as described in Figure 6A. Note that GFP-Snc1p was mainly intracellular (top) and underphosphorylated (bottom) in all strains defective for the VpsClC complex tested. (B) BY4741 (WT) and yJMG476 (vps33Δ) cells were analyzed for their ability to recycle the fluorescent membrane dye FM4-64, as described in Materials and Methods. Fluorescence was measured in triplicate for each strain, every 6 s for 10 min on a spectrofluorimeter. Results are expressed as a percentage of the initial fluorescence ± SD. (C) yJMG498 (pep12Δ), yJMG506 (vam3Δ), and yJMG526 (vam3Δpep12Δ) cells transformed with pJMG118 (tpi-GFP-Snc1, URA3, and CEN) were cultured and analyzed for GFP-Snc1p recycling as described in Figure 6A. Note that GFP-Snc1p displayed a similar distribution and phosphorylation defect in vam3Δpep12Δ cells and VpsClC-deficient cells (see A).
Thus, cells defective for the VpsClC complex display multiple recycling defects. According to the already known double function of this complex, these defects could result from a trafficking block either to the LE, from the LE or both. Alternatively, these phenotypes may reflect a third unknown function of the Vps ClC complex, in the direct flow of membrane from endosomes to the plasma membrane. To distinguish between these possibilities, we investigated the recycling of Snc1p in cells specifically defective in trafficking to the LE (pep12Δ), from the LE (vam3 and ypt7Δ), or both (pep12Δ vam3Δ). As expected, GFP-Snc1p was present mainly at the plasma membrane in pep12Δ cells deficient in trafficking to the LE (Figure 7C; Lewis et al., 2000). Similarly, GFP-Snc1p is normally recycled to the plasma membrane in cells defective for the vacuolar syntaxin Vam3p (Figure 7C) and in ypt7Δ cells (unpublished data), which are known to be specifically defective in trafficking between the LE and vacuole. As reported previously, a strain lacking both PEP12 and VAM3 is a phenocopy of VpsClC mutant cells in terms of vacuolar fragmentation, thermosensitivity, and the transport of vacuolar cargoes (Peterson and Emr, 2001; unpublished data). Importantly, the recycling of both GFP-Snc1p (Figure 7C) and FM4-64 (unpublished data) is highly impaired in pep12Δvam3Δ cells to a similar extent as in VpsClC mutant cells (compare with Figure 7A). This result indicates that the general recycling defect observed in VpsClC mutant cells is a secondary effect of the simultaneous impairment of the two functions of the VpsClC complex in trafficking to and from the LE.
DISCUSSION
We show here that the accumulation of Fur4p at the plasma membrane of VpsClE mutant cells is due to the efficient recycling of the permease. This recycling event does not require prior deubiquitination of Fur4p. On its way back to the plasma membrane, the permease bypasses the Golgi apparatus, unlike the well-characterized recycling cargo Snc1p. In our attempts to identify factors involved in the recycling of Fur4p, we found an unexpected phenotype associated with the loss of function of the VpsClC complex: cells lacking this complex display impaired recycling of Fur4p, Snc1p, and the dye FM4-64.
Internalization Defect or Enhanced Recycling?
The accumulation of receptors and transporters at the plasma membrane is classically interpreted as a consequence of defects in the internalization step of endocytosis. Alternatively, such phenotype could result from activated recycling. We show here that the accumulation of Fur4p at the plasma membrane of ESCRT mutant cells is due neither to enhanced secretion nor to a defect in internalization, but to efficient recycling of the protein. This result is consistent with the demonstration that EGFR is efficiently recycled at the plasma membrane of tsg101/hVps23-deficient fibroblasts, leading to prolonged EGFR signaling, which may contribute to the tumorigenic phenotype displayed by these cells (Babst et al., 2000).
The difficulty to distinguish between an internalization defect and enhanced recycling is illustrated by several published examples. For example, npi3/bro1 mutant cells were initially identified as deficient in down-regulation at the plasma membrane of both the general amino acid permease Gap1p and Fur4p (Springael et al., 2002). It was recently reported that the stabilization of Gap1p at the plasma membrane of bro1Δ cells is lost in bro1Δypt6Δ cells, suggesting that Gap1p is efficiently internalized and recycled in bro1Δ cells, in a Ypt6p-dependent manner (Nikko et al., 2003). The accumulation at the plasma membrane of a number of transporters and receptors in cells deficient for the Npi1p/Rsp5p ubiquitin-ligase had led to the widely accepted model in which the ubiquitination at the plasma membrane of endocytic cargoes triggers their internalization (Rotin et al., 2000). However, Npi1p/Rsp5p also is required for the ubiquitination and subsequent vacuolar targeting of biosynthetic cargoes of the VPS pathway, indicating that this E3-ligase can act in endosomal/post-Golgi compartments (Blondel et al., 2004; Dunn et al., 2004; Hettema et al., 2004; Katzmann et al., 2004; Morvan et al., 2004). An alternative model would postulate that the apparent stabilization at the plasma membrane of a subset of Rsp5p substrates in Rsp5p-deficient cells is the result of a constitutive internalization and efficient recycling of these proteins. Verification of this model requires a kinetic assay that allows an uncoupling of endocytosis from the recycling of those cargo proteins. Our protocol for monitoring recycling of the uracil permease based on carbon source availability fulfills this criterion. This assay is quantitative and reliable, thanks to the dual monitoring of the recycled pool of Fur4p by means of both fluorescence microscopy and uracil uptake measurement. In addition, the ability of Fur4p to transport toxic analogs of uracil is of great potential value for screening for new components of the recycling machinery.
Multiple Recycling Pathways in Yeast
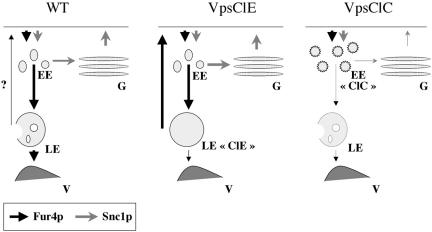
Blocking of the endocytic pathway at the level of MVB formation (in ESCRT mutants) results in the efficient recycling of Fur4p. Does the permease also recycle in wt cells? In carbon starvation/addition experiments, we observed a faint but reproducible increase in uracil uptake after the addition of glucose to wt cells (Figure 4B), but the pool of Fur4p recycling in these cells was far too small to be detected by fluorescence microscopy. Although we cannot rule out the possibility that, under particular experimental conditions, Fur4p massively recycles in wt cells, we suggest that normally most of the internalized protein is targeted for vacuolar degradation when the endocytic pathway is functional (Figure 8).
Figure 8.
Working model. Fur4p (black arrow) and Snc1p (gray arrow) recycle via different pathways. The thickness of the arrows reflects the intensity of the traffic. Left, in wt cells: Fur4p follows the endocytic pathway through EE, LE, and is degraded in the vacuole (V), with a small amount possibly recycling (?). Snc1p constitutively cycles between EE, the Golgi apparatus (G) and the plasma membrane (PM). Middle, in VpsClE mutant cells: after endocytosis, Fur4p recycles efficiently from the ClE compartment to the PM. Snc1p recycling is not affected. Right, in VpsClC mutant cells: after endocytosis, Fur4p and Snc1p are blocked in a modified early endosomal compartment (EE “ClC”) from which they cannot recycle to the plasma membrane. See text for details.
In cells deficient for the ESCRT machinery, recycling may occur from the class E compartment or from the limiting membrane of the vacuole. In mammalian cells, conventional lysosomes have been demonstrated to fuse with the plasma membrane (Andrews, 2002). Similarly, in S. cerevisiae, Nikko et al. (2003) have proposed that Gap1p recycles from the vacuolar membrane because a lack of the vacuolar syntaxin Vam3p abolishes the stabilization of Gap1p observed at the plasma membrane of bro1Δ cells. We think this is unlikely to occur in the case of Fur4p, because the recycling of this protein still occurs in vps37Δypt7Δ cells where LE to vacuole transport is blocked. Instead, we favor a model in which Fur4p recycles from the membrane of the ClE compartment. In addition, the recycling of Fur4p in VpsClE cells is not affected by mutations impairing transport to and from the Golgi apparatus (rcy1Δ, vps51Δ, vps29Δ, sec14-1), indicating that in contrast to Snc1p the permease bypasses this compartment (Figure 8). Our data constitute the first evidence for a direct recycling pathway from endosomes to the plasma membrane and are consistent with the observation that VpsClE mutant cells are defective in the retrograde transport of TGN resident proteins from the LE/ClE compartment to the Golgi apparatus (Bilodeau et al., 2002).
We also demonstrated efficient recycling of Fur4p in pep12Δ cells, presumably from the early endosomal compartment. We tried to investigate whether this recycling pathway also bypassed the Golgi apparatus by constructing double mutant cells defective in both Pep12p and the genes required for EE-to-Golgi transport (RCY1, VPS51), but these mutants were not viable (unpublished data). Similarly, the deletion of YPT6 encoding the RAB protein required for VFT/GARP mediated fusion to the Golgi is synthetic-lethal with several other mutants deficient for Snc1p recycling (Lafourcade et al., 2004). This raised the possibility that recycling of membrane proteins via the Golgi may be an essential process for viability regulated by at least two distinct trafficking pathways.
Sorting in the Class E Compartment
Ubiquitination is required for the internalization of Fur4p. On its way to the vacuole, Fur4p is deubiquitinated in the LE compartment in a Doa4p-dependent manner, but impairing the deubiquitination of MVB cargoes targeted to the vacuolar lumen has no effect on the trafficking of these cargoes (Dupré and Haguenauer-Tsapis, 2001). This raises the question whether Fur4p needs to be deubiquitinated when the permease is recycled back to the plasma membrane in ClE mutant cells. Although we were unable to directly address the ubiquitination status of Fur4-GFP during the very rapid kinetics of recycling of this permease, we were able to show that impairing the deubiquitination of Fur4p (by using Doa4p-deficient cells or a form of the permease that cannot be deubiquitinated) has no effect on the recycling of this protein. Similarly, the ligand-dependent uptake and recycling of the truncated receptor Ste3-Δ365 is not regulated by means of its ubiquitination status (Chen and Davis, 2002). It has therefore been suggested that recycling may be a default pathway, i.e., that does not require a specific signal.
It should be noted that, in ESCRT mutant cells, the biosynthetic cargoes of the MVB display different steady-state localizations: CPS and Phm5p are mostly at the vacuolar membrane, whereas Sna3p is blocked exclusively in the ClE compartment (Katzmann et al., 2001; Reggiori and Pelham, 2002). These data indicate that active sorting events occur in the ClE compartment: biosynthetic cargoes are either blocked in the ClE compartment (Sna3p) or targeted to the vacuolar membrane (Phm5p, Cps), whereas receptors and transporters (Gap1p, Fur4p) are recycled back to the plasma membrane. The signals on the cargoes and the machinery involved in these sorting events are still unknown. The nexin proteins, which interact with both cargoes and components of the trafficking machinery, are good candidates for involvement in such a sorting function (Hettema et al., 2003).
Role of the Vps Class C Complex in Recycling
We observed the intracellular accumulation of recycling cargoes (Snc1p and Fur4p) and a general slowing of bulk membrane recycling in VpsClC mutant cells. These phenotypes probably result from the dual function of this complex in trafficking to and from the LE, because vam3Δpep12Δ cells share the same recycling defects. A simple explanation for the recycling defect of Fur4p in the VpsClC-VpsClE double mutant is that the permease cannot reach the ClE compartment from which it would normally recycle. Alternatively, the ClE compartment may be nearly absent in the VpsClCVpsClE double mutant as recently suggested by Subramanian et al. (2004). However, the endosomal structures in the VpsClC mutant cells, theoretically corresponding to EE, are probably not functional in recycling, as the recycling of both Snc1p and FM4-64 also is impaired in these cells. We therefore suggest that the loss of VpsClC complex function leads to an accumulation of endocytosed cargoes in nonfunctional early endosomal structures (Figure 8).
Components of the Vps class C complex were recently identified in Drosophila, plants, and mammals, suggesting that this complex is widely conserved in eukaryotes (Sevrioukov et al., 1999; Rojo et al., 2003; Suzuki et al., 2003). Most of these studies focused on Vps33a and Vps33b, the two mammalian paralogs of the yeast SM (Sec1/Munc18) protein Vps33p. Several phenotypes associated with the loss of function of Vps33a and Vps33b do not fit with the classical role of the VpsClC complex in lysosomes. For example, mice lacking Vps33a (bf mice)—an animal model for Hermansky-Pudlak syndrome—display hypopigmentation but, surprisingly, no clear abnormalities in lysosomal function (Suzuki et al., 2003). In addition, it was recently reported that mutations in hVps33b cause arthrogryposis-renal dysfunctioncholestasis (ARC) (Gissen et al., 2004). In cells from individuals with ARC, several plasma membrane proteins normally restricted to the apical membrane of polarized cells were mislocalized throughout the interior of the cell (Devonald et al., 2003; Gissen et al., 2004). According to our data, it is tempting to speculate that some of the phenotypes associated with ARC result from a recycling problem.
In summary, our results illustrate the unexpected variety of recycling routes in S. cerevisiae. In mammalian cells, the diversity of recycling pathways is well established, but the molecular mechanisms underlying these processes are still poorly understood (Maxfield and McGraw, 2004). The yeast model may be of considerable value in this respect. In addition, our data provide a key to understand some of the changes in distribution of plasma membrane proteins observed in cases of VpsClC and ESCRT complex deficiencies in higher eukaryotic cells.
Acknowledgments
We thank H. Pelham, H. Riezman, S. Friant, and M. Hochstrasser for plasmids and yeast strains. We also thank members of the RHT laboratory and M. Blondel for helpful discussions and critical reading of the manuscript. Many thanks to C. Volland for preliminary experiments on Fur4p recycling. This work was supported by the Centre National dela Recherche Scientifique. A.B. and M.F. are supported by a Ph.D. fellowship from the “Ministère de l'enseignement et de la recherche” and a postdoctoral fellowship (EFFEXPORT contract QLRT 2001-00533), respectively.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04-05-0420. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0420.
References
- Amerik, A.Y., Nowak, J., Swaminathan, S., and Hochstrasser, M. (2000). The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein sorting and endocytic pathways. Mol. Biol. Cell 11, 3365-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, N. (2002). Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J. Cell Biol. 158, 389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst, M., Odorizzi, G., Estepa, E.J., and Emr, S.D. (2000). Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1, 248-258. [DOI] [PubMed] [Google Scholar]
- Bensen, E.S., Yeung, B.G., and Payne, G.S. (2001). Ric1p and the Ypt6p GTPase function in a common pathway required for localization of trans-Golgi network membrane proteins. Mol. Biol. Cell 12, 13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilodeau, P.S., Urbanowski, J.L., Winistorfer, S.C., and Piper, R.C. (2002). The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4, 534-539. [DOI] [PubMed] [Google Scholar]
- Blondel, M.O., Morvan, J., Dupre, S., Urban-Grimal, D., Haguenauer-Tsapis, R., and Volland, C. (2004). Direct sorting of the yeast uracil permease to the endosomal system is controlled by uracil binding and Rsp5p-dependent ubiquitylation. Mol. Biol. Cell 15, 883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., and Davis, N.G. (2000). Recycling of the yeast a-factor receptor. J. Cell Biol. 151, 731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L., and Davis, N.G. (2002). Ubiquitin-independent entry into the yeast recycling pathway. Traffic 3, 110-123. [DOI] [PubMed] [Google Scholar]
- Davis, N.G., Horecka, J.L., and Sprague, J.G.F. (1993). Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J. Cell Biol. 122, 53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonald, M.A., Smith, A.N., Poon, J.P., Ihrke, G., and Karet, F.E. (2003). Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat. Genet 33, 125-127. [DOI] [PubMed] [Google Scholar]
- Dunn, R., Klos, D.A., Adler, A.S., and Hicke, L. (2004). The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J. Cell Biol. 165, 135-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupré, S., and Haguenauer-Tsapis, R. (2001). Deubiquitination step in the endocytic pathway of yeast plasma membrane proteins: crucial role of Doa4p ubiquitin isopeptidase. Mol. Cell. Biol. 21, 4482-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan, J.-M., and Haguenauer-Tsapis, R. (1997). Ubiquitin Lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 16, 5847-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan, J.M., Moreau, V., André, B., Volland, C., and Haguenauer-Tsapis, R. (1996). Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J. Biol. Chem. 271, 10946-10952. [DOI] [PubMed] [Google Scholar]
- Galan, J.M., Wiederkehr, A., Seol, J.H., Haguenauer-Tsapis, R., Deshaies, R.J., Riezman, H., and Peter, M. (2001). Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol. Cell. Biol. 21, 3105-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissen, P., et al. (2004). Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat. Genet. 36, 400-404. [DOI] [PubMed] [Google Scholar]
- Gruenberg, J., and Maxfield, F.R. (1995). Membrane transport in the endocytic pathway. Curr. Opin. Cell Biol. 7, 552-563. [DOI] [PubMed] [Google Scholar]
- Gurunathan, S., David, D., and Gerst, J. (2002). Dynamin and clathrin are required for the biogenesis of a distinct class of secretory vesicles in yeast. EMBO J. 21, 602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay, E., and Schekman, R. (2002). A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J. Cell Biol. 156, 271-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema, E.H., Lewis, M.J., Black, M.W., and Pelham, H.R. (2003). Retromer and the sorting nexins Snx4/41/42 mediate distinct retrieval pathways from yeast endosomes. EMBO J. 22, 548-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema, E.H., Valdez-Taubas, J., and Pelham, H.R. (2004). Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 23, 1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, Z., Fatheddin, P., and Graham, T.R. (2002). An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell 13, 3162-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann, D., Odorizzi, G., and Emr, S. (2002). Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell. Biol., 893-905. [DOI] [PubMed]
- Katzmann, D.J., Babst, M., and Emr, S.D. (2001). Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145-155. [DOI] [PubMed] [Google Scholar]
- Katzmann, D.J., Sarkar, S., Chu, T., Audhya, A., and Emr, S.D. (2004). Multivesicular body sorting: ubiquitin ligase Rsp5 is required for the modification and sorting of carboxypeptidase S. Mol. Biol. Cell 15, 468-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade, C., Galan, J.M., Gloor, Y., Haguenauer-Tsapis, R., and Peter, M. (2004). The GTPase-activating enzyme Gyp1p is required for recycling of internalized membrane material by inactivation of the Rab/Ypt GTPase Ypt1p. Mol. Cell. Biol. 24, 3815-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, M.J., Nichols, B.J., Prescianotto-Baschong, C., Riezman, H., and Pelham, H.R. (2000). Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 11, 23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Kane, T., Tipper, C., Spatrick, P., and Jenness, D.D. (1999). Yeast mutants affecting possible quality control of plasma membrane proteins. Mol. Cell. Biol. 19, 3588-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M.S., A., M.I., Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- Luo, W., and Chang, A. (2000). An endosome-to-plasma membrane pathway involved in trafficking of a mutant plasma membrane ATPase in yeast. Mol. Biol. Cell 11, 579-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal, C., Dupre, S., and Urban-Grimal, D. (2002). Casein kinase I controls a late step in the endocytic trafficking of yeast uracil permease. J. Cell Sci. 115, 217-226. [DOI] [PubMed] [Google Scholar]
- Marchal, C., Haguenauer-Tsapis, R., and Urban-Grimal, D. (2000). Casein kinase I-dependent phosphorylation within a PEST sequence and ubiquitination at nearby lysines, signal endocytosis of yeast uracil permease. J. Biol. Chem. 275, 23608-23614. [DOI] [PubMed] [Google Scholar]
- Maxfield, F.R., and McGraw, T.E. (2004). Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5, 121-132. [DOI] [PubMed] [Google Scholar]
- Moreau, V., Galan, J.-M., Devilliers, G., Haguenauer-Tsapis, R., and Winsor, B. (1997). The yeast actin-related protein Arp2p is required for the internalization step of endocytosis. Mol. Biol. Cell 8, 1361-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan, J., Froissard, M., Haguenauer-Tsapis, R., and Urban-Grimal, D. (2004). The ubiquitin ligase Rsp5p is required for modification and sorting of membrane proteins into multivesicular bodies. Traffic 5, 383-392. [DOI] [PubMed] [Google Scholar]
- Nikko, E., Marini, A.M., and Andre, B. (2003). Permease recycling and ubiquitination status reveal a particular role for Bro1 in the multivesicular body pathway. J. Biol. Chem. 278, 50732-50743. [DOI] [PubMed] [Google Scholar]
- Peterson, M.R., and Emr, S.D. (2001). The class C Vps complex functions at multiple stages of the vacuolar transport pathway. Traffic 2, 476-486. [DOI] [PubMed] [Google Scholar]
- Reggiori, F., and Pelham, H.R. (2002). A transmembrane ubiquitin ligase required to sort membrane proteins into multivesicular bodies. Nat. Cell Biol. 4, 117-123. [DOI] [PubMed] [Google Scholar]
- Rojo, E., Zouhar, J., Carter, C., Kovaleva, V., and Raikhel, N.V. (2003). A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100, 7389-7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotin, D., Staub, O., and Haguenauer-Tsapis, R. (2000). Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases [In Process Citation]. J. Membr. Biol. 176, 1-17. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. (1997). Molecular Cloning. A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Schmeizle, K., and Hall, M. (2000). TOR, a central controller of cell Growth. Cell 103, 253-262. [DOI] [PubMed] [Google Scholar]
- Sevrioukov, E.A., He, J.P., Moghrabi, N., Sunio, A., and Kramer, H. (1999). A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol. Cell 4, 479-486. [DOI] [PubMed] [Google Scholar]
- Shaw, J.D., Cummings, K.B., Huyer, G., Michaelis, S., and Wendland, B. (2001). Yeast as a model system for studying endocytosis. Exp. Cell Res. 271, 1-9. [DOI] [PubMed] [Google Scholar]
- Sherman, F., Fink, G., and Hicks, J.B. (1986). Methods in Yeast Genetics. A Laboratory Manual, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Siniossoglou, S., and Pelham, H.R. (2001). An effector of Ypt6p binds the SNARE Tlg1p and mediates selective fusion of vesicles with late Golgi membranes. EMBO J. 20, 5991-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siniossoglou, S., and Pelham, H.R. (2002). Vps51p links the VFT complex to the SNARE Tlg1p. J. Biol. Chem. 277, 48318-48324. [DOI] [PubMed] [Google Scholar]
- Springael, J.Y., Nikko, E., Andre, B., and Marini, A.M. (2002). Yeast Npi3/Bro1 is involved in ubiquitin-dependent control of permease trafficking. FEBS Lett. 517, 103-109. [DOI] [PubMed] [Google Scholar]
- Srivastava, A., and Jones, E.W. (1998). Pth1/Vam3p is the syntaxin homolog at the vacuolar membrane of Saccharomyces cerevisiae required for the delivery of vacuolar hydrolases. Genetics 148, 85-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian, S., Woolford, C.A., and Jones, E.W. (2004). The Sec1/Munc18 protein, Vps33p, functions at the endosome and the vacuole of Saccharomyces cerevisiae. Mol. Biol. Cell 15, 2593-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, T., Oiso, N., Gautam, R., Novak, E.K., Panthier, J.J., Suprabha, P.G., Vida, T., Swank, R.T., and Spritz, R.A. (2003). The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc. Natl. Acad. Sci. USA 100, 1146-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski, J., and Piper, R.C. (2001). Ubiquitin sorts proteins into the lumenal degradative compartment of the late endosome/vacuole. Traffic 2, 622-630. [DOI] [PubMed] [Google Scholar]
- Valdivia, R.H., Baggott, D., Chuang, J.S., and Schekman, R.W. (2002). The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2, 283-294. [DOI] [PubMed] [Google Scholar]
- Volland, C., Urban-Grimal, D., Géraud, G., and Haguenauer-Tsapis, R. (1994). Endocytosis and degradation of the yeast uracil permease under adverse conditions. J. Biol. Chem. 269, 9833-9841. [PubMed] [Google Scholar]
- Whyte, J.R., and Munro, S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627-2637. [DOI] [PubMed] [Google Scholar]
- Wiederkehr, A., Avaro, S., Prescianotto-Baschong, C., Haguenauer-Tsapis, R., and Riezman, H. (2000). The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 149, 397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]