Abstract
The study determined if administration of Vernonia amygdalina and Carica papaya plants provides synergistic effects in ameliorating plasmodium infection in mice. Thirty mice (17.88–25.3 g) were divided into 6 groups of 5 mice each. Group 1 was normal control, while groups 2–6 were intraperitoneally inoculated 2.5 × 107 Plasmodium berghei parasitized red blood cell, followed by daily administration of 350 mg/kg aqueous leaf extracts after establishment of infection. Group 2 was disease control, while group 6 was treated with standard drug for four consecutive days. The results showed significant (P < 0.05) reduction in percentage of parasite load between the infected treatment groups and disease control group at day 3 after infection, which remained consistent until the end of the experiment. All infected treated groups showed significant (P < 0.05) increases in RBC and PCV recovery compared to the disease control, with the exception of WBC. There was insignificant (P > 0.05) change in mean body weight of all treated groups except in disease control group. Histological studies of the infected mice indicate recovery of hepatic cells from congested black pigmentation. The reduction in parasite load and recovery of hepatic cell damage/hematological parameters were induced by these plant extracts. This highlighted the important usage of the plant in traditional remedy of malaria infection.
1. Introduction
Malaria remains one of the deadliest infectious diseases, caused by protozoan parasite of the genus Plasmodium [1]. Available therapeutic agents are already limited in their efficacy, while drug resistance threatens the ability to prevent and treat the infection [1]. Malaria is the leading cause of illness and death in sub-Saharan Africa with an annual mortality of approximately one million children under five [2]. In Nigeria and the rest of the world, malaria infection continues to pose a major health challenge. In view of resistance of the parasite to antimalarial drug therapy, which leads to drug failure, new drugs or drug combinations are urgently required for the treatment of malaria infections from traditional medicinal plants [3]. Traditional medicinal plants play an important role in the medical system in Nigeria; however, plant materials remain an important resource to combat serious diseases in the world. Currently used plants have always been considered to be a possible alternative and rich source of new drugs, and some of the antimalarial drugs in use today such as quinine and artemisinin were either obtained directly from plants or developed using chemical structures of plant-derived compounds as templates [4].
The uses of synthetic antimalarial drugs are among the main ways to treat malaria and are effective in controlling parasite load. However, the practical application of the majority of these therapeutic agents remains restricted owing to their limited action, pharmacokinetic properties, secondary failure rate, and accompanying side effects [5]. With the increasing incidence of the disease in urban/rural population throughout the world, there is clear need for development of indigenous, inexpensive botanical sources for antimalarial crude or purified drugs [5].
Vernonia amygdalina Del belongs to the family Asteraceae; its common name is bitter leaf. It is a perennial shrub of 2–5 m in height that grows throughout tropical Africa [6]. The leaf and stem are acclaimed to be one of the most used medicinal plants in traditional practice [7]. Carica papaya Linn belongs to the Caricaceae family and is commonly known as pawpaw [8]. The leaf of Carica papaya in combination with Vernonia amygdalina is used traditionally to treat infection [7, 9]. In traditional medicine, herbal practitioners use V. amygdalina and C. papaya in Nigeria as anthelmintic, antimalaria, digestive tonic, appetizer, antihemolytic, antifungal, and antijaundice agents. The leaf infusion is used in treatment of urinary disorders and gonorrhea. The fresh leaves are used in dressing of wounds or as antidysentery agent and against chronic diarrhea or as sedative and as cure for pile and wounds of the urinary tracts and several other ailments [9]. The plant extracts have been scientifically proven to function as antibacterial [7], antifungal [7, 10], anticancer/tumor [11, 12], and antiplasmodial agents [13, 14].
While the physiological role of some plant active principle(s) has been intensively studied and their functions in health and disease conditions have been recognized, it is still a focus of pharmaceutical research in the development of agent that can effectively treat malaria and many other diseases, hence the need to study the efficacy of V. amygdalina and C. papaya in living systems in order to foster information regarding its medical applications in malarial infection.
2. Materials and Methods
2.1. Plant Samples Collection and Identification
Fresh leaves of Vernonia amygdalina and Carica papaya were collected in November 2015, from Federal Low Cost Housing Estate, Makurdi, Nigeria. The plants were identified and authenticated by a taxonomist in the Department of Biological Sciences, University of Agriculture, Makurdi, Nigeria.
2.2. Experimental Animals
Adult mice (17.88–25.3 g) of both sexes were obtained from the Laboratory Animal House, College of Health Sciences, Benue State University, Makurdi, Nigeria. The animals were acclimatized for 2 weeks under standard environmental conditions. The temperature and humidity were maintained at 25°C and 50%, respectively. Dark and light cycles were maintained at 12 hrs each. They had access to standard commercial rat pellets (UAC Grand Cereal Ltd., Jos, Nigeria) and water ad libitum. The animals were used in accordance with the guideline and recommendation of the ethics committee on the use of animals for research of the University of Agriculture, Makurdi, Nigeria.
2.3. Equipment/Reagents
The following equipment and reagents were used: Olympus microscope, hemocytometer with aspirating pipette, capillary tube, microhematocrit centrifuge, PCV reader, cover slip and slides, and water bath. Turk's fluid for WBC, Dacie's fluid for RBC, and Giemsa stain were purchased from Sigma-Andrich Chemical Company (St. Louis, USA). All other chemicals and reagents used for this study were of analytical grade.
2.4. Preparation of Plant Sample
The collected samples were rinsed in clean water and shade dried at room temperature for two weeks. The dry plants sample was ground into powder using pestle and mortar. The powder obtained was then used to prepare the extracts.
2.5. Aqueous Extraction
Exactly 100 g of the dried powder was weighed out and soaked in the solvent (water) with the solute-solvent proportion of 1 : 10. The extraction was by cold maceration at room temperature with intermittent shaking at 3-hour intervals for 48 hours. Whatman filter paper of 8 μm was used to filter the extract. The filtrate obtained was concentrated in temperature regulated water bath at 45°C.
2.6. Lethality (LD50) Test
The mean lethal dose (LD50) of the aqueous extracts was determined in mice (weighing 20–30 g) using the arithmetic-geometric-harmonic (AGH) methods of rough estimation as modified by Saganuwan [15].
2.7. Malaria Parasite
The malaria parasite (Chloroquine sensitive Plasmodium berghei (Nk65)) was obtained from the Faculty of Veterinary Medicine, Ahmadu Bello University (ABU), Zaria.
2.8. Parasite Inoculation
Plasmodium berghei parasitized erythrocytes were obtained from the tail of the donor mice and were diluted with 0.9% normal saline. Mice were inoculated intraperitoneally with 0.5 mL blood suspension containing 2.5 × 107 parasitized erythrocytes on day 0 and were monitored for manifestation of parasitemia for 4 days without treatment. The mice were randomly divided into 6 groups of five (5) mice per group and treated for 4 consecutive days with daily doses of the extracts (350 mg/kg b.w) and standard antimalarial drug (halofantrine, 25 mg/kg b.w) by oral route.
2.9. Animal Grouping and Treatment
Group 1: negative control, not infected with P. berghei and not treated
Group 2: positive control, infected with P. berghei but not treated
Group 3: infected with P. berghei and treated with 350 mg/kg C. papaya extract
Group 4: infected with P. berghei and treated with 350 mg/kg V. amygdalina extract
Group 5: infected with P. berghei and treated with 350 mg/kg combined extracts of C. papaya and V. amygdalina in ratio 1 : 1
Group 6: infected with P. berghei and treated with antimalarial drug (halofantrine, 25 mg/kg)
2.10. Hematological Analysis
The percentages of parasitized erythrocytes levels were determined as described by Brown [16], using a microscopic examination of thin blood smears made on microscopic slide. The packed cell volume was assayed according to the method described by Coles [17]. The RBC and WBC count was estimated according to the protocol of Brown [16], using the Neubauer haemocytometer.
2.11. Histological Analysis
At the end of the experiment, all the mice were anaesthetized using chloroform and bled by cardiac puncture. The hepatic tissues were dissected out of all the mice and washed on ice cold saline immediately. A portion of the tissue was fixed in 10% formalin fixative solution for histological studies as described by Strate et al. [18].
2.12. Statistical Analysis
The analysis was carried out in triplicate for all determinations and the results were expressed as mean ± SEM. The SPSS program (version 20.0 SPSS Inc., Chicago, IL, USA) was used for the analysis of variance, followed by the new Duncan's multiple range tests for multiple comparisons of the means [19]. P < 0.05 between mean values was considered statistically significant.
3. Results
The results of this study show that aqueous leaf extracts of V. amygdalina and C. papaya displayed antimalarial activity in an infected mouse when compared to the infected, untreated control. Figure 1 reveals the percentage of parasitized erythrocytes of mice infected with P. berghei. The groups infected with P. berghei developed parasitemia after infection. The percentage of parasitized erythrocytes in the infected untreated group (Group 2) was significantly (P < 0.05) increased on day 9 compared to postinfection day (within group) and other groups (between groups), respectively. The significant (P < 0.05) reduction in parasite load was more pronounced in groups 3 and 6, respectively.
Figure 1.
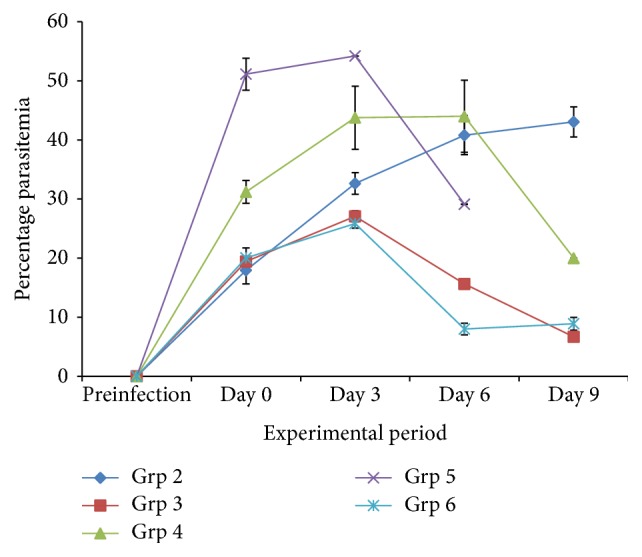
The percentage of parasitized erythrocytes in mice infected with P. berghei and treated with aqueous leaf extracts of V. amygdalina and C. papaya, and halofantrine.
Table 1 shows the mean RBC, PCV, and WBC count of mice infected with P. berghei and subsequently treated with aqueous extracts of C. papaya, V. amygdalina, combined C. P. + V. A., and halofantrine. There was significant (P < 0.05) decrease in mean RBC count and PCV in all the experimental groups after infection compared to normal control group. However, as treatment commenced, there was an observed significant (P < 0.05) increase in mean RBC count and PCV within groups in all the treated groups (except RBC in group 4) compared to the infected, untreated control group. The mean white blood cells count of all the groups infected with P. berghei was significantly (P < 0.05) increased after infection compared to the normal control. Increase in WBC was significantly reduced upon administration of the different regimen of extracts to infected groups.
Table 1.
The red blood cell and white blood cell count of mice infected with P. berghei and treated with aqueous leaf extracts of V. amygdalina, C. papaya, and halofantrine.
| Parameters | Groups | Preinfection | Day 0 | Day 3 | Day 6 | Day 9 |
|---|---|---|---|---|---|---|
| RBC (×1012/l) | GRP I | 13.60 ± 0.47b | 13.08 ± 0.53b | 11.70 ± 0.74ab | 10.88 ± 0.27a | 11.48 ± 0.62a |
| GRP II | 12.10 ± 0.70c | 6.50 ± 1.92b | 1.23 ± 0.21a | 1.40 ± 0.51a | 2.15 ± 0.33a | |
| GRP III | 9.84 ± 0.49c | 5.44 ± 0.24b | 2.90 ± 0.05a | 4.23 ± 0.11a | 5.23 ± 0.18b | |
| GRP IV | 10.26 ± 0.47c | 5.42 ± 0.72b | 2.90 ± 0.72a | 2.37 ± 0.29a | 5.57 ± 0.23b | |
| GRP V | 12.80 ± 1.37c | 4.42 ± 0.19b | 0.93 ± 0.23a | 2.30 ± 0.31a | ||
| GRP VI | 11.56 ± 0.21e | 2.70 ± 0.22b | 1.63 ± 0.27a | 5.45 ± 0.17c | 6.45 ± 0.22d | |
|
| ||||||
| PCV (%) | GRP I | 50.40 ± 1.46b | 44.20 ± 2.33ab | 44.20 ± 4.80ab | 46.20 ± 1.95ab | 38.60 ± 2.15a |
| GRP II | 52.00 ± 1.87d | 43.20 ± 0.73c | 30.33 ± 4.37b | 33.50 ± 1.50b | 17.50 ± 2.50a | |
| GRP III | 48.60 ± 1.40c | 43.20 ± 0.73c | 20.75 ± 1.85a | 41.50 ± 2.53bc | 32.75 ± 5.34b | |
| GRP IV | 43.20 ± 2.52b | 44.60 ± 0.81b | 29.50 ± 2.06a | 26.33 ± 5.78a | 30.00 ± 0.00a | |
| GRP V | 47.20 ± 1.71d | 39.20 ± 1.24c | 23.00 ± 0.00b | 18.00 ± 0.00a | ||
| GRP VI | 49.80 ± 0.48d | 34.80 ± 1.85b | 27.50 ± 2.90a | 46.50 ± 1.50cd | 45.50 ± 0.50c | |
|
| ||||||
| WBC (×109/l) | GRP I | 4.68 ± 0.59ab | 4.32 ± 0.56a | 4.56 ± 0.55ab | 7.04 ± 0.32bc | 6.04 ± 0.63c |
| GRP II | 4.20 ± 1.86a | 6.75 ± 1.24a | 17.93 ± 4.45a | 13.00 ± 0.25ab | 13.20 ± 0.38b | |
| GRP III | 6.08 ± 0.83a | 6.30 ± 0.59ab | 8.13 ± 1.75ab | 6.73 ± 1.17b | 8.93 ± 0.38b | |
| GRP IV | 6.36 ± 1.32ab | 5.92 ± 1.16a | 11.27 ± 1.89c | 10.33 ± 5.34bc | 5.80 ± 0.00ab | |
| GRP V | 8.00 ± 1.76a | 13.90 ± 0.65b | 15.2 ± 0.00b | 7.60 ± 0.00a | ||
| GRP VI | 4.68 ± 1.42a | 15.36 ± 1.42c | 12.10 ± 1.89b | 4.60 ± 0.51ab | 7.80 ± 0.13ab | |
Values are expressed as mean ± SEM of five replicate determinations. Values with different superscript along the rows are significantly different at P < 0.05.
The body weight of all the experimental groups was presented in Figure 2. There was significant (P < 0.05) increase in the body weight gain throughout the postinfection period in groups 1, 3, and 6 compared to the preinfection day. However, no significant increase was observed in groups 2, 4, and 5 when compared to the preinfection day.
Figure 2.
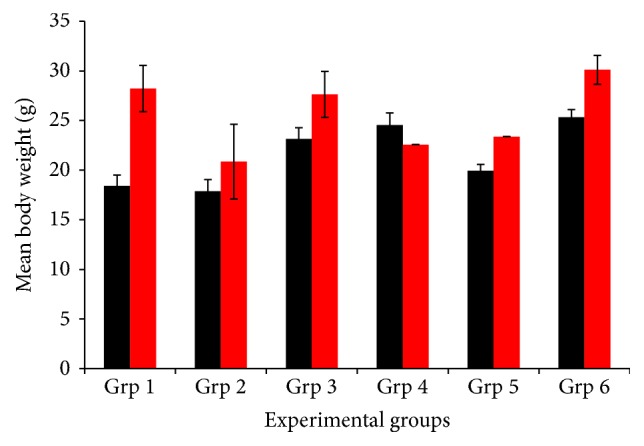
The mean body weight of mice infected with P. berghei and treated with aqueous leaf extracts of V. amygdalina, C. papaya, and halofantrine. Black colour indicates preinfection; red colour indicates day 9, except in Grp 5 (indicating day 6).
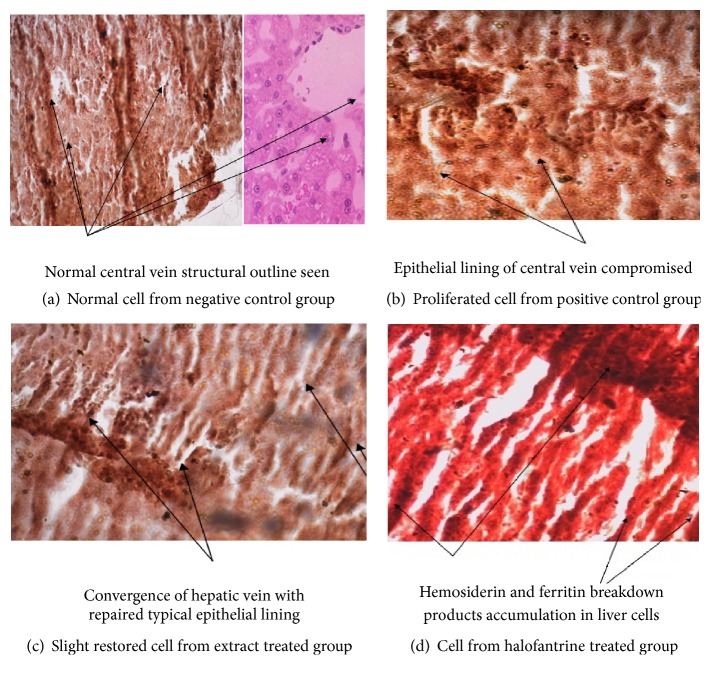
Figure 3 shows the histological section of the hepatocyte of mice infected with P. berghei and treated with aqueous extracts of C. papaya, V. amygdalina, combined C. P. + V. A., and halofantrine. Figure 3(a) is the normal control mice with normal architecture of hepatic cells and reference standard. Figure 3(b) is the malarial control mice with complete depletion of hepatic cells by P. berghei. Here, the figure revealed prominent sinusoids and sinusoidal spaces, along with areas of necrosis. Figure 3(c) is the representative hepatocyte from infected mice treated with 350 mg/kg b.w of the extracts. In this figure, there was a slight restoration of diffused proliferated hepatic cells. Figure 3(d) is the infected mice treated with 25 mg/kg b.w halofantrine; this shows slight restoration of diffused proliferated hepatic cells.
Figure 3.
Representative central vein of the hepatic cells of the parasitic mice treated with leaf extracts of V. amygdalina, C. papaya, and halofantrine.
4. Discussion
The existence of experimental animal models of a disease aids not only in the understanding of the pathophysiology of such disease, but also in the development of drug candidate [20].
Therefore, screening for antimalarial activity of plant crude extracts is the first step in isolation of new molecules with potent activity [21, 22]. The results clearly indicate that the individual administration of aqueous leaf extract of C. papaya, V. amygdalina, and the combination of both plants significantly (P < 0.05) decreased parasite load in mice and enhanced their survival. There are many bioactive constituents present in both extracts and hence, at present, it is not certain, which is/are responsible for the observed effects. However, some reports have shown that flavonoids, tannins, saponins, and other phytoconstituents may play some roles in the inhibition of malaria parasites in infected animals [23, 24]. The observed parasitemia in the experimental mice when treated separately with single doses and with a combined dose of C. papaya and V. amygdalina synergistically decreases along with halofantrine on day 9 after establishment of infection, compared with the disease control which is characterized by excessive parasite growth, exaggerated immune reactions, or a combination of both causing severe pathology and death, which is detrimental to both parasite and host. Thus, the findings of Saganuwan et al. [25] that many Nigerian plants can be used for the treatment of malaria and that infection with P. berghei is almost fatal with death occurring within 1–3 weeks are corroborated[25].
The decrease in PCV and RBC and increase in WBC of all the infected groups after infections are in conformity with previous reports, documenting reduced serum PCV, RBC, and an increased WBC levels in malarial subjects [5]. In this study, administration of aqueous leaf extracts of C. papaya, V. amygdalina, and the combined extracts significantly reduced WBC and triggered an increase in PCV as a result of increased production of RBC, thus, suppressing hemolytic damage to RBC.
The increase in body weight of the infected groups of mice and the noninfected group disagrees with the report of Saganuwan and Onyeyili [5], indicating that P. berghei decreases body weight. The increase in body weight observed in this study may be due to constant feeding of the animals during the experimental period.
Histological examination of the liver cells in the treated groups showed the inability of the extract to clear the entire parasite inclusion from circulation and repair the hepatocyte, which agrees with the work of Stenad et al. [26], who stated that hepatic inclusions are defined as intracellular aggregates of stainable substances which represent established hallmarks of their respective human disorder. Signs of regeneration/repairs of tissues using medicinal plants have been reported in other works [27, 28], which are consistent with the present study. Thus, tissue repairs or therapy may offer therapeutic benefit in disease conditions.
5. Conclusion
In conclusion, the finding revealed that the reduction in parasite load and recovery of hepatic cell damage/hematological parameters were induced by the plant extracts and thus gave credence to the usage of the plant traditionally for the treatment of malaria.
Acknowledgments
The authors express gratitude to Dr. S. A. Saganuwan of the Department of Veterinary Physiology and Pharmacology, University of Agriculture, Makurdi, for his support.
Competing Interests
The authors have declared that no competing interests exist.
Authors' Contributions
This work was carried out in collaboration between all authors. Oche Okpe designed the study, wrote the protocol, and interpreted the data. Vincent A. Upev and Omiagocho T. Isaac anchored the field study, gathered the initial data, and performed preliminary data analysis. Nathan Habila and Joseph Ikwebe managed the literature searches and produced the initial draft. Stanley I. R. Okoduwa critically revised the draft for intellectual content. All authors read and approved the final manuscript.
References
- 1.Kato N., Comer E., Sakata-Kato T., et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature. 2016;538(7625):344–349. doi: 10.1038/nature19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owusu-Agyei S., Asante K. P., Adjuik M., et al. Epidemiology of malaria in the forest-savanna transitional Zone of Ghana. Malaria Journal. 2009;8(1, article 220) doi: 10.1186/1475-2875-8-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoareau L., DaSilva E. J. Medicinal plants: a re-emerging health aid. Electronic Journal of Biotechnology. 1999;2(2):56–69. [Google Scholar]
- 4.Basco L. K., Mitaku S., Skaltsounis A.-L., et al. In vitro activities of furoquinoline and acridone alkaloids against Plasmodium falciparum . Antimicrobial Agents and Chemotherapy. 1994;38(5):1169–1171. doi: 10.1128/aac.38.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saganuwan A. S., Onyeyili P. A. Comparative toxicological effects of oral and intraperitoneal aqueous extracts of Abrusprecatorius in musmusculus . Herbapolonica. 2011;57(3) [Google Scholar]
- 6.Egedigwe C. A. Effect of dietary incorporation of Vernonia amygdalina and Vernoniacolorata on blood lipid profile and relative organ weights in albino rats [M.S. dissertation] Department of Biochemistry, Mouau; 2010. [Google Scholar]
- 7.Erasto P., Grierson D. S., Afolayan A. J. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina . Journal of Ethnopharmacology. 2006;106(1):117–120. doi: 10.1016/j.jep.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Bhattachrjee S. K. Carica papaya . In: Jain S., editor. Hand Book of Medicinal Plant. 3rd. Jaipur, India: Pointer; 2001. [Google Scholar]
- 9.Iwu M. M. Empirical investigation of dietary plants used in Igbo- Ethnomedicine. In: Iwu M. M., editor. Plants in Indigenous Medicine and Diet. New York, NY, USA: Nina Etkined Redgrove; 1986. pp. 131–150. [Google Scholar]
- 10.Alabi D. A., Oyero L. A., Amusa N. A. Fungitoxic and phytotoxic effect of Vernonia amygdalina Del., Bryophyllum pinnantus Kurz, Ocimum gratissimum (Closium) L and Eucalypta globules (Caliptos) Labill water extracts on cowpea and cowpea seedling pathogens in Ago-Iwoye, South Western Nigeria. World Journal of Agricultural Science. 2005;1:70–75. [Google Scholar]
- 11.Oyugi D. A., Luo X., Lee K. S., Hill B., Izevbigie E. B. Activity markers of the antibreast carcinoma cell growth fractions of Vernoniaamygdalina extracts. Proceedings of the Society for Experimental Biology and Medicine. 2009;10:p. 3181/0811. doi: 10.3181/0811-RM-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard C. B., Izevbigie E. B., Opata M. M. Inhibition of paclitaxel- resistant MCF7Rag cell growth by Vernonia amygdalina extract. Proceedings of the 1st AACR International Conference on Molecular Diagnostics in Cancer Therapeutic Development; 2006; Chicago, Ill, USA. pp. 12–15. [Google Scholar]
- 13.Abosi A. O., Raseroka B. H. In vivo antimalarial activity of Vernonia amygdalina . British Journal of Biomedical Science. 2003;60(2):89–91. doi: 10.1080/09674845.2003.11783680. [DOI] [PubMed] [Google Scholar]
- 14.Iwalokun B. A. Enhanced antimalarial effects of chloroquine by aqueous Vernonia amygdalina leaf extract in mice infected with chloroquine resistant and sensitive Plasmodium berghei strains. African Health Sciences. 2008;8(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Saganuwan S. A. Arithmetic-Geometric-Harmonic (AGH) method of rough estimation of median lethal dose (Ld50) using up- and- down procedure. Journal of Drug Metabolism and Toxicology. 2005;6(2, article 180) doi: 10.4172/2157-7609.1000180. [DOI] [Google Scholar]
- 16.Brown B. Hematology Principles and Procedures. 2nd. Philadelphia, Pa, USA: Lea and Febiger; 1976. [Google Scholar]
- 17.Coles E. H. Vertinary Clinical Pathology. 2nd. W. B. Saunders; 1986. [Google Scholar]
- 18.Strate T., Mann O., Kleinhans H., et al. Microcirculatory function and tissue damage is improved after therapeutic injection of bovine hemoglobin in severe acute rodent pancreatitis. Pancreas. 2005;30(3):254–259. doi: 10.1097/01.mpa.0000157481.22155.2d. [DOI] [PubMed] [Google Scholar]
- 19.Duncan D. E. Multiple range and multiple F tests. Biometric. 1955;11(2):1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 20.Okpe O., Abdullahi A. S., Ihuoma O., Nkeonye O. L., Ilechukwu C., Nweke O. Hypoglycaemic and hypolipidaemic effects of ethanolic and aqueous leaf extract of Vitexdoniana in normoglycemic albino rats. Global Advanced Research Journal of Microbiology. 2012;1(10):173–179. [Google Scholar]
- 21.Ma C., Zhang H. J., Tan G. T. Antimalarial compounds from Grewia bilamellata . Journal of Natural Products. 2006;69(3):346–350. doi: 10.1021/np050313d. [DOI] [PubMed] [Google Scholar]
- 22.Njoroge G. N., Bussmann R. W. Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya) Journal of Ethnobiology and Ethnomedicine. 2006;2, article 8 doi: 10.1186/1746-4269-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igile G. O., Oleszek W., Jurzysta M., Burda S., Fafunso M., Fasanmade A. A. Flavonoids from Vernonia amygdalina and their antioxidant activities. Journal of Agricultural and Food Chemistry. 1994;42(11):2445–2448. doi: 10.1021/jf00047a015. [DOI] [Google Scholar]
- 24.Udensi E., Ijeh I., Ogbonna U. Effect of traditional processing on the phytochemical and nutrient composition of some local Nigerian leafy vegetables. Journal of Science and Technology. 2002;8:37–40. [Google Scholar]
- 25.Saganuwan S. A., Onyeyili P. A., Etuk E. U. Immuno modulatory potentials and histopathological effects of aqueous extract of Abrus precatorius leaf in mus musculus. Journal of Hematology Research. 2014;1(2):54–62. doi: 10.12974/2312-5411.2014.01.02.3. [DOI] [Google Scholar]
- 26.Stenad P., Nuraldeen R., Guldiken N., et al. Broad spectrum of hepatocyte inclusion in human, animals and experimental models. A Physiological Sciences. 2013;3(4) doi: 10.1002/cphy.c120032. [DOI] [PubMed] [Google Scholar]
- 27.Shittu I., Emmanuel A., Nok A. J. Antimalaria effect of the ethanolic stem bark extracts of Ficus platyphylla del. Journal of Parasitology Research. 2011;2011:5. doi: 10.1155/2011/618209.618209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oche O., Sani I., Chiaka N. G., Samuel N. U., Samuel A. Pancreatic islet regeneration and some liver biochemical parameters of leaf extracts of Vitex doniana in normal and streptozotocin-induced diabetic albino rats. Asian Pacific Journal of Tropical Biomedicine. 2014;4(2):124–130. doi: 10.1016/s2221-1691(14)60220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]