Abstract
Background
The observer perspective causes patients with social anxiety disorder (SAD) to excessively inspect their performance and appearance. This study aimed to investigate the neural basis of distorted self-face recognition in non-social situations in patients with SAD.
Methods
Twenty patients with SAD and 20 age- and gender-matched healthy controls participated in this fMRI study. Data were acquired while participants performed a Composite Face Evaluation Task, during which they had to press a button indicating how much they liked a series of self-faces, attractively transformed self-faces, and attractive others' faces.
Results
Patients had a tendency to show more favorable responses to the self-face and unfavorable responses to the others' faces compared with controls, but the two groups' responses to the attractively transformed self-faces did not differ. Significant group differences in regional activity were observed in the middle frontal and supramarginal gyri in the self-face condition (patients < controls); the inferior frontal gyrus in the attractively transformed self-face condition (patients > controls); and the middle frontal, supramarginal, and angular gyri in the attractive others' face condition (patients > controls). Most fronto-parietal activities during observation of the self-face were negatively correlated with preference scores in patients but not in controls.
Conclusion
Patients with SAD have a positive point of view of their own face and experience self-relevance for the attractively transformed self-faces. This distorted cognition may be based on dysfunctions in the frontal and inferior parietal regions. The abnormal engagement of the fronto-parietal attentional network during processing face stimuli in non-social situations may be linked to distorted self-recognition in SAD.
Keywords: Social anxiety disorder, Face evaluation, Self-face recognition, Attractiveness, fMRI
Highlights
-
•
We studied neural responses to attractive self-face images in SAD.
-
•
Patients with SAD showed more favorable responses to the self-face.
-
•
Distorted self-recognition in SAD was related to fronto-parietal dysfunctions.
-
•
Patients with SAD had abnormal attentional network-based self-face recognition.
1. Introduction
Social anxiety disorder (SAD) is characterized by marked fear in social situations, particularly those involving possible scrutiny by others (American Psychiatric Association, 2000). SAD develops at an early age and often leads to subsequent depressive disorder, drug abuse, or other psychiatric disorders (Sadock et al., 2014). Concerning the generation and maintenance of social anxiety, several researchers have proposed explanatory models (Clark and Wells, 1995, Rapee and Heimberg, 1997), which emphasize the problems of attention in SAD. According to these models, patients allocate attentional resources toward detecting an external social threat and monitoring their internal mental processes. External social threats in patients with SAD include not only negative faces (Clark and McManus, 2002), but also neutral facial expression of others (Cooney et al., 2006). An important factor of the internal process in patients with SAD is “self-focused attention”, which is an awareness of self-referent, internally generated information regarding bodily states, emotions, thoughts, and memories (Ingram, 1990). Self-focused attention impedes the opportunity for disconfirmation of negative expectations in social situations (Clark and Wells, 1995), and is related to anxiety level (Woody and Rodriguez, 2000) and poor social performance (Alden et al., 1992, McManus et al., 2008).
Another important factor in attention problems is the ‘observer perspective’, which allows a subject to see him- or herself from the perspective of another as an actor in social situations. This is in contrast to a field perspective in which the individual views a situation from their own perspective (Wells et al., 1998). Since patients with SAD observe their appearance, social performance, and the details of what is going on around them, they might excessively monitor their mental images using the observer perspective (Coles et al., 2001, D'Argembeau et al., 2006, Wells et al., 1998). Because patients with SAD hold distorted negative mental representations of themselves due to their negative self-aspects, memory retrieval using the observer perspective interrupts activation of the cortical representation of the embodied self (Eich et al., 2009). Through this process, patients with SAD heighten their levels of self-criticism and negative emotions (Ickes et al., 1973). Collectively, an important pathology of SAD is distorted mental image, which is composed of not only social performance, but also general appearance.
Facial appearance plays an important role in representing the self, as people interpret others' feelings or intentions based on facial expressions (Oikawa et al., 2012). When people look at facial images, many processes operate sequentially, including the perception of images, attention to images, retrieval of image-related memory, and comparison of a mental representation to the presented images (Legrand and Ruby, 2009). In particular, because the observer perspective leads patients with SAD to monitor their mental images in detail, patients might process facial images in different ways compared to healthy people, and thus they have more distorted body image (Izgiç et al., 2004). When examining the social value of the appearance of the face, attractiveness needs to be considered. For example, physically attractive people tend to be more receptive to a good evaluation of their abilities (Clifford and Walster, 1973, Landy and Sigall, 1974). It has been reported that patients with SAD tend to judge themselves to be less physically attractive (Montgomery et al., 1991). Despite this rationale, the underlying mechanism of distorted perception of facial appearance and attractiveness in patients with SAD has not been studied. Due to the development of advanced computer morphing technology, we are now able to easily manipulate facial pictures in order to study the influence of facial appearance on cognitive processing in patients with SAD.
In fact, facial stimuli have been used in studies for SAD, in which faces of others have been used as social threats (Blair et al., 2008, Klumpp et al., 2012, Phan et al., 2013). One study of SAD using self-faces investigated neural responses to the observable self in an anxiety-provoking situation and found that deactivation in the dorsal prefrontal and parietal cortices might be related to dysfunction in controlling anxiety in SAD (Pujol et al., 2013). These two regions are also known to be main components of the cortical attentional network (Corbetta et al., 2000, Corbetta and Shulman, 2002) and to be engaged in self-face recognition (Morita et al., 2008, Platek et al., 2008, Sugiura et al., 2005, Sugiura et al., 2006). However, since there is no other study of self-recognition in non-social situations in SAD, it is not certain whether the neural disturbance in SAD is affected by a self-recognition process. It should be noted that patients with SAD tend to have a negative baseline self-aspect and thus have a pattern of self-criticism in non-social situations as well as social situations (Morrison and Heimberg, 2013).
In the current study, we used functional magnetic resonance imaging (fMRI) to address how self-recognition in non-social situations can be distorted in patients with SAD. Based on the fact that people recognize themselves even if their faces are slightly modified (Uddin et al., 2005), we designed the Composite Face Evaluation Task to evaluate a facial recognition style for this investigation. A novel point of the task was to present attractively transformed self-faces using a composition technique, in order to understand the viewpoint of participants' facial appearances. Based on previous reports (Cooney et al., 2006, Izgiç et al., 2004, Montgomery et al., 1991), we hypothesized that patients with SAD would dislike their own facial appearance due to their negative self-aspect and also dislike attractive others' faces because of recognizing them as social threats. On the other hand, we predicted that their likable responses to the attractively transformed self-face would be increased as the dislikable faces are changed. In addition, we predicted that the abnormal engagement of the fronto-parietal attentional network in self-face recognition in non-social situations would be linked to the distorted self-face processing style in SAD.
2. Methods and materials
2.1. Participants
A total of 20 patients with SAD (10 men and 10 women; mean age = 23.6 ± 2.0 years) and 20 age- and gender-matched healthy controls (10 men and 10 women; mean age 23.6 ± 2.3 years) participated in this study. Participants were recruited from the community through an advertisement on the internet message board of the local universities and the internet board for undergraduate students who were seeking part-time job. All patients met the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for SAD (American Psychiatric Association, 2000) without any other lifetime comorbid psychiatric diagnoses. Patients were also drug- and psychotherapy-naïve for the management of SAD. All participants were right-handed, as assessed by the Annett Handedness Inventory (Annett, 1970), and had no history of other medical or neurological illness. Patients and healthy controls did not significantly differ in duration of education (15.5 ± 0.6 years and 14.9 ± 1.5 years, respectively). The study was approved by the institutional review board of Yonsei University Gangnam Severance Hospital, and written informed consent was obtained from all participants before the study began.
The degree of social anxiety was assessed using the Liebowitz Social Anxiety-Self-Report (LSAS-SR) (Fresco et al., 2001). Patients had significantly higher LSAS-SR scores than controls (patients: 84.8 ± 17.1, controls: 24.2 ± 6.7, t = 14.61, p < 0.01). In addition, the Self Consciousness Scale (SCS) was used to explore the relation between self-consciousness and appearance. The SCS consists of subscales of “private self” (attending to one's inner thoughts and feelings), “public self” (a general awareness of the self as social object), and “social anxiety” (a discomfort in the presence of others) (Fenigstein et al., 1975). Patients and controls showed no significant difference on the private self-subscale (24.2 ± 6.5 and 22.1 ± 6.5, respectively) or public self-subscale (19.9 ± 4.5 and 17.0 ± 4.9, respectively), but the social anxiety subscale score was significantly higher in patients than in controls (17.9 ± 4.2 and 5.9 ± 3.6, respectively; t = 9.59, p < 0.01).
2.2. Task stimuli and experimental procedure
During the fMRI scan, participants completed the Composite Face Evaluation Task as shown in Fig. 1. Visual stimuli consisted of three sets of 10 composite face pictures and a group of 10 contrast pictures. The composite face pictures were produced by merging face pictures of the self and a stranger of the same sex using Abrosoft FantaMorph before the scanning. The composite ratio was 9:1 for the self-faces, 5:5 for the attractively transformed self-faces, and 1:9 for the attractive other faces, which were referred to as “90% self-analogous face (SAF)”, “50% SAF”, and “10% SAF”, respectively. The contrast pictures were 10 emoticons, referred to as “EMO”, displaying positive or negative emotions.
Fig. 1.
The Composite Face Evaluation Task, which included five runs with six blocks of 20 s each. The experimental blocks consisted of 10 images of 90%, 50%, or 10% self-analogous face (SAF), which were produced by merging face pictures of the self and a stranger, or 10 emoticon (EMO) pictures. The six composite faces in the figure were not a picture used in the real experiment and were demonstrated for display to help an understanding of the composite ratio. Face pictures of three persons for the self and two strangers, whose faces were selected from Korean Facial Expressions of Emotion (Park et al., 2011), were used for the production of the composite faces.
The self-face pictures were taken on the day of scanning. Strangers' pictures were prepared prior to the experiment and were pretested for attractiveness. In this pretest, a different set of healthy participants (9 men and 14 women; mean age 23.5 ± 2.3 years) scored the attractiveness of 100 male and 100 female stranger pictures on a scale of − 3 (very unattractive) to 3 (very attractive). Mean attractiveness scores were − 0.2 ± 1.3 and − 0.5 ± 1.4 for men and women, respectively. The pictures of the 10 most attractive strangers of each gender were chosen to produce the task stimuli. The median value of these selected pictures was 1.2 (maximum 1.8 to minimum 0.5) for men and 0.8 for women (maximum 1.9 to minimum 0.6). In order to validate the attractive transformation of 50% SAF, another set of healthy participants (11 men and 10 women; mean age 24.4 ± 2.2 years) evaluated the attractiveness of composite images of the self and 10 selected attractive others using the same scale (from − 3 to 3). Mean score was significantly higher in 50% SAF than in 90% SAF (0.57 ± 0.8 and − 0.11 ± 1.2, respectively; t = 2.81, p = 0.011).
The Composite Face Evaluation Task was comprised of five runs with six 20-s blocks. Each run consisted of four experimental blocks (90% SAF, 50% SAF, 10% SAF, and EMO) and two rest blocks with a fixation crossbar placed between two consecutive experimental blocks. The order of the four experimental blocks was pseudo-randomized among the runs. In every experimental block, 10 different pictures from the corresponding category were displayed for 1.9 s each, separated by a fixation crossbar screen for 0.1 s. In each trial, participants were asked to choose their preferred picture by pressing a button indicating ‘likable,’ ‘mediocre,’ or ‘dislikable,’ and responses were automatically saved.
2.3. Image acquisition
Imaging data were acquired on a 3.0 Tesla scanner (Achieva; Philips Medical System). Thirty contiguous axial slices were collected using a gradient-echo echo-planar imaging sequence depicting the blood oxygenation level-dependent signal (echo time = 30 ms; repetition time = 2000 ms; flip angle = 90°; field of view = 220 mm; slice thickness = 4 mm, gap = 1 mm; and image matrix = 128 × 128). Five dummy scans were run to allow for signal equilibrium before the image acquisition started. Axial 1.2-mm-thick T1-weighted images (echo time = 4.6 ms; repetition time = 9.7 ms; flip angle = 30°; field of view = 220 mm; and image matrix = 256 × 256) were also collected using a turbo field echo sequence.
2.4. Behavioral data analysis
Responses for each picture category were analyzed using a generalized linear mixed model with logit link (Rao et al., 2007), which can be applied to repeated measures analyses and reflects the random effect. Statistical analysis of behavioral data was completed with a significance level set at 0.05. We then transformed the responses into a ‘preference score’ by assigning ‘1’ to ‘likable,’ ‘0’ to ‘mediocre,’ and ‘− 1’ to ‘dislikable’ and calculated the intraclass correlation coefficient (ICC) using a two-way mixed model in order to evaluate response repeatability for the same pictures across five runs. We defined an ICC of < 0.40 as poor agreement, 0.40–0.75 as good agreement, and 0.76–1.00 as excellent agreement (Fleiss et al., 2013). The preference scores were also used to perform correlation analysis between behavioral and neural responses.
2.5. Neuroimaging data analysis
Preprocessing and analysis of the imaging data were performed using Statistical Parametric Mapping, version 8 (SPM8; Wellcome Department of Cognitive Neurology, London, UK). After the first five volumes for dummy scans were discarded, all fMRI data corrected for non-simultaneous interleaved slice acquisition within each volume. Then, head movement effects were corrected by realigning the images. These functional images were coregistered to the T1-weighted image for each subject and then spatially normalized using nonlinear transformation functions obtained by registering individual T1-weighted images to a standard template. The spatially normalized functional data were smoothed with a Gaussian kernel of 8 mm full-width-at-half-maximum.
In the single-subject analysis, generalized linear model analysis of expected signal change for the four types of stimuli (90% SAF, 50% SAF, 10% SAF and EMO) was performed using the canonical hemodynamic response function. A high-pass filter with a cutoff period of 128 s was used to eliminate the artefactual low-frequency trend. The motion component, consisting of six time series representing head motion, was regressed out. To exclude the signal change provoked by a common visual stimulus, we constructed contrast images by subtracting “EMO” from “90% SAF”,“50% SAF”, and “10% SAF”, which were referred to as the 90% SELF, 50% SELF, and 10% SELF conditions, respectively.
The resulting first-level contrast images were used in subsequent second-level group analyses. One-sample t-tests were performed using SPM8 in order to acquire brain activation maps in each group of patients and controls. Full factorial analysis of variance was applied to investigate two main effects of group (patients and controls) and condition (90% SELF, 50% SELF, and 10% SELF) as well as an interaction effect between them. Additionally, in order to check the interference effect of using the EMO condition as a baseline, we constructed contrast images for all conditions using the null condition as a baseline and performed full factorial analysis of variance. Statistical inferences were conducted at a threshold of AlphaSim corrected p < 0.05, which corresponded to a voxel-level threshold p < 0.001 and cluster size k > 61 voxels. The cluster size was determined through a Monte Carlo simulation with 10,000 iterations. For the entire clusters that were identified as significant interactions in this analysis, we extracted parameter estimates of regional activity using MarsBaR version 0.42. Then, post-hoc analyses were conducted to explore the direction of interaction effects using a linear mixed model and Pearson correlations of regional activities with behavioral responses such as score preference and clinical scales including the LSAS-SR and SCS. Results were considered significant at p < 0.05 corrected with Benjamini-Hochberg procedure.
3. Results
3.1. Behavioral responses
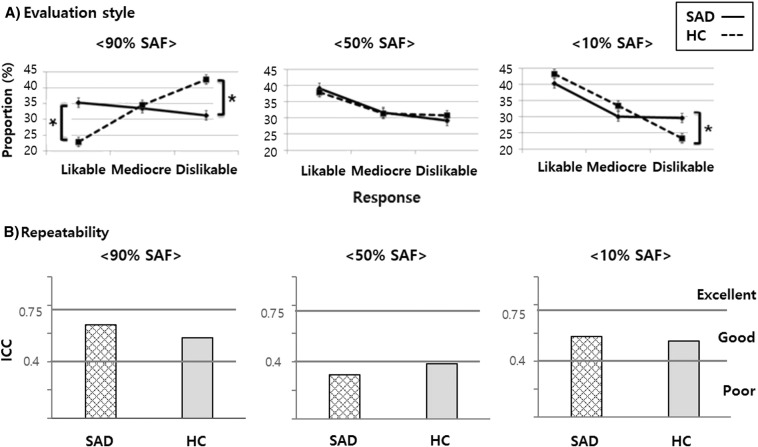
Fig. 2A displays the proportions of responses during Composite Face Evaluation in each visual stimulus type. Likable responses significantly varied with the picture type in controls (F3, 413 = 33.58, p < 0.0001; 10% SAF > 50% SAF > 90% SAF), but not significantly in patients. For the 90% SAF pictures, controls showed a significant difference (F2, 413 = 38, p < 0.0001) among the three responses (‘likable,’ 22.9 ± 1.4%; ‘mediocre,’ 34.5 ± 1.6%; and ‘dislikable,’ 42.6 ± 1.7%), whereas in patients differences were not significant (likable, 35.3 ± 1.5%; mediocre, 33.5 ± 1.5%; and dislikable, 31.2 ± 1.5%). For the 50% and 10% SAF pictures, the most common response was ‘likable’ in both groups, which showed a significant difference among the three responses (patients: F2, 413 = 11.43, p < 0.0001 and controls: F2, 413 = 6.22, p < 0.005 in the 50% SAF pictures; patients: F2, 413 = 16.05, p < 0.0001 and controls: F2, 413 = 38.87, p < 0.0001 in the 10% SAF pictures). Significant group difference was observed in the ‘likable’ and ‘dislikable’ responses to the 90% SAF pictures (t = 5.07, p < 0.0001 and t = − 5.82, p < 0.0001, respectively) and in the ‘dislikable’ responses to the 10% SAF pictures (t = 3.04, p < 0.005).
Fig. 2.
Behavioral responses to composite faces.
*Significant threshold p-value < 0.016, adjusted by Bonferroni's correction. SAF, self-analogous face; SAD, social anxiety disorder; HC, healthy controls; ICC, intraclass correlation coefficient.
The ICCs of the preference scores for each category of pictures in each group are presented in Fig. 2B. Both patient and control groups showed ICCs of good agreement in the 90% SAF pictures (0.658 and 0.568, respectively) and 10% SAF pictures (0.575 and 0.544, respectively), but ICCs reflecting poor agreement were found for the 50% SAF pictures (0.311 and 0.386, respectively).
3.2. Neural responses to the composite face stimuli in each group
The brain regions specifically activated according to the condition in each group are presented in Table 1. In the 90% SELF condition, healthy controls showed significant activations in wide areas including the right prefrontal cortex, bilateral inferior parietal lobules, bilateral posterior cingulate cortex (PCC), and bilateral inferior occipital gyrus, whereas patients showed relatively small regions of activation in the right prefrontal cortex, bilateral PCC, and bilateral inferior occipital gyri. In the 50% SELF condition, however, relatively broad areas of activation were noted in the inferior frontal gyrus and bilateral PCC/precuneus in patients rather than in controls. In the 10% SELF condition, the groups showed no activation differences in the prefrontal cortex, but large activation in the right PCC/precuneus in patients was contrasted with small activation in the same region in controls.
Table 1.
Neural responses to the different composite faces in patients with social anxiety disorder (SAD) and healthy controls.
| SAD (N = 20) |
Controls (N = 20) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | BA | k | Coordinates |
Tmax | Region | BA | k | Coordinates |
Tmax | |||||
| x | y | z | x | y | z | |||||||||
| 90% SELF (Self) | ||||||||||||||
| R. Middle frontal G. | 46 | 160 | 42 | 38 | 10 | 5.8 | R. Middle frontal G. | 10 | 4527 | 42 | 44 | 10 | 8.0 | |
| R. Inferior frontal G. | 44 | 52 | 12 | 8 | 5.3 | R. Inferior frontal G. | 45 | 38 | 30 | − 4 | 8.7 | |||
| R. Medial frontal G. | 6 | 398 | 6 | 28 | 54 | 5.3 | ||||||||
| L. Middle frontal G. | 9 | 169 | − 40 | 20 | 54 | 5.1 | ||||||||
| R. Supramarginal G. | 9 | 768 | − 40 | 20 | 54 | 5.1 | ||||||||
| L. Angular G. | 39 | 1034 | − 58 | − 58 | 26 | 6.3 | ||||||||
| L. Middle temporal G. | 21 | 83 | − 56 | − 8 | − 28 | 5.0 | ||||||||
| R. Inferior occipital G. | 18 | 273 | 22 | − 94 | − 4 | 6.8 | R. Inferior occipital G. | 18 | 410 | 20 | − 94 | − 6 | 8.6 | |
| L. Inferior occipital G. | 18 | 239 | − 18 | − 94 | − 8 | 6.2 | L. Inferior occipital G. | 18 | 283 | − 18 | − 98 | − 8 | 9.1 | |
| R. Posterior cingulate G. | 23 | 735 | 8 | − 48 | 28 | 4.8 | R. Posterior cingulate G. | 31 | 1033 | 6 | − 42 | 36 | 5.8 | |
| L. Posterior cingulate G. | 30 | − 6 | − 44 | 26 | 5.0 | L. Posterior cingulate G. | 31 | − 2 | − 46 | 30 | 5.3 | |||
| L. Precuneus | 31 | − 8 | − 58 | 28 | 5.2 | L. Precuneus | 31 | − 12 | − 44 | 38 | 5.2 | |||
| 50% SELF (Attractively transformed self) | ||||||||||||||
| R. Inferior frontal G. | 47 | 1779 | 40 | 36 | 14 | 6.4 | R. Middle frontal G. | 46 | 683 | 42 | 40 | 12 | 7.0 | |
| 47 | 32 | 28 | − 2 | 5.9 | R. Precentral G. | 6 | 203 | 48 | 4 | 24 | 6.6 | |||
| R. Angular G. | 39 | 274 | 46 | − 62 | 28 | 5.3 | ||||||||
| R. Inferior occipital G. | 18 | 676 | 22 | − 94 | − 8 | 10.4 | R. Inferior occipital G. | 18 | 545 | 22 | − 88 | − 8 | 9.4 | |
| L. Inferior occipital G. | 18 | 508 | − 20 | − 98 | − 12 | 9.0 | L. Inferior occipital G. | 18 | 367 | − 14 | − 96 | − 12 | 10.0 | |
| L. Precuneus | 7 | 1303 | − 2 | − 62 | 36 | 6.6 | L. Posterior cingulate G. | 23 | 530 | 0 | − 54 | 22 | 6.0 | |
| R. Posterior cingulate G. | 31 | 4 | − 54 | 28 | 5.5 | |||||||||
| 10% SELF (Attractive other) | ||||||||||||||
| R. Angular G. | 39 | 180 | 46 | − 58 | 28 | 4.1 | R. Inferior occipital G. | 18 | 583 | 22 | − 90 | − 8 | 11.0 | |
| R. Inferior occipital G. | 18 | 719 | 24 | − 92 | − 10 | 11.8 | L. Inferior occipital G. | 18 | 356 | − 16 | − 96 | − 12 | 10.2 | |
| L. Inferior occipital G. | 18 | 571 | − 24 | − 88 | − 10 | 7.6 | R. Posterior cingulate G. | 23 | 214 | 2 | − 54 | 22 | 5.1 | |
| R. Posterior cingulate G. | 23 | 1289 | 4 | − 54 | 26 | 6.5 | ||||||||
| R. Precuneus | 31 | 4 | − 64 | 32 | 5.7 | |||||||||
BA, Brodmann's area; k, voxel number; R., right; L., left; and G., gyrus
3.3. Activation regions showing group effect and group-by-condition interaction effect
The results from full factorial analysis of variance are presented in Supplementary Table 1. There was no significant main effect of group. As shown in Fig. 3, however, a significant group-by-condition interaction effect was observed in the right middle frontal gyrus [F2, 114 = 13.16, p < 0.001], right inferior frontal gyrus [F2, 114 = 9.59, p < 0.001], supramarginal gyrus [F2, 114 = 11.05, p < 0.001], and angular gyrus [F2, 114 = 8.25, p < 0.001]. When regional activities extracted in these four regions were compared in each condition, significant group differences were observed in the middle frontal gyrus (F1, 76 = 5.50, p = 0.02) and supramarginal gyrus (F1, 76 = 4.10, p = 0.04) in the 90% SELF condition; the inferior frontal gyrus (F1, 76 = 13.46, p < 0.001) in the 50% SELF condition; and the middle frontal gyrus (F1, 76 = 6.33, p = 0.01), supramarginal gyrus (F1, 76 = 8.29, p < 0.01), and angular gyrus (F1, 76 = 4.91, p = 0.03) in the 10% SELF condition. Meanwhile, a significant group-by-condition interaction effect when the null condition was used as a baseline is listed in Supplementary Table 2, showing results similar to the regions presented in Fig. 3. In these contrasts, a significant group difference in regional activity was observed only in the right supramarginal gyrus in the 50% SAF condition (Supplementary Table 3).
Fig. 3.
Regional activation showing the significant group-by-condition interaction effect during Composite Face Evaluation and their correlations with behavioral responses.
The fronto-parietal regions (A, B, D) showed a similar pattern of signal change across conditions. Specifically, activity in the inferior frontal gyrus significantly differed for 50% SELF: higher in patients than in controls. *p < 0.05; **p < 0.05, corrected with Benjamini-Hochberg procedure. SAF, self-analogous face; SAD, social anxiety disorder; HC, healthy controls; L, left; R, right.
When regional activities were compared among the conditions, the two groups showed different patterns, as displayed in Table 2. In controls, all regional activities were significantly increased in the 90% SELF condition compared to the 50% and 10% SELF conditions, and supramarginal gyrus activity was significantly increased in the 50% SELF condition compared to the 10% SELF condition (t = 3.74, p < 0.01). In patients, however, angular gyrus activity was significantly decreased in the 90% SELF condition compared to the 50% SELF conditions (t = − 3.20, p < 0.01), and inferior frontal gyrus activity was significantly increased in the 50% SELF condition compared to the 10% SELF condition (t = 3.79, p < 0.01).
Table 2.
Comparison of regional condition-specific activities in patients with social anxiety disorder (SAD) and healthy controls.a
| Condition | SAD (N = 20) | t-Value | Controls (N = 20) | t-Value |
|---|---|---|---|---|
| Middle frontal gyrus | ||||
| 90% SELF vs 50% SELF | − 0.04 ± 0.03 | − 2.20 | 0.12 ± 0.03 | 3.90⁎ |
| 90% SELF vs 10% SELF | − 0.07 ± 0.03 | − 1.31 | 0.17 ± 0.03 | 5.55⁎ |
| 50% SELF vs 10% SELF | − 0.03 ± 0.03 | − 0.89 | 0.05 ± 0.03 | 1.65 |
| Inferior frontal gyrus | ||||
| 90% SELF vs 50% SELF | − 0.10 ± 0.05 | − 2.07 | 0.25 ± 0.05 | 3.94⁎ |
| 90% SELF vs 10% SELF | 0.08 ± 0.05 | 1.72 | 0.18 ± 0.05 | 5.29⁎ |
| 50% SELF vs 10% SELF | 0.18 ± 0.05 | 3.79⁎ | 0.06 ± 0.05 | 1.35 |
| Supramarginal gyrus | ||||
| 90% SELF vs 50% SELF | − 0.17 ± 0.046 | − 0.38 | 0.17 ± 0.05 | 3.65⁎ |
| 90% SELF vs 10% SELF | 0.04 ± 0.046 | 0.94 | 0.34 ± 0.05 | 7.33⁎ |
| 50% SELF vs 10% SELF | 0.06 ± 0.046 | 1.31 | 0.17 ± 0.05 | 3.74⁎ |
| Angular gyrus | ||||
| 90% SELF vs 50% SELF | − 0.16 ± 0.05 | − 3.20⁎ | 0.15 ± 0.05 | 3.11⁎ |
| 90% SELF vs 10% SELF | − 0.10 ± 0.05 | − 1.95 | 0.23 ± 0.05 | 4.74⁎ |
| 50% SELF vs 10% SELF | 0.06 ± 0.05 | 1.25 | 0.08 ± 0.05 | 1.62 |
Significant threshold p-value < 0.016, adjusted by Bonferroni's correction.
Difference in % signal change among the three face conditions was described as estimated mean ± standard error.
In the 90% SELF condition, three regional activities were negatively correlated with preference scores in patients (the middle frontal gyrus: r = − 0.588, p < 0.01; the inferior frontal gyrus: r = − 0.727, p < 0.01; and the angular gyrus: r = − 0.572, p < 0.01), but no significant correlation was found in controls. No regional activities in the 50% and 10% SELF condition were significantly correlated with preference scores in either group. In addition, there were no significant relationships between regional activities and LSAS-SR or SCS scores.
4. Discussion
In the present study, we aimed to elucidate the problem of processing self-related stimuli, particularly face images, by manipulating attractiveness level in patients with SAD. We observed characteristic behaviors in patients with SAD, who were more favorable to the self-face, more unfavorable to the others' faces and similarly responsive to the attractively transformed self-faces, compared to age- and sex-matched healthy controls. These behaviors-related functional neural changes in patients with SAD were observed in fronto-parietal regions regardless of the face conditions.
In the behavioral results, contrary to our expectation, patients showed a more favorable response to the self-face than did controls. This result suggests that patients with SAD may have distorted self-face recognition in the direction of positivity. Given that body image is distorted in patients with SAD (Izgiç et al., 2004), this positive distortion may reflect compensation of their negative self-aspect rather than actual confidence in their appearance. Alternatively, this result may stem from the nature of our task, in which the self and attractive others are contrasted. Responses in controls tended to be unfavorable to the self-face and favorable to the attractive others' faces, suggesting that people generally view themselves harshly. Attractive others' faces might be the basis of hierarchical social values, which could have made controls feel inferior because of the contrast effect (Brown and Gallagher, 1992, Cash et al., 1983, Gutierres et al., 1999, Kenrick et al., 1994, Oikawa et al., 2012, Richins, 1991). Comparatively, because patients felt attractive others' faces to be threatening, the contrast effect might be weakened and thus unfavorable responses to the self-face might not be increased. In case of responses to the attractively transformed self-faces, controls reacted more positively compared with the self-face in that ‘likable’ responses were significantly increased in 50% SAF pictures than in 90% SAF pictures. Although responses to 50% SAF pictures in patients were similar to those in controls, the underlying mechanism may be different because responses to 90% and 10% SAF pictures differed between the two groups. The favorable responses in controls may be due to satisfaction with an attractive transformation, whereas those in patients may be attributed to compensation for negative self-aspect and the reduction of threats from strangers.
Our finding of multiple strong responses to the self-face in the frontal and parietal regions in controls is consistent with previous reports that these regions are involved in self-face recognition (Morita et al., 2008, Platek et al., 2004, Platek et al., 2008, Sugiura et al., 2005, Sugiura et al., 2006, Uddin et al., 2007). However, these fronto-parietal responses were absent or reduced in patients during evaluations of the self-face. Instead, strong fronto-parietal responses in patients were characteristic during evaluation of the attractively transformed self-faces. These differences might be linked to abnormalities in the processing of self-related stimuli because neither group showed fronto-parietal activations while evaluating attractive others. In addition, these fronto-parietal abnormalities might be part of a neural basis of patients' behavioral responses, suggesting a distortion of self-face recognition in the direction of positivity.
In particular, decreased right middle frontal and right supramarginal activities while evaluating the self-face in patients compared with controls were confirmed in the post-hoc tests of the significant group-by-condition interaction effect. The middle frontal and supramarginal gyri are the main components of the ventral attention network, which is associated with the reflexive reorienting of attention that enables a person to reorient to the salient or behaviorally relevant stimuli in the environment (Corbetta et al., 2008, Downar et al., 2001, Hutchinson et al., 2009). Our task contained repeated evaluations of personal preference, for which selective and sustained attention abilities on different images were important. Therefore, the finding of deficient fronto-parietal responses to the self-face in patients might be related to a defect in attentive processing. Self-face images seem to be less likely to capture patient attention.
An alternative explanation may be a deficit in recognizing the self-face. When we produced the morphed face images, the images of frontal view were used to facilitate the process of self-face recognition (Jiang et al., 2006, Pourtois et al., 2005, Suddendorf and Butler, 2013). Basically, slightly distorted 90% SAF pictures were good for representing the self, while 50% SAF pictures were stimuli of confused identity (Uddin et al., 2005). It has been demonstrated that watching faces can induce motor imagery and activate the motor system in prefrontal and inferior parietal regions, suggesting the existence of a causal relationship between mirror neuron systems and self-recognition (Uddin et al., 2005). Mapping the self-face onto one's own motor system may produce a better match than mapping another person's face, so that neural activity is increased with self-stimuli. The middle frontal and supramarginal gyri are highly related to discrimination of the self and others (Platek et al., 2006, Platek and Kemp, 2009). The middle frontal gyrus also plays a role in evaluation within a social context rather than at a perceptual level (Devue and Brédart, 2011). In these contexts, our results suggest that low middle frontal and supramarginal activities in patients might be related to deficits in self-recognition.
Correlations between the behavioral data and fMRI data allow us to more easily understand the self-face evaluation pattern. Neural activation in the fronto-parietal area, where a significant group-by-condition interaction effect was observed, was negatively correlated with preference scores for the self-face in patients but not in controls. This could mean that patients evaluated their faces more highly when the pictures were less provocative and failed to capture their attention, implying that patients with SAD might assess their own pictures based on subjective, pre-defined images of themselves rather than an objective view. If that is the case, it would be interesting to analyze these pre-defined images. This can be addressed from patients' responses in the 50% SELF condition. Compared with controls, patients showed increased inferior frontal activity when evaluating the attractively transformed self-faces. A previous study has suggested that the inferior frontal gyrus reflects self-relevance or relevance to the standard self and is activated more when evaluating self-face as positive (Morita et al., 2008). Considering the role of the inferior frontal gyrus, we can assume that controls may have felt the attractively transformed self-faces to be good but irrelevant to themselves, whereas patients may have felt those faces to be suitable for themselves despite being also felt good. Based on this assumption, we can infer that patients with SAD conceptualize a likable pre-defined image similar to the attractively transformed self-faces, and their positive evaluation of the self-face is related to overestimation rather than confidence.
We also found that the middle frontal, supramarginal, and angular gyri were more highly activated for the attractive other's face in patients than in controls. Previous studies that used others' faces with negative affect to provoke anxiety found increased amygdala activity in patients with SAD (Goldin et al., 2009a, Goldin et al., 2009b) which was not observed in our study. It should be noted that our task was specialized to assess baseline cognitive processes by excluding emotional reactivity and presenting others' faces with neutral valence. Increased activity in the ventral attentional system without provoked social anxiety suggests that patients with SAD are concerned about others' faces even in neutral situations. This excessive attention toward others may be based on patients' negative reactions to attractive others' faces, which they rated as more negative than healthy controls. A previous study demonstrated that patients with SAD thought that people negatively evaluated others (Stopa and Clark, 1993), suggesting that such a belief might influence them to appraise others negatively.
Meanwhile, our experimental task was ascertained to be reliable as reflected by the range of good agreement for ICC levels of the self-face and attractive others' faces in both groups. Although the attractive transformation of 50% SAF was confirmed in the pretest, its ICC level was ranged in poor agreement. This result might be reasonable because participants' preferences would not be established definitely for 50% SAF, which possessed the quality of ambiguity or complexity and the responses could be changed in evaluation blocks. Although the value of ICC for 50% SAF was low, its average preference scores were positively weighted. In other words, both groups showed various responses to 50% SAF dependent on the character of a visual stimulus, but overall responses leaned toward ‘likable’. Therefore, the level of ICC representing the consistency of preference would not affect the level of attractiveness. This fMRI investigation on the baseline cognitive style without the effect of social anxiety or threats enables us to better understand the core pathology of SAD, which is a chronic or lasting illness rather than a situational or provoked illness and involves neural-level problems in self-referential processing in non-social situations.
There are several limitations in our study. First, because the sample size of each group was small and participant age was restricted to the early 20s, the generalizability of our results to people in different age groups is limited. Second, it is uncertain whether the abnormal behavioral responses in patients are associated with cognitive deficits because neurocognitive ability was not assessed. Third, the attractiveness of others' faces was evaluated in a pretest using a separate sample in order to select the visual stimuli but was not repeated with the participants of the present study because their task was to evaluate preference. It was expected that participants would also perceive others' faces to be attractive, but whether they did so was not verified. Fourth, all facial stimuli were composed of a neutral expression. The experimental situation could have been more demanding and more stressful for patients in that neutral faces were not emotionally neutral (Lee et al., 2008). In addition, because emoticons for the control condition had positive and negative emotions, the imbalance between facial and emoticon emotions could have influenced the imaging results.
In conclusion, we used fMRI to examine the neural basis of distorted self-recognition in non-social situations in patients with SAD when performing the Composite Face Evaluation Task, which allowed us to examine the viewpoint of participants' facial appearance. The main neuroimaging finding was a significant group difference in fronto-parietal regions, such as the middle frontal and supramarginal gyri in the self-face condition, the inferior frontal gyrus in the attractively transformed self-face condition, and the middle frontal, supramarginal, and angular gyri in the attractive other's face condition. These results suggest that distorted self-recognition in patients with SAD is based on dysfunctions in the fronto-parietal attentional network, which may be an underlying mechanism of behavioral characteristics that they had a positive point of view of their own face and experienced self-relevance for the attractively transformed self-faces. Further research is needed to test whether or not the distorted positive self-images in patients would be maintained without contrast with the attractive others' faces.
Financial disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. NRF-2013R1A2A2A03068342).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.04.010.
Appendix A. Supplementary data
Supplementary Table 1 Summary of full factorial analysis of variance for brain activity during the different composite face-conditions in patients with social anxiety disorder and healthy controls. Supplementary Table 2 Regional activation showing the significant group-by-condition interaction effect during composite face evaluation when using the null condition as a baseline. Supplementary Table 3 Percent signal changes in the regions showing the significant group-by-condition interaction effect during composite face evaluation, which were obtained using the null condition as a baseline and compared in the full conditions.
References
- Alden L.E., Teschuk M., Tee K. Public self-awareness and withdrawal from social interactions. Cogn. Ther. Res. 1992;16(3):249–267. [Google Scholar]
- American Psychiatric Association . fourth ed. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. (Text Revision). [Google Scholar]
- Annett M. A classification of hand preference by association analysis. Br. J. Psychol. 1970;61(3):303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- Blair K., Shaywitz J., Smith B.W., Rhodes R., Geraci M., Jones M., McCaffrey D., Vythilingam M., Finger E., Mondillo K., Jacobs M., Charney D.S., Blair R.J., Drevets W.C., Pine D.S. Response to emotional expressions in generalized social phobia and generalized anxiety disorder: evidence for separate disorders. Am. J. Psychiatry. 2008;165(9):1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.D., Gallagher F.M. Coming to terms with failure: private self-enhancement and public self-effacement. J. Exp. Soc. Psychol. 1992;28:3–22. [Google Scholar]
- Cash T.F., Cash D.W., Butters J.W. “Mirror, mirror, on the wall…?” Contrast effects and self-evaluations of physical attractiveness. Personal. Soc. Psychol. Bull. 1983;9(3):351–358. [Google Scholar]
- Clark D.M., McManus F. Information processing in social phobia. Biol. Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- Clark D.M., Wells A. Social phobia: diagnosis, assessment, and treatment. In: Heimberg R.G., Liebowitz M.R., Hope D.A., Schneier F.R., editors. A Cognitive Model of Social Phobia. Guilford; New York: 1995. pp. 69–93. [Google Scholar]
- Clifford M.M., Walster E. The effect of physical attractiveness on teacher expectations. Sociol. Educ. 1973;46(2):248–258. [Google Scholar]
- Coles M.E., Turk C.L., Heimberg R.G., Fresco D.M. Effects of varying levels of anxiety within social situations: relationship to memory perspective and attributions in social phobia. Behav. Res. Ther. 2001;39(6):651–665. doi: 10.1016/s0005-7967(00)00035-8. [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Atlas L.Y., Joormann J., Eugène F., Gotlib I.H. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res. Neuroimaging. 2006;148:55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Kincade J.M., Ollinger J.M., McAvoy M.P., Shulman G.L. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 2000;3(3):292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A., Van der Linden M., d'Acremont M., Mayers I. Phenomenal characteristics of autobiographical memories for social and non-social events in social phobia. Memory. 2006;14(5):637–647. doi: 10.1080/09658210600747183. [DOI] [PubMed] [Google Scholar]
- Devue C., Brédart S. The neural correlates of visual self-recognition. Conscious. Cogn. 2011;20(1):40–51. doi: 10.1016/j.concog.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. NeuroImage. 2001;14(6):1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Eich E., Nelson A.L., Leghari M.A., Handy T.C. Neural systems mediating field and observer memories. Neuropsychologia. 2009;47(11):2239–2251. doi: 10.1016/j.neuropsychologia.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Fenigstein A., Scheier M.F., Buss A.H. Public and private self-consciousness: assessment and theory. J. Consult. Clin. Psychol. 1975;43(4):522–527. [Google Scholar]
- Fleiss J.L., Levin B., Paik M.C. third ed. John Wiley & Sons; New York: 2013. Statistical Methods for Rates and Proportions. [Google Scholar]
- Fresco D., Coles M., Heimberg R.G., Liebowitz M.R., Hami S., Stein M.B., Goetz D. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol. Med. 2001;31(6):1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Goldin P., Manber-Ball T., Werner K., Heimberg R., Gross J.J. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol. Psychiatry. 2009;66(12):1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., Manber T., Hakimi S., Canli T., Gross J.J. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Arch. Gen. Psychiatry. 2009;66(2):170–180. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierres S.E., Kenrick D.T., Partch J.J. Beauty, dominance, and the mating game: contrast effects in self-assessment reflect gender differences in mate selection. Personal. Soc. Psychol. Bull. 1999;25:1126–1134. [Google Scholar]
- Hutchinson J.B., Uncapher M.R., Wagner A.D. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn. Mem. 2009;16(6):343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickes W.J., Wicklund R.A., Ferris C.B. Objective self-awareness and self-esteem. J. Exp. Soc. Psychol. 1973;9(3):202–219. [Google Scholar]
- Ingram R.E. Self-focused attention in clinical disorders: review and a conceptual model. Psychol. Bull. 1990;107(2):156–176. doi: 10.1037/0033-2909.107.2.156. [DOI] [PubMed] [Google Scholar]
- Izgiç F., Akyuz G., Dogan O., Kugu N. Social phobia among university students and its relation to self-esteem and body image. Can. J. Psychiatr. 2004;49:630–634. doi: 10.1177/070674370404900910. [DOI] [PubMed] [Google Scholar]
- Jiang F., Blanz V., O'Toole A.J. Probing the visual representation of faces with adaptation: a view from the other side of the mean. Psychol. Sci. 2006;17:493–500. doi: 10.1111/j.1467-9280.2006.01734.x. [DOI] [PubMed] [Google Scholar]
- Kenrick D.T., Neuberg S.L., Zierk K.L., Krones J.M. Evolution and social cognition: contrast effects as a function of sex, dominance, and physical attractiveness. Personal. Soc. Psychol. Bull. 1994;20(2):210–217. [Google Scholar]
- Klumpp H., Angstadt M., Phan K.L. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol. Psychol. 2012;89(1):273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy D., Sigall H. Beauty is talent: task evaluation as a function of the performer's physical attractiveness. J. Pers. Soc. Psychol. 1974;29(3):299–304. [Google Scholar]
- Lee E., Kang J.I., Park I.H., Kim J.J., An S.K. Is a neutral face really evaluated as being emotionally neutral? Psychiatry Res. 2008;157(1–3):77–85. doi: 10.1016/j.psychres.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Legrand D., Ruby P. What is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychol. Rev. 2009;116(1):252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- McManus F., Sacadura C., Clark D.M. Why social anxiety persists: an experimental investigation of the role of safety behaviours as a maintaining factor. J. Behav. Ther. Exp. Psychiatry. 2008;39(2):147–161. doi: 10.1016/j.jbtep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Montgomery R.L., Haemmerlie F.M., Edwards M. 1991. Social, Personal, and Interpersonal Deficits in Socially Anxious People. (Journal of Social Behavior & Personality) [Google Scholar]
- Morita T., Itakura S., Saito D., Nakashita S., Harada T., Kochiyama T., Sadato N. The role of the right prefrontal cortex in self-evaluation of the face: a functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008;20(2):342–355. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Morrison A., Heimberg R. Social anxiety and social anxiety disorder. Annu. Rev. Clin. Psychol. 2013;9:249–274. doi: 10.1146/annurev-clinpsy-050212-185631. [DOI] [PubMed] [Google Scholar]
- Oikawa H., Sugiura M., Sekiguchi A., Tsukiura T., Miyauchi C.M., Hashimoto T., Takano-Yamamoto T., Kawashima R. Self-face evaluation and self-esteem in young females: an fMRI study using contrast effect. NeuroImage. 2012;59(4):3668–3676. doi: 10.1016/j.neuroimage.2011.10.098. [DOI] [PubMed] [Google Scholar]
- Park J.Y., Oh J.M., Kim S.Y., Lee M.K., Lee C.R., Kim B.R., An S.K. Section of Affect & Neuroscience, Institute of Behavioral Science in Medicine, Yonsei University College of Medicine; Seoul, Korea: 2011. Korean Facial Expressions of Emotion (KOFEE) [Google Scholar]
- Phan K.L., Coccaro E.F., Angstadt M., Kreger K.J., Mayberg H.S., Liberzon I., Stein M.B. Corticolimbic brain reactivity to social signals of threat before and after sertraline treatment in generalized social phobia. Biol. Psychiatry. 2013;73(4):329–336. doi: 10.1016/j.biopsych.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek S., Keenan J., Gallup G., Mohamed F. Where am I? The neurological correlates of self and other. Cogn. Brain Res. 2004;19(2):114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Platek S., Wathne K., Tierney N., Thomson J. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Res. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Platek S.M., Kemp S.M. Is family special to the brain? An event-related fMRI study of familiar, familial, and self-face recognition. Neuropsychologia. 2009;47(3):849–858. doi: 10.1016/j.neuropsychologia.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Platek S.M., Loughead J.W., Gur R.C., Busch S., Ruparel K., Phend N., Panyavin I.S., Langleben D.D. Neural substrates for functionally discriminating self-face from personally familiar faces. Hum. Brain Mapp. 2006;27(2):91–98. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G., Schwartz S., Seghier M.L., Lazeyras F., Vuilleumier P. Portraits or people? Distinct representations of face identity in the human visual cortex. J. Cogn. Neurosci. 2005;17:1043–1057. doi: 10.1162/0898929054475181. [DOI] [PubMed] [Google Scholar]
- Pujol J., Giménez M., Ortiz H., Soriano-Mas C., López-Solà M., Farré M., Deus J., Merlo-Pich E., Harrison B.J., Cardoner N., Navinés R., Martín-Santos R. Neural response to the observable self in social anxiety disorder. Psychol. Med. 2013;43(4):721–731. doi: 10.1017/S0033291712001857. [DOI] [PubMed] [Google Scholar]
- Rao C.R., Miller J.P., Rao D.C. Elsevier; 2007. Handbook of Statistics: Epidemiology and Medical Statistics. [Google Scholar]
- Rapee R.M., Heimberg R.G. A cognitive-behavioral model of anxiety in social phobia. Behav. Res. Ther. 1997;35(8):741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Richins M.L. Social comparison and the idealized images of advertising. J. Consum. Res. 1991;18(1):71–83. [Google Scholar]
- Sadock B.J., Sadock V.A., Ruiz P. 11th ed. 2014. Kaplan & Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. (Wolters Kluwer Health) [Google Scholar]
- Stopa L., Clark D.M. Cognitive processes in social phobia. Behav. Res. Ther. 1993;31(3):255–267. doi: 10.1016/0005-7967(93)90024-o. [DOI] [PubMed] [Google Scholar]
- Suddendorf T., Butler D.L. The nature of visual self-recognition. Trends Cogn. Sci. 2013;17:121–127. doi: 10.1016/j.tics.2013.01.004. (Regul. Ed.) [DOI] [PubMed] [Google Scholar]
- Sugiura M., Sassa Y., Jeong H., Miura N., Akitsuki Y., Horie K., Sato S., Kawashima R. Multiple brain networks for visual self-recognition with different sensitivity for motion and body part. NeuroImage. 2006;32(4):1905–1917. doi: 10.1016/j.neuroimage.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Sugiura M., Watanabe J., Maeda Y., Matsue Y., Fukuda H., Kawashima R. Cortical mechanisms of visual self-recognition. NeuroImage. 2005;24(1):143–149. doi: 10.1016/j.neuroimage.2004.07.063. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Iacoboni M., Lange C., Keenan J.P. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends Cogn. Sci. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kaplan J.T., Molnar-Szakacs I., Zaidel E., Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. NeuroImage. 2005;25(3):926–935. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Wells A., Clark D.M., Ahmad S. How do I look with my mind's eye: perspective taking in social phobic imagery. Behav. Res. Ther. 1998;36(6):631–634. doi: 10.1016/s0005-7967(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Woody S.R., Rodriguez B.F. Self-focused attention and social anxiety in social phobics and normal controls. Cogn. Ther. Res. 2000;24(4):473–488. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Summary of full factorial analysis of variance for brain activity during the different composite face-conditions in patients with social anxiety disorder and healthy controls. Supplementary Table 2 Regional activation showing the significant group-by-condition interaction effect during composite face evaluation when using the null condition as a baseline. Supplementary Table 3 Percent signal changes in the regions showing the significant group-by-condition interaction effect during composite face evaluation, which were obtained using the null condition as a baseline and compared in the full conditions.