Abstract
Working memory ability matures through puberty and early adulthood. Deficits in working memory are linked to the risk of onset of neurodevelopmental disorders such as schizophrenia, and there is a significant temporal overlap between the peak of first episode psychosis risk and working memory maturation. In order to characterize the normal working memory functional maturation process through this critical phase of cognitive development we conducted a systematic review and coordinate based meta-analyses of all the available primary functional magnetic resonance imaging studies (n = 382) that mapped WM function in healthy adolescents (10–17 years) and young adults (18–30 years). Activation Likelihood Estimation analyses across all WM tasks revealed increased activation with increasing subject age in the middle frontal gyrus (BA6) bilaterally, the left middle frontal gyrus (BA10), the left precuneus and left inferior parietal gyri (BA7; 40). Decreased activation with increasing age was found in the right superior frontal (BA8), left junction of postcentral and inferior parietal (BA3/40), and left limbic cingulate gyrus (BA31). These results suggest that brain activation during adolescence increased with age principally in higher order cortices, part of the core working memory network, while reductions were detected in more diffuse and potentially more immature neural networks. Understanding the process by which the brain and its cognitive functions mature through healthy adulthood may provide us with new clues to understanding the vulnerability to neurodevelopmental disorders.
Keywords: Working memory, Neurodevelopment, Brain activation, fMRI, Neurodevelopmental disorders, Schizophrenia
Highlight
-
•
Healthy working memory functional maturation process in adolescence
-
•
Brain activation increased with age in higher order cortices.
-
•
Activation decreased in more diffuse and potentially more immature networks.
-
•
Provide new clues to understanding vulnerability to neurodevelopmental disorders
1. Introduction
1.1. Rationale
Adolescence represents a time of both dynamic cognitive development and a period when neurodevelopmental disorders such as schizophrenia often first emerge clinically (Brenhouse and Andersen, 2011, Paus et al., 2008). Late adolescence and early adulthood are periods of active neuronal maturation in higher order integrative cortices such as the superior temporal, posterior parietal, and prefrontal regions (Schweinsburg et al., 2005) that may underpin age related improvements in cognitive performance (Olesen et al., 2003). One such cognitive ability is working memory (WM). WM is a temporary holding store for information no longer available in the external environment. Healthy WM functioning is critical for a variety of executive functions (Baddeley, 1998), while WM deficits are linked to disorders such as schizophrenia (Mark and Toulopoulou, 2016, Park and Gooding, 2014, Toulopoulou et al., 2007), and meet endophenotypic criteria for that disorder (Mark and Toulopoulou, 2016). These links with schizophrenia may be particularly relevant given that the maturation of WM coincides so closely with the time when many patients first develop symptoms of schizophrenia (Catts et al., 2013).
To understand the possible links between WM functional maturation in late adolescence and early adulthood and the emergence of neurodevelopmental disorders it is important to first establish the basis for healthy WM development. This has the advantage of circumventing the potentially confounding effects of ill-related factors (e.g. treatment) that make it difficult to interpret results in patients alone. WM performance improves throughout adolescence as subjects employ better executive and rehearsal strategies and improve processing speed (Kwon et al., 2002, Luciana et al., 2005).
There is a body of work that relates to executive functioning, specifically WM in healthy children and the transition period from childhood to adolescence (Garon et al., 2008). Furthermore, a meta-analysis of fMRI responses across a range of WM tasks in healthy adults leads to the proposal of a core network, that includes the dorsolateral prefrontal and the inferior and superior parietal cortices (Rottschy et al., 2012, Thomas et al., 1999). Subcortical structures include anterior insula (Rottschy et al., 2012), basal ganglia (Olesen et al., 2003), hippocampus (Finn et al., 2010), and the cerebellum (Ciesielski et al., 2006). However, the transition period of WM development between adolescence and adulthood remains under-researched and it is not currently well mapped. Regarding WM development, much of the ‘mature’ circuitry is ‘functionally’ in place by middle childhood but continues to refine as the child gets older (Brahmbhatt et al., 2010), though this process remains unclear. Evidence shows that WM development is linked to a shift from visuospatial or motor network activation toward executive network activation with increasing age (Ciesielski et al., 2006, Klingberg et al., 2002, Scherf et al., 2006). However, more contradictory results have been found for executive network activation and task-related responses. For example, WM development has been linked both to increased (Durston et al., 2006) and decreased executive network activation and continued refinement of fronto-parietal regions with age (Geier et al., 2009, Klingberg, 2006, Schweinsburg et al., 2005). Similarly, it has been associated with enhanced sustained and diminished task-related response (Velanova et al., 2009), but also the opposite (Brahmbhatt et al., 2010, Burgund et al., 2006). One review proposed that WM development first involved an integration of childhood compensatory network with the more mature performance enhancing regions, and then a greater degree of localisation within those regions (Bunge and Wright, 2007).
1.2. Objectives
Taken together, these data suggest that WM related neural activation patterns change through adolescence although the timing, site, and manner of those changes remain unclear. To address these questions we performed a coordinate based meta-analysis that integrated the results of all available fMRI neuroimaging studies that investigated WM function with age in healthy adolescents and young adults from a systematic review of the literature. We decided to adopt a broad and inclusive rather than overly focused age window for the analyses, because the timing of normal development and cortical maturation is itself indistinct. This strategy allowed us to map activity across a broad range of ages and developmental time frames. Due to a lack of longitudinal studies, we solely included studies containing a cross sectional design that maps WM as a function of increasing age. Pooling data from multiple studies provides an opportunity to address some of neuroimaging's traditional problems such as small sample size and study-specific ‘noise’. We chose the Activation Likelihood Estimation (ALE) method that offers a well-established process for quantitative voxel wise random effects meta-analysis (Eickhoff et al., 2012, Turkeltaub et al., 2002, Turkeltaub et al., 2012). Based on the available literature, we hypothesized that the meta-analysis would primarily reveal an increase of activation in higher order integrative cortices, or association areas linked to the fully matured core WM network and a decrease in activation in ancillary brain regions, that might serve a less mature WM network.
2. Methods
2.1. Protocol and registration
We followed the Preferred Reporting Items for Systematic Reviews and Meta Analyses (Moher et al., 2009) method.
2.2. Information source and search
We first searched peer-reviewed papers published in English through MEDLINE, PubMed, PsychINFO and Cochrane Library, including all potential articles from inception to July 2014. The following search terms were used: ‘Working Memory’ and ‘healthy adolescence/adolescents/developmental trajectories’ and “neuroimaging/fMRI”. The search was conducted without any language restrictions. Other reviews and articles were hand searched for relevant studies not identified from the computerized literature search. Authors were contacted for further co-ordinate information where necessary.
2.3. Eligibility criteria
The following inclusion criteria were used: (a) Participants were healthy adolescents (age: 10–17 years) and adults (age: 18–30 years) (b) participants' age was provided as either an independent continuous or categorical variable (c) WM studies used a within or between-subject design (d) WM performance was indexed by a quantitative, well validated and reliable WM measurement, respectively a form of n-back task (Owen et al., 2005) or delayed matching to sample task (Rodriguez and Paule, 2009) (e) WM studies contrasted the WM task with a resting baseline (passive) or a sensory-motor (active) control condition that did not include a WM component (f) used fMRI with a voxel based approach, and (h) reported results as stereotactic coordinates in Talairach or Montreal Neurological Institute space.
2.4. Data collection process
Two reviewers (JAA, RZ) screened and assessed the studies returned from the search using the above criteria. Both reviewers independently extracted and analyzed the data from the included papers.
2.5. Publication Bias and coordinate-based meta-analysis
Publication-trends and biases in neuroimaging literature can affect ALE-analysis as the inclusion of a particular coordinate is conditioned on the fact that significant findings are prioritized for publication (Jennings and Van Horn, 2012). However, coordinate-based neuroimaging meta-analysis differs from most other forms of meta-analyses by assessing the spatial convergence between reported coordinates rather than quantifying the pooled effect sizes, which may be biased by non-published small effects and published type I error in small samples. Thus including an estimated number of unpublished results would not have any impact on the assessment of spatial convergence performed by ALE. The result is that a coordinate based rather than effect size, meta-analysis should be less susceptible to publication bias, while still acknowledging them as a quantitative integration of the available data (Rottschy et al., 2012).
2.6. Outcome measures
We categorized the WM tasks on the basis of the experimental paradigm used. We focused on two commonly used tasks the n-back and delayed matching-to-sample task. In the n-back, stimuli are presented consecutively, at each stimulus the participant is asked to identify whether the current stimulus is the same as the previous (1-back) or the second to last (2-back) etc. In delayed matching-to-sample task a single stimulus (the sample) is presented, after a variable delay the participant has to identify the sample from a set of distractors. Examples of this task include the “Sternberg task” (Sternberg, 1969) and the oculomotor delayed-response task (Funahashi and Takeda, 2002). Within these two major experimental paradigms, tasks differed in a number of ways, but principally by the means of presentation, usually aural or visual, the nature of the stimuli e.g. letters, numbers, words or the property to be remembered e.g. location or identity.
2.7. Meta-analytic approach
ALE is a robust method of quantitative meta-analysis of fMRI data implemented in “GingerALE 2.3.2” (http://www.brainmap.org/ale/). Coordinates, reported in, either Talairach or MNI standard space of each significant peak for all eligible subjects constituted the input to the meta-analyses (Eickhoff et al., 2012). Montreal Neurological Institute coordinates were converted to Talairach space using the validated icbm2tal transformation (Lancaster et al., 2007). Only one contrast was reported per study to ensure statistical independence.
Foci were restricted to the reported contrast between the experimental WM task with either resting baseline or a sensory-motor control. Age was coded as an independent variable. Two principle-analyses were conducted; firstly we looked at overall WM performance (visuospatial and verbal WM tasks together) as the dependent variable. Secondly we repeated the analyses but separately for visuospatial and verbal WM. Thus, in total six separate meta-analyses were conducted; three that identified areas where activation increased with increasing subject age and three where activation decreased with increasing age for each of overall WM, visuospatial WM, and verbal WM. Each activation focus from each study was modeled as the centre of Gaussian probability distribution with full width half-maxima of 10 mm3. These were subsequently summed to create a statistical map that estimated the likelihood of activation for each voxel from the entire set of studies. After combining the probabilities, a critical significant ALE score threshold was determined; the ALE maps were thresholded at p < 0.05 using a non-parametric False Discovery Rate (FDR) correction with a threshold of pN < 0.05. Statistical significance was determined using a permutation test (= 5000) of randomly created foci and clusters below a volume of 100 mm3 were excluded. ALE results were overlaid onto an optimized individual anatomical T1-template (www.brainmap.og/ale/Colin1.1.nii) and cluster centers were anatomically located in Mango (http://ric.uthscsa.edu/mango) (Lancaster et al., 2010).
3. Results
3.1. Search
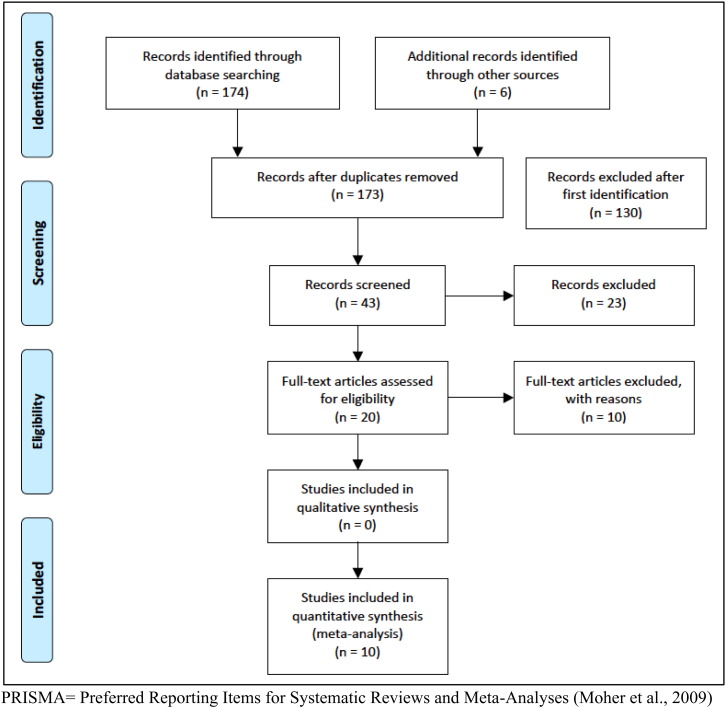
After removing duplicates, the initial search returned 173 articles that were screened by title and abstract and 130 articles were immediately excluded. After more detailed screening of the remaining 43 articles, 20 met inclusion criteria. A full-text assessment excluded a further ten because (1) ALE compatible coordinates were not available despite efforts to contact the authors directly; or (2) used region of interest analyses; or (3) did not provide age as an independent continuous or dichotomous variable, or (4) contained only load rather than task effects. There were no available studies with within-subject design. Thus only studies with between-subject design were included. In total ten articles fulfilled all the inclusion criteria and proceeded to the meta-analysis. See Fig. 1.
Fig. 1.
PRISMA flow chart of literature search.
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Moher et al., 2009).
3.2. Study and sample characteristics
Of the ten studies included in the meta-analysis, six assessed visuospatial WM, one focused on visual WM only, one on verbal WM only, and two on both visuospatial WM and verbal WM. Five of the studies treated age as a continuous variable. The remaining studies as categorical variable with arbitrarily defined ranges (e.g. 13–17 and 18–30; 11–15 and 20–28; 9–13 and 18–23). Nine of the ten studies that included data from 218 subjects, identified 60 foci of increased brain activation, while six studies, that included data on 164 subjects reported 22 foci that showed decreased brain activation (See Table 1).
Table 1.
Overview of Study Characteristics.
| Study | N | f/m ratio | Age-range | Age variable* | WM-material |
Activation profile |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WM-typea | WM taskb | Control taskc | Stimulus-type | Operation | # of Foci increased | # of Foci decreasee | |||||
| Nagel et al. (2013) | 67 | f | 10–16 | Cont. | VS & verbal | N-Back | Active | Letters | Location & identity verification | 1 (n = 45) | 3 (n = 56) |
| Brahmbhatt et al. (2010) | 35 | f | 9–13 18–23 |
Categ. | VS | N-Back | Active | Letters | Order verification | 3 (n = 17) | 4 (n = 17) |
| Thomason et al. (2009) | 27 | / | 7–12 20–30 |
Categ. | VS & verbal | DMTS | Active | Letters/ Shapes |
Location & Identity verification |
27 (n = 14) | 6 (n = 14) |
| Geier et al. (2009) | 46 | f | 13–17 18–30 |
Categ. | VS | DMTS | Active | Shapes | Location verification | 4 (n = 15) | |
| O'Hare et al. (2008) | 18 | 11–15 20–28 |
Categ. | Verbal | DMTS | Passive | Letters | Identity verification | 6 (n = 8) | ||
| Olesen et al. (2007) | 24 | / | 12–25 | Cont. | VS | DMTS | Active | Shapes | Location verification | 3 (n = 11) | |
| Ciesielski et al. (2006) | 27 | m | 10 12–28 |
Categ. | Visual | N-Back | Passive | Shapes | Object verification | 5 (n = 27) | |
| Schweinsburg et al. (2005) | 49 | 25/24 | 12–17 | Cont. | VS | N-Back | Active | Abstract Lines | Identity verification | 5 (n = 49) | 4 (n = 49) |
| Kwon et al. (2002) | 23 | 14/9 | 7–22 | Cont. | VS | N-Back | Active | Letters | Location verification | 4 (n = 34) | |
| Klingberg et al. (2002) | 13 | 4/9 | 9–18 | Cont. | VS | DMTS | Active | Shapes | Location verification | 6 (n = 13) | 1 (n = 13) |
*Conti. = Continuous variable; categ. = Categorical variable.
WM-Type: Visuospatial (VS) WM and verbal WM.
WM Task: N-back task and delayed matching-to-sample (DMTS) task.
Control task: active = sensory-motor control; passive = baseline.
Number of foci and corresponding sample size.
Number of foci and corresponding sample size.
3.3. Regions of increased brain activation with age
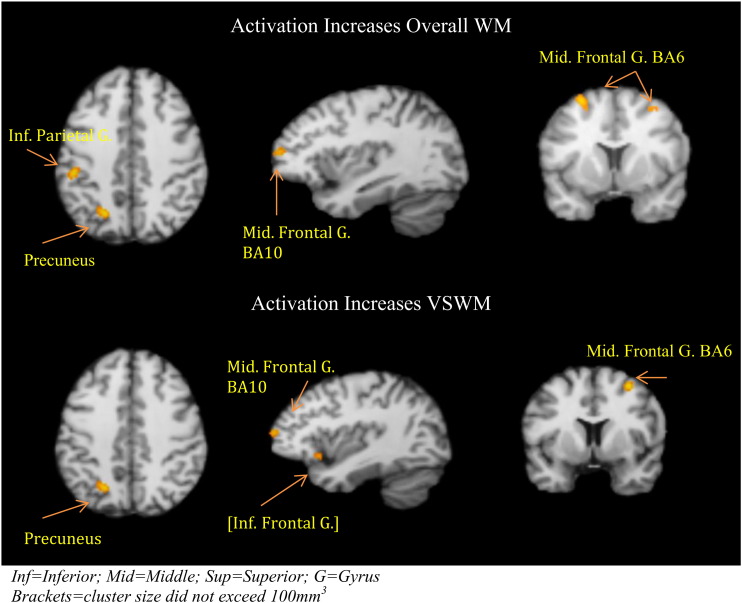
Nine studies of overall WM function identified increased brain activation in 60 foci that were restricted to five distinct brain regions when minimum cluster size criteria were applied, during WM tasks with increasing age. These were the middle frontal cortex bilaterally (Brodmann area: BA6); left precuneus (BA7); left parieto-temporal cortex, the inferior parietal gyrus (BA40); and left middle frontal cortex (BA10).
Seven studies reported on visuospatial WM and identified 39 foci, of which two, the right middle frontal cortex (BA6) and the left precuneus (BA7), exceeded minimum cluster volume. No cluster for verbal WM exceeded the minimum volume of > 100 mm3 (See Table 2 and Fig. 2).
Table 2.
Regions of increased brain activation for different WM categories with age (Cluster-level inference = 0.05; FDR pN = 0.05, permutation threshold = 5000, cluster included > 100 mm3).
| Hemisphere | Lobe | Gyrus | BA | Volume (mm3) | ALE | Centera |
|||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Total WM | Right | Frontal | Middle frontal | 6 | 480 | 0.0129 | 32 | 2 | 52 |
| Left | Parietal | Precuneus | 7 | 424 | 0.0109 | − 24 | − 62 | 36 | |
| Left | Parietal | inferior parietal | 40 | 400 | 0.0107 | − 46 | − 32 | 40 | |
| Left | Frontal | Middle frontal | 6 | 368 | 0.0107 | − 26 | 6 | 58 | |
| Left | Frontal | Middle frontal | 10 | 304 | 0.0101 | − 38 | 54 | 10 | |
| VSWM | Right | Frontal | Middle frontal | 6 | 432 | 0.0132 | 32 | 2 | 52 |
| Left | Parietal | Precuneus | 7 | 424 | 0.0109 | − 24 | − 62 | 36 | |
Center of mass in Talairach coordinates.
Fig. 2.
Activation increases with increasing age for respectively overall WM and VSWM.
Inf = Inferior; Mid = Middle; Sup = Superior; G = Gyrus.
Brackets = cluster size did not exceed 100 mm3.
3.4. Regions of decreased brain activation with age
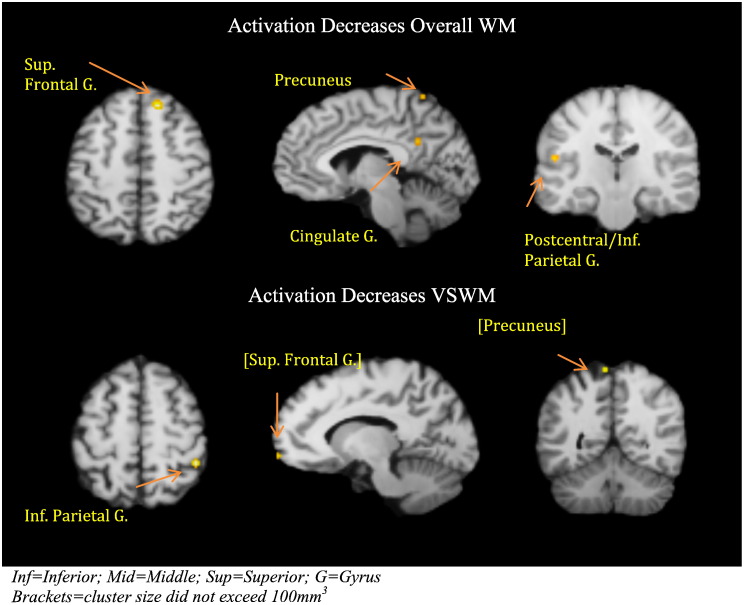
Six experiments identified decreased brain activation with increasing age in overall WM performance in 22 foci in three distinct brain regions; the right superior frontal gyrus including the frontal eye field (BA8), the left junction of the postcentral gyrus and inferior parietal (BA3/40), and left cingulate gyrus (BA31).
When restricted to visuospatial WM, five experiments of 110 subjects identified 17 foci. Only one region exceeded the minimum cluster size; the right inferior parietal lobe (BA40). No cluster for verbal WM exceeded the minimum volume. See Table 3 and Fig. 3 for detailed information.
Table 3.
Regions of decreased brain activation for different WM categories with age (Cluster-level inference = 0.05; FDR pN = 0.05; permutation threshold = 5000; cluster included > 100 mm3).
| Hemisphere | Lobe | Gyrus | BA | Volume (mm3) | ALE | Centera |
|||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Total WM | Right | Frontal | Superior frontal | 8 | 392 | 0.0118 | 16 | 32 | 46 |
| Left | Parietal | Postcentral/inferior parietal | 3/40 | 112 | 0.0097 | − 50 | − 24 | 14 | |
| VSWM | Left | Limbic | Cingulate | 31 | 104 | 0.0095 | − 2 | − 52 | 26 |
| Right | Parietal | Inferior parietal | 40 | 160 | 0.0095 | 44 | − 43 | 52 | |
Center of mass in Talairach coordinates.
Fig. 3.
Activation decreases with increasing age for respectively overall WM and VSWM.
Inf = Inferior; Mid = Middle; Sup = Superior; G = Gyrus.
Brackets = cluster size did not exceed 100 mm3.
4. Discussion
In this meta-analysis of all the available fMRI data we set out to assess the developmental changes that take place in WM functional circuits during adolescence and early adulthood. We found that there were no suitable longitudinal studies available and that within cross sectional designs, cognitive development appeared to be supported by increases in brain activation in key WM nodes and activation decreases in others. This is in line with previous developmental work that found both hypo- and hyper activation in adolescents relative to adults in regions significantly important in executive functioning and, therefore, providing evidence for the varied nature of not yet fully developed processes. Age-related increases in activation are interpreted as enhanced maturity in accessing performance-enhancing regions, whereas decreases in responses with age are viewed as indicating that a specific circuitry is no longer needed with age (Geier et al., 2009, Luna et al., 2001, Scherf et al., 2006).
4.1. Increased brain activation with age
4.1.1. Fronto-parietal network
Within the frontal lobes, the middle frontal gyrus bilaterally (BA6) showed increased activation with increasing subject age. This is consistent with earlier meta-analyses that identified this area as a region in the core WM network in healthy adults (Owen et al., 2005, Rottschy et al., 2012, Wager and Smith, 2003). The middle frontal gyrus has a critical role to the execution of complex motor responses (Nachev et al., 2008) and though frequently implicated, its role in more overt cognitive tasks is less well understood (Picard and Strick, 2001, Schubotz and von Cramon, 2003). Specifically, during cognitive tasks BA6 involvement has often been conceptualized as a concomitant latent motor process (Haxby et al., 2000). It may be that the middle frontal gyrus is involved in higher-level control processes critical to motor self-control (Seitz et al., 2009). The majority of WM tasks in this meta-analysis required the participants to make a motor response, typically a button push. That process required planning and response inhibition of motor activity that may develop in line with increased activation in BA6 with increasing subject age.
Activation in the left anterior middle prefrontal cortex (BA10) increased with increasing subject age. This region is linked to a monitoring and control function. The BA10 may support cognitive shifting between maintenance or updating and selection, manipulation, or monitoring functions, optimizing efficiency and task performance. This brain region may thus play a role in goal or process learning and selection rather than data processing in memory per se (Fletcher and Henson, 2001). A complimentary view is that the anterior middle prefrontal cortex supports the coordination of information processing and transfer between the multiple cognitive operations within WM (Ramnani and Owen, 2004). Based on this view we propose that the increased activation in the BA10 with age is linked in broad terms to the maturation of strategic processing.
Activation in the left inferior parietal lobe (BA40) and left precuneus (BA7) increased with increasing age. Both regions are frequently implicated in WM studies (Owen et al., 2005) and in rule-guided conditional reasoning operations, that require the application of a wide range of cognitive resources to the maintenance of WM (Liu et al., 2012, Reverberi et al., 2010). Furthermore, the BA7 might be related to the representation and manipulation of spatial relations among different object features. (Cavanna and Trimble, 2006). In addition, consistent with the complex nature of the WM tasks in this meta-analysis, increased activation in parietal areas (BA40, BA7) with increasing age might be explained by the fact that the inferior parietal cortex also plays an important role in enhanced domain-general executive processes such as rapid switching of attention (Ravizza et al., 2004). This is in line with a review that suggests that shifting attention may be a component of many executive processes involved in the control of WM. Thus, maintaining information in WM while processing other information, manipulating information in WM, and selecting which information among several sources should be stored in WM are all relatively complex processes that require shifting among both perceptual items and those stored in WM (Wager et al., 2004).
Complimentary data suggest links between improving WM performance and structural indices in the fronto-parietal cortex. For example greater white matter integrity in superior fronto-parietal regions, adjacent to cortical regions implicated in WM have been associated with better WM performance (Klingberg, 2006, Nagy et al., 2004). Similarly, a multi-modal diffusion tensor imaging and fMRI study found that the degree of white matter maturation is positively correlated with brain activity in superior fronto and intraparietal cortex, which contribute to the WM functional network (Olesen et al., 2003).
Our meta-analysis identified regions in the core adult WM network where activation increased with age from adolescence to adulthood (Owen et al., 2005). It is likely that the increased activation with age in the middle frontal cortex (BA6), anterior prefrontal cortex (BA10), and left inferior parietal lobe and left precuneus (BA40 + BA7) is indicative of the progressive recruitment of these task related frontal and parietal regions that underpin the functional maturation of the adult WM network (Bunge and Wright, 2007, Klingberg, 2006, Schweinsburg et al., 2005).
4.2. Decreased brain activation with age
4.2.1. Fronto-parietal network and limbic lobe
Besides its role in eye movement, the posterior dorsolateral prefrontal gyrus (BA8) contributes to the conditional allocation of attention to competing environmental stimuli (Petrides, 2005). Related to the maintenance of visuospatial attention during WM, it has been suggested that the activity in this region particularly plays an important role were delays are imposed between a stimulus and a response to that stimulus (Owen, 2000). One possible explanation for the reduced brain activation in BA8 with increasing participant age might be that this region is strongly associated with maintenance. For it has been suggested that younger participants might be more sensitive in the delay phase relying more on BA8 compared to older participants (Geier et al., 2009).
The left junction of the postcentral and inferior parietal gyrus (BA3/40) supports complex linguistic processes, especially attention to phonological relations (Hirshorn and Thompson-Schill, 2006, Small and Burton, 2002). Many of the WM paradigms included in these meta-analyses used letters and the participants might have used active verbal rehearsal strategies to follow rules and keep the information active in the WM store to enhance optimal task performance (Smith and Jonides, 1998). Furthermore, the decrease in activation as subjects aged, and as cognitive strategies matured, could be interpreted as that participants became less reliant on visual analysis processing (Kwon et al., 2009).
Activation in the left posterior cingulate gyrus (BA31) was reduced with age. While the region is highly functionally connected there is no consensus about its cognitive role (Leech and Sharp, 2014) and it is not implicated in the core WM network in healthy adults (Owen et al., 2005, Rottschy et al., 2012). One possible explanation is that the posterior cingulate is active in the default mode network. The default network mode is rapidly deactivated during a variety of externally directed tasks and when attention is focussed in for example a WM task (Leech and Sharp, 2014). Its role has been linked to monitoring change and facilitating shifts in behavior, where low activity is linked to continued operation with the prevailing cognitive set, and increases with investigation, exploration, flexibility and new learning (Pearson et al., 2011). It might be that younger subjects experience greater novelty with WM tasks compared to older subjects.
The significance of local reductions in brain activation with increasing age remains a subject of conjecture. The three regions identified by this meta-analysis, the caudal dorsolateral prefrontal cortex (BA8), the junction of the postcentral and inferior parietal gyrus (BA3/40), and the posterior cingulate gyrus (BA31) are not implicated in the core WM network in healthy adults (Owen et al., 2005, Rottschy et al., 2012). It is possible that these regions form part of a compensatory network that supports the still maturing WM network. With increasing age WM performance increases, the underpinning WM network becomes more refined and there is less need to activate the compensatory network (Bunge and Wright, 2007). Supporting evidence comes from a region of interest study that investigated the cross-sectional changes in functional WM brain circuitry from childhood to adulthood. Its results support the hypothesis that younger subjects rely primarily on a more ventromedial network including the caudate nucleus and anterior insula and less on the core adult WM regions such as the dorsal lateral prefrontal cortex and inferior parietal region (i.e. supramarginal gyrus). As subjects age the WM network matures by integrating premotor response preparation and executive circuitry into a specialized refined core WM network (Scherf et al., 2006).
4.3. Methodological considerations
Our meta-analysis has a number of limitations. The first and most important was the lack of suitable within-subject longitudinal studies. Therefore, we solely included studies with a between-subject design. In order to speak about true maturational processes, there is an immediate need for future research to deploy longitudinal designs in neurodevelopmental informative healthy and high-risk populations, to address this research gap. Secondly, we included data from two classes of experimental paradigm; the n-back task and delayed matching-to-sample tasks. These differed according to task subtype, control task, memory modality and stimulus types. By including data from such a diverse group of experiments there might be confounding brain activation related to dissimilar underlying processes and strategies involved during the separate WM tasks. Future meta-analyses with access to greater number of studies over time should focus both on single experimental paradigms that specify the underlying WM processes such as encoding, maintenance or manipulation of stimuli. To eliminate performance related variation in brain activity, some cross-sectional studies used simple tasks or grouped subjects based on individual performance. However, this approach leaves certain critical cognitive operations untapped, which might lead to biases in the investigation of the true higher order cortices development. Indeed, a recent paper suggested that chronological age might be too limited an index to measure relevant developmental changes in brain function and suggested “functional age” as an alternative (Satterthwaite et al., 2013). The fourth limitation was linked to how subjects' chronological age was characterized. Some studies treated age as a continuous and others as a categorical variable, e.g. “children”, “adolescents”. This creates several problems. Firstly the definition of age categories often differed between studies, with no commonality of definition. Secondly, by collapsing into categories, researchers lose data richness and the ability to precisely track developmental changes over time. Finally using age as a continuous variable facilitates comparisons between studies Thus, we conclude that age should be dealt with as a continuous variable in future studies. Another limitation is related to the gender-ratios of the included studies. A recent coordinate based meta-analysis explored the effects of gender on the functional WM network and found common elements but also differences between the genders. Specifically, females tended to activate more limbic (amygdala and hippocampus) and prefrontal structures (right inferior frontal gyrus), while males activated a distributed network including more parietal components (Hill et al., 2014). Future work needs to pay attention to this effect and incorporate it at the study design and analytic stage. Lastly, based on our exploratory approach, we only included between-subject voxel-based studies to avoid a user bias in defining the regions of interest and therefore excluded studies that used region of interest methods. The next step will be a confirmation of brain regions that showed an increase and decrease in activation based on the voxel based design. This should preferably be done in a longitudinal study using the region of interest method.
4.4. Implications for neurodevelopmental disorders
Adolescence is a time of substantial concomitant refinement of cognitive processes and physical maturation of neural circuitry underlying cognitions, such as WM. These changes are usually beneficial and optimize the brain for the challenges ahead, but they can also confer a vulnerability to certain types of psychopathology, such as schizophrenia (Paus et al., 2008). Overall, WM deficit is a trait characteristic of schizophrenia present across a range of clinical stages, notably from initial vulnerability to the chronic state (Park and Gooding, 2014). Understanding the basis of schizophrenia therefore requires a comprehensive knowledge of how the brain is put together. Since the adolescent brain is in a state of flux it may be possible to stabilize the adolescent brain of those at risks so that any disruption is only transitory and so that chronic schizophrenia does not emerge (Paus et al., 2008). Specifically, neuroimaging data can help in the development of neuroanatomical models of, for example, cognitive processes that are based on findings from developmental psychology. Therefore, imaging studies of healthy adolescents are very important to help us construct age appropriate structural and functional brain templates. Because both common genetic and environmental risk factors affect healthy subjects as well as patients, studies using healthy participants can often avoid the confounders associated with manifest illness and offer the hope to identify mechanisms that lie before the emergence of illness (Meyer-Lindenberg, 2010).
5. Conclusion
The current meta-analysis of healthy children and adolescents confirmed late maturational changes in activation in WM-associated regions. Evidence of activation increases was found in performance-enhancing fronto-parietal higher order cortices part of the core WM network. Furthermore, decreases in compensatory or supportive brain regions were also seen. Given that adolescence is a vulnerable period in which the initial symptoms of neurodevelopmental disorders tend to manifest, it is paramount to establish a robust understanding of the healthy functional development of WM. On the basis of this, abnormalities could be detected both faster and with greater certainty. Ideally, this paper will kindle further scientific inquiry into healthy neural circuitry development, explored through key cognitive paradigms such as WM, and thus, move a step closer to the earlier detection of neurodevelopmental disorders. Such detection is likely to contribute to more effective intervention strategies and ultimately, better health outcomes for our patients.
References
- Baddeley A. Recent developments in working memory. Curr. Opin. Neurobiol. 1998;8(2):234–238. doi: 10.1016/s0959-4388(98)80145-1. (Retrieved from http://www.sciencedirect.com/science/article/pii/S0959438898801451) [DOI] [PubMed] [Google Scholar]
- Brahmbhatt S.B., White D.A., Barch D.M. Developmental differences in sustained and transient activity underlying working memory. Brain Res. 2010;1354:140–151. doi: 10.1016/j.brainres.2010.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H.C., Andersen S.L. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 2011;35(8):1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Burgund E.D., Lugar H.M., Miezin F.M., Schlaggar B.L., Petersen S.E. The development of sustained and transient neural activity. Neuroimage. 2006;29(3):812–821. doi: 10.1016/j.neuroimage.2005.08.056. [DOI] [PubMed] [Google Scholar]
- Catts V.S., Fung S.J., Long L.E., Joshi D., Vercammen A., Allen K.M.…Shannon Weickert C. Rethinking schizophrenia in the context of normal neurodevelopment. Front. Cell. Neurosci. 2013;7:60. doi: 10.3389/fncel.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Lesnik P.G., Savoy R.L., Grant E.P., Ahlfors S.P. Developmental neural networks in children performing a Categorical N-Back Task. Neuroimage. 2006;33(3):980–990. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Durston S., Davidson M.C., Tottenham N., Galvan A., Spicer J., Fossella J.A., Casey B.J. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn A.S., Sheridan M.A., Kam C.L., Hinshaw S., D'Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J. Neurosci. 2010;30(33):11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.C., Henson R.N. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124(Pt 5):849–881. doi: 10.1093/brain/124.5.849. (Retrieved from http://brain.oxfordjournals.org/content/brain/124/5/849.full.pdf) [DOI] [PubMed] [Google Scholar]
- Funahashi S., Takeda K. Information processes in the primate prefrontal cortex in relation to working memory processes. Rev. Neurosci. 2002;13(4):313–345. doi: 10.1515/revneuro.2002.13.4.313. [DOI] [PubMed] [Google Scholar]
- Garon N., Bryson S.E., Smith I.M. Executive function in preschoolers: a review using an integrative framework. Psychol. Bull. 2008;134(1):31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Geier C.F., Garver K., Terwilliger R., Luna B. Development of working memory maintenance. J. Neurophysiol. 2009;101(1):84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Petit L., Ungerleider L.G., Courtney S.M. Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. Neuroimage. 2000;11(2):145–156. doi: 10.1006/nimg.1999.0527. [DOI] [PubMed] [Google Scholar]
- Hill A.C., Laird A.R., Robinson J.L. Gender differences in working memory networks: a BrainMap meta-analysis. Biol. Psychol. 2014;102:18–29. doi: 10.1016/j.biopsycho.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshorn E.A., Thompson-Schill S.L. Role of the left inferior frontal gyrus in covert word retrieval: neural correlates of switching during verbal fluency. Neuropsychologia. 2006;44(12):2547–2557. doi: 10.1016/j.neuropsychologia.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Jennings R.G., Van Horn J.D. Publication bias in neuroimaging research: implications for meta-analyses. Neuroinformatics. 2012;10(1):67–80. doi: 10.1007/s12021-011-9125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. Development of a superior frontal–intraparietal network for visuo-spatial working memory. Neuropsychologia. 2006;44(11):2171–2177. doi: 10.1016/j.neuropsychologia.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Klingberg T., Forssberg H., Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. J. Cogn. Neurosci. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H., Reiss A.L., Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc. Natl. Acad. Sci. U. S. A. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y.J., Lee J.K., Shin D.H., Jeong J.S. Changes in brain activation induced by the training of hypothesis generation skills: an fMRI study. Brain Cogn. 2009;69(2):391–397. doi: 10.1016/j.bandc.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutierrez D., Martinez M., Salinas F., Evans A., Zilles K.…Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Cykowski M.D., McKay D.R., Kochunov P.V., Fox P.T., Rogers W.…Mazziotta J. Anatomical global spatial normalization. Neuroinformatics. 2010;8(3):171–182. doi: 10.1007/s12021-010-9074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang M., Jou J., Wu X., Li W., Qiu J. Neural bases of falsification in conditional proposition testing: evidence from an fMRI study. Int. J. Psychophysiol. 2012;85(2):249–256. doi: 10.1016/j.ijpsycho.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Luciana M., Conklin H.M., Hooper C.J., Yarger R.S. The development of nonverbal working memory and executive control processes in adolescents. Child Dev. 2005;76(3):697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J.…Sweeney J.A. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13(5):786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Mark W., Toulopoulou T. Cognitive intermediate phenotype and genetic risk for psychosis. Curr. Opin. Neurobiol. 2016;36:23–30. doi: 10.1016/j.conb.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468(7321):194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nagel B.J., Herting M.M., Maxwell E.C., Bruno R., Fair D. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 2013;82(1):58–68. doi: 10.1016/j.bandc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z., Westerberg H., Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004;16(7):1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- O'Hare E.D., Lu L.H., Houston S.M., Bookheimer S.Y., Sowell E.R. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42(4):1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen P.J., Nagy Z., Westerberg H., Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cogn. Brain Res. 2003;18(1):48–57. doi: 10.1016/j.cogbrainres.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Olesen P.J., Macoveanu J., Tegner J., Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb Cortex. 2007;17(5):1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Owen A.M. The role of the lateral frontal cortex in mnemonic processing: the contribution of functional neuroimaging. Exp. Brain Res. 2000;133(1):33–43. doi: 10.1007/s002210000398. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Gooding D.C. Working memory impairment as an endophenotypic marker of a schizophrenia diathesis. Schizophr. Res. Cogn. 2014;1(3):127–136. doi: 10.1016/j.scog.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J.M., Heilbronner S.R., Barack D.L., Hayden B.Y., Platt M.L. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn. Sci. 2011;15(4):143–151. doi: 10.1016/j.tics.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N., Strick P.L. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11(6):663–672. doi: 10.1016/s0959-4388(01)00266-5. (Retrieved from http://www.sciencedirect.com/science/article/pii/S0959438801002665) [DOI] [PubMed] [Google Scholar]
- Ramnani N., Owen A.M. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Ravizza S.M., Delgado M.R., Chein J.M., Becker J.T., Fiez J.A. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Reverberi C., Cherubini P., Frackowiak R.S., Caltagirone C., Paulesu E., Macaluso E. Conditional and syllogistic deductive tasks dissociate functionally during premise integration. Hum. Brain Mapp. 2010;31(9):1430–1445. doi: 10.1002/hbm.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J., Paule M. Working memory delayed response tasks in monkeys. In: Buccafusco J.J., editor. Methods of Behavior Analysis in Neuroscience. Chapter 12. 2nd edition. CRC Press; Boca Raton (FL): 2009. [Google Scholar]
- Rottschy C., Langner R., Dogan I., Reetz K., Laird A.R., Schulz J.B.…Eickhoff S.B. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60(1):830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Erus G., Ruparel K., Elliott M.A., Gennatas E.D.…Gur R.E. Functional maturation of the executive system during adolescence. J. Neurosci. 2013;33(41):16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf K.S., Sweeney J.A., Luna B. Brain basis of developmental change in visuospatial working memory. J. Cogn. Neurosci. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schubotz R.I., von Cramon D.Y. Functional–anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. Neuroimage. 2003;20(Suppl. 1):S120–S131. doi: 10.1016/j.neuroimage.2003.09.014. (Retrieved from http://www.sciencedirect.com/science/article/pii/S1053811903005330) [DOI] [PubMed] [Google Scholar]
- Schweinsburg A.D., Nagel B.J., Tapert S.F. fMRI reveals alteration of spatial working memory networks across adolescence. J. Int. Neuropsychol. Soc. 2005;11(5):631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz R.J., Franz M., Azari N.P. Value judgments and self-control of action: the role of the medial frontal cortex. Brain Res. Rev. 2009;60(2):368–378. doi: 10.1016/j.brainresrev.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Small S.L., Burton M.W. Functional magnetic resonance imaging studies of language. Curr. Neurol. Neurosci. Rep. 2002;2(6):505–510. doi: 10.1007/s11910-002-0037-y. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Jonides J. Neuroimaging analyses of human working memory. Proc. Natl. Acad. Sci. U. S. A. 1998;95(20):12061–12068. doi: 10.1073/pnas.95.20.12061. (Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC21765/pdf/pq012061.pdf) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiments. Am. Sci. 1969;57(4):421–457. [PubMed] [Google Scholar]
- Thomas K.M., King S.W., Franzen P.L., Welsh T.F., Berkowitz A.L., Noll D.C.…Casey B.J. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10(3 Pt 1):327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Race E., Burrows B., Whitfield-Gabrieli S., Glover G.H., Gabrieli J.D. Development of spatial and verbal working memory capacity in the human brain. J Cogn Neurosci. 2009;21(2):316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulopoulou T., Picchioni M., Rijsdijk F., Hua-Hall M., Ettinger U., Sham P., Murray R. Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch. Gen. Psychiatry. 2007;64(12):1348–1355. doi: 10.1001/archpsyc.64.12.1348. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–780. doi: 10.1006/nimg.2002.1131. (Retrieved from http://www.sciencedirect.com/science/article/pii/S1053811902911316) [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eickhoff S.B., Laird A.R., Fox M., Wiener M., Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. J. Neurosci. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Smith E.E. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Jonides J., Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]