Abstract
Background:
Premenstrual syndrome (PMS) is one of the important health problems with high incidence in young women. The exact cause of this syndrome is not clear and some theories have been declared from hormonal factors to nutritional disorders.
Objective:
We investigated the correlation between serum zinc and antioxidant status with PMS.
Materials and Methods:
In this case-control study, forty eight young girls were selected from a total sample of 110 students residing at university dormitories including PMS (n=23) and healthy (n=25) groups based on PMS questionnaire. Dietary intake questionnaire and blood samples were collected from all participants. Serum total antioxidant capacity (TAC) and zinc concentrations were also measured.
Results:
Serum TAC and zinc concentrations were lower in PMS patients compared with healthy groups (p<0.01 and p<0.05, respectively). Healthy controls consumed lower servings of hydrogenated oils (p<0.05). There were significant differences in terms of muscle mass between the PMS and healthy groups (p<0.05). Both serum TAC and zinc levels were negatively correlated to PMS scores (r=-0.39, p<0.05 and r= -0.36; p<0.05, respectively).
Conclusion:
This study shows that higher TAC and zinc serum levels are associated with lower risk of PMS. PMS cases have more hydrogenated oils than their normal counterparts.
Key Words: Premenstrual syndrome, Total antioxidant capacity, Serum zinc concentrations, Young women
Introduction
Premenstrual syndrome (PMS) is a mixture of physical, behavioral, and mental symptoms that is felt before the onset of menstrual periods (1). PMS is more common in young women, and WHO has considered PMS as a public health threat in the modern societies (2). PMS comprises two major affective and mental sub groups (3). Approximately 70-90% of women in their fertility period show at least one of the PMS symptoms (4). The prevalence of PMS is 47.8% in the world. Lower and higher rates of PMS are seen in Europe and Asia respectively (5, 6).
The prevalence of PMS has been reported in different university dormitories of Iran; for example: 96.6% in Arak, 85% in Kerman, 84.62% in Tehran, 54.9% in Bandar Abbas, and 27.8% in Yazd (7, 8). The American College of Obstetricians and Gynecologists has published a set of diagnostic criteria for PMS, such as depression, anxiety, breast tenderness, extremities swelling, headache and abdominal bloating during the five days before menses for three consecutive menstrual cycles (9). However, the exact cause of this syndrome is not completely identified. There are some theories declaring that the genetic vulnerability, sensitivity to hormonal instabilities, and changes in brain neurotransmitters might alter PMS phenomenon (10). Due to high costs and the lack of effectiveness of medications for treatment, many patients tend to practice alternative therapies like dietary supplements, vitamins, and minerals (11).
Several lines of evidence claim that nutrients could affect the mood and behavioral disorders which happen as PMS consequence. Zinc as an essential nutrient for living organisms has a key role in more than 300 enzymes function (12). Low zinc status leads to learning and behavioral deprivation and mood disorders (13). Moreover, zinc has an important function in progesterone binding, prolactin secretion, opiates action, gonadal secretion and regulation of the menstrual cycle (14, 15). Some studies revealed that the level of serum zinc in luteal phase is significantly lower than follicular phase in normal women (16). Similarly, compared to the healthy people, serum levels of zinc in this phase are reported lower in PMS patients (17).
In healthy people, oxidant and antioxidant activities are in equilibrium. Losing this equilibrium can cause oxidative stress (OS) and it may lead to more than 100 diseases (18). Some studies showed that the level of total antioxidant capacity (TAC) is reduced in PMS patients but others indicated no significant difference in the antioxidant and lipid peroxidation levels between control and PMS patient (19, 20).
Since zinc deficiency has shown a high prevalence, especially in the south of Iran and also, the role of TAC is still controversial, this study was aimed to investigate the association between serum zinc concentrations and body antioxidant status with PMS in young dormitory female students (12).
Materials and methods
Participants and procedures
At first, an analytical cross-sectional study was carried out on 110 medical students (age 21-31 years old) in dormitories of Ahwaz Jundishapur University of Medical Sciences. They were assessed based on PMS questionnaire (Rossignol and Bonnlander scores) in the fall and winter of 2014. Scores <2, 2-16, 17-33, and >33 were considered as normal, mild, moderate, and severe PMS, respectively (21, 22).
Then, 23 students who were identified as moderate to severe PMS were selected as the study group (cases), and 25 students who had not experienced any of premenstrual symptoms were selected as healthy group (controls). Diagnosis of PMS was confirmed by the Daily Symptom Rating Scale (DSR) that included 22 symptoms, each of which was rated based on a 0-3 scale (0=without any symptom, 1=slight, 2=average, and 3=severe). Two groups completed the DSR for 2 consecutive months (23).
Food intake was measured by a validated 86-item semi-quantitative food frequency questionnaire (FFQ) (24). Blood samples were obtained from each subject in 3rd week (i.e. luteal phase) of menstrual cycle for assessing the serum zinc and TAC concentrations (19). Research protocol was approved by the Medical Ethics Committee at Jundishapur University of Medical Sciences, Ahvaz, Iran. (Ethical approval code: IR.AJUMS.REC.1394.61).
Measurement of serum TAC levels, serum zinc concentrations and anthropometric indices
Serum TAC was measured by ELISA assay (LDN® Labor Diagnostika Nord GmbH, Germany) based on peroxidase reaction and followed by a color reaction of chromogenic substrate tetramethylbenzidine. The change in color was calculated calorimetrically at 450 nm and expressed as “mMol/L” (25). According to kit protocol the expected values were: <1 mMol/L: antioxidative capacity is too low, 1-1.3 mMol/L: is borderline antioxidative capacity, >1.3 mMol/L: is sufficient antioxidative capacity. Serum zinc concentrations were measured by atomic absorption flame method (Chemtech, CTA 3000, England), using wavelength of 213.9 nm and slit width of 0.7 nm. Serum zinc levels <70 μg/dL was regarded as deficiency (26). For anthropometric assessment, percentage of body fat (BF), basal metabolic rate (BMR), muscle mass, weight and viscera fat were obtained by bioelectrical impedance analysis (BIA) method using OMRON device BF-511. Body mass index was also calculated as weight (kg) divided by height (m2). The waist and hip size were measured by a non-stretchable meter.
Statistical analysis
In this study, data analysis was done by SPSS software version 22. Kulmogrov-Smirnov test was applied so as to show normal distribution of data. Chi-square test was employed to analyze the qualitative data. Besides, comparisons were made, using independent t-test. In order to assess the linear correlation, Pearson’s coefficient was measured. Statistical significance was considered at p<0.05.
Results
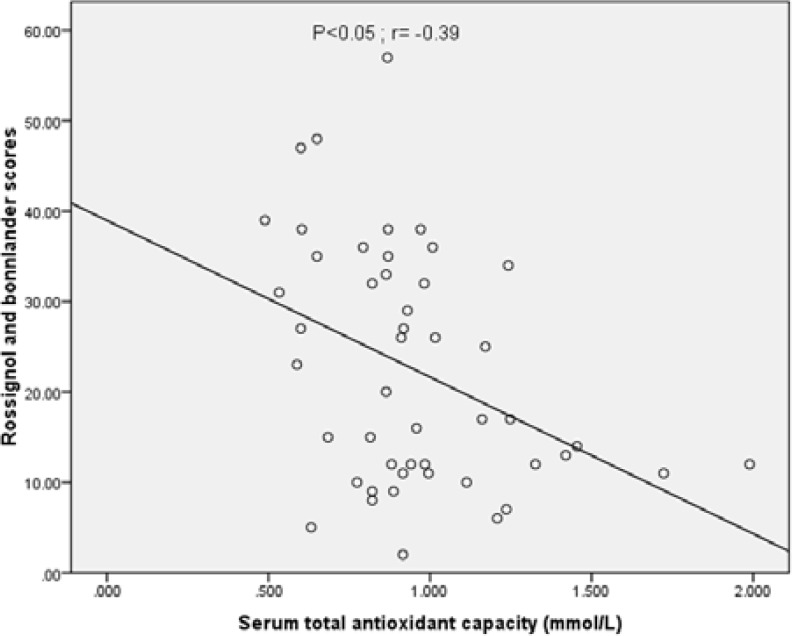
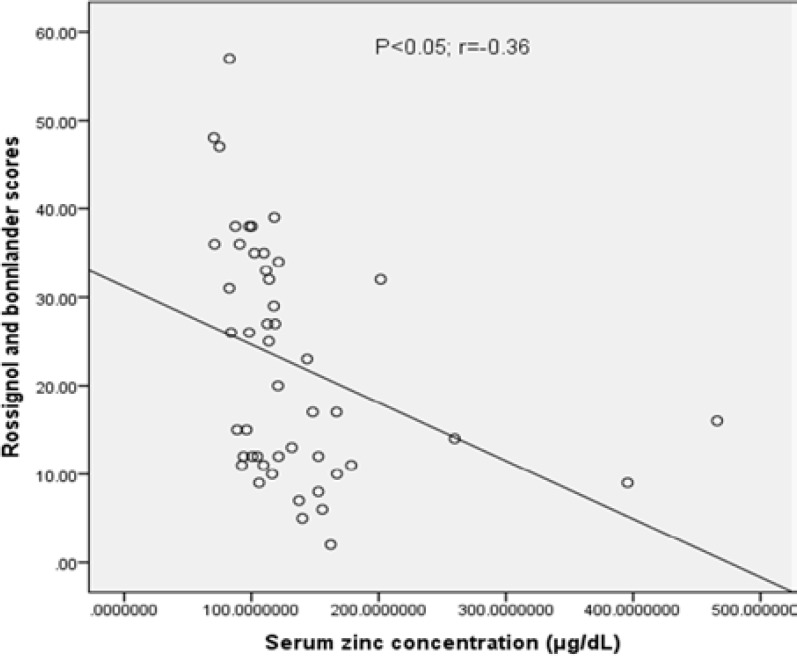
As shown in table I, there are no significant differences between the PMS and healthy groups in terms of basic characteristics (age and BMR). However, serum levels of TAC and serum zinc were lower in PMS group (p<0.01). There are significant differences in terms of muscle mass between the PMS and healthy groups (p<0.05, Table II). Consumption of hydrogenated oils was higher in PMS group compared with healthy group (p<0.05, Table III). There were no differences regarding the intake of other food items. Figure 1 indicates a negative linear regression between serum TAC and PMS scores (r= -0.39; p<0.05). The correlation between serum zinc concentrations and PMS scores was also analyzed and a negative linear regression was observed between serum zinc and PMS scores (r=-0.36; p<0.05; Figure 2).
Table I.
Basic characteristics of the study group
| Criteria | PMS (n=23) | Healthy (n=25) | p-value |
|---|---|---|---|
| Age (years) | 24.17 ± 0.55 | 23.64 ± 0.60 | 0.52 |
| TAC ( mmol/L) | 0.81 ± 0.041 | 1.075 ± 0.06 | 0.00 * |
| Serum zinc ( μg/dL) | 108.20 ± 3.73 | 153.8 ± 18.77 | 0.026* |
| BMR (Kcal) | 1297.16 ±20.01 | 1256.43 ±16.05 | 0.12 |
| PMS score | 33.91 ± 1.80 | 11.92 ± 1.11 | < 0.00*** |
TAC: total antioxidant capacity
BMR: Basal metabolic rate
P<0.05;
P<0.001 PMS vs. healthy group, Independent t-test was used. Data are shown as mean ± SEM.
Table II.
Comparison of anthropometric indices between the study groups
| Criteria | Healthy (n=25) | PMS (n=23) | p-value |
|---|---|---|---|
| BMI | 21.19 ± 0.61 | 22.90 ± 0.67 | 0.7 |
| Body fat percent (%) | 30.24 ± 1.27 | 34.29 ± 1.39 | 0.08 |
| Muscle mass (%) | 27.24 ± 0.50 | 25.58 ± 0.48 | 0.04* |
| Visceral fat (%) | 4.00 ±0.20 | 3.43 ±0.25 | 0.08 |
| Waist (cm) | 82.78 ±1.84 | 77.44 ±1.64 | 0.07 |
| Hip (cm) | 98.48 ±1.18 | 90.56 ± 0.98 | 0.06 |
BMI: Body mass index
P<0.05 PMS vs. healthy group, Independent t-test was used Data are shown as mean ± SEM.
Table III.
Intake of selected food items as servings in study groups
| Selected food items | Servings | Healthy (n=25) | PMS (n=23) | p-value |
|---|---|---|---|---|
| Cakes | Daily | 1.07 ± 0.15 | 1.09 ± 0.16 | 0.9 |
| Snacks | Daily | 2.91 ± 0.90 | 2.19 ± 0.67 | 0.5 |
| Butter | Weekly | 0.90 ± 0.35 | 1.40 ± 0.40 | 0.4 |
| Fruits | Daily | 1.64 ± 0.16 | 1.10 ±0.15 | 0.2 |
| Fresh vegetables | Daily | 5.03 ± 0.85 | 5.03 ± 0.73 | 0.9 |
| Cooked vegetables | Daily | 2.47 ± 0.32 | 2.84 ± 0.44 | 0.5 |
| Soft drinks | Weekly | 3.51 ± 1.14 | 7.58± 1.99 | 0.09 |
| Eggs | Weekly | 3.22± 0.54 | 2.48 ± 0.47 | 0.3 |
| Red meats | Daily | 1.24± 0.16 | 1.79 ± 0.28 | 0.1 |
| Chicken | Daily | 2.35± 0.43 | 1.80 ± 0.22 | 0.3 |
| Fish | Monthly | 7.78 ± 2.06 | 5.90 ± 1.17 | 0.4 |
| Fats | Daily | 0.86 ± 0.24 | 0.40 ± 0.114 | 0.06 |
| Refined grains | Daily | 5.43 ± 2.85 | 4.40 ± 1.11 | 0.7 |
| Whole grains | Daily | 9.17± 0.93 | 8.90 ± 0.89 | 0.8 |
| Sweets/candies | Weekly | 3.88 ± 0.88 | 6.80 ± 1.36 | 0.7 |
| Sugars | Daily | 3.72 ± 1.35 | 4.00 ± 1.01 | 0.8 |
| ice creams | Weekly | 5.10 ± 1.37 | 3.70 ± 1.07 | 0.4 |
| Tea and coffee | Daily | 1.95 ± 0.193 | 1.70 ± 0.39 | 0.5 |
| Olive oils | Daily | 3.40 ± 1.2 | 3.70 ± 1.51 | 0.3 |
| Hydrogenated oils | Daily | 0.48 ± 0.28 | 2.37 ± 0.58 | 0.00** |
| Vegetables oils | Daily | 7.01 ± 0.61 | 8.01 ± 0.60 | 0.3 |
P<0.01 PMS vs. healthy group, Independent t-test was used
Data are shown as mean ± SEM.
Figure 1.
Correlation between PMS scores and serum total antioxidant capacity (µg/deciliter) in PMS patients (r=-0.39; p<0.05).
Figure 2.
Correlation between PMS scores and serum zinc concentrations (µg/deciliter) in PMS patients (r=-0.36; p<0.05).
Discussion
Despite several decades of research, the PMS pathophysiology is still unknown. Many supplementary diets and micronutrients potentially influence the improvement of this syndrome through modifications in neurotransmitters and hormones (27). Nevertheless, few studies have focused on the effects of micronutrients on PMS (28, 29). Here, we studied the correlation between serum TAC and zinc levels with PMS.
PMS has a wild spectrum of physical and psychological problems. Major depression and anxiety are the most common psychological problems (23). Several studies evaluated the role of OS in these problems (18). Some researchers showed a high significant increase in malondialdehyde (as OS parameter) concentrations in plasma of the patients with depression (30-32). Other research obtained significantly reduced level of TAC in these patients. A decline in TAC is also associated with increased production of free radicals and decreased levels of antioxidant defenses (33). On the other hand, some researchers agree that hydrogenated oils lead to OS in metabolism of females bodies (33, 34).
Longhi et al found that hydrogenated soybean oils diet was associated with OS in Wistar rats (34). We also showed that women with PMS eat about 5 times more hydrogenated oil servings in their daily meals that may cause increased OS and following by mental disorder. This study also showed that the muscle mass, as an anthropometric indices, was higher in healthy group than in PMS. The muscle mass could be regarded as an index of protein consumption or physical activity. Our data did not show any significant difference between two groups in protein consumption. On the other hand, previous studies revealed that the exercise improved PMS disorders so, increased muscle mass in our normal group might be related to exercise (36, 37).
To date, there are various discussions on the effects of antioxidants on PMS. In some studies, Kalia et al and Balat et al investigated the antioxidants levels, such as SOD, glutathione, ceruloplasmin and lipid peroxidation product-MDA in PMS patients (20, 38). They reported no significant differences between the control and PMS patients in antioxidant levels. On the other hand, some studies showed that TAC decreased on the 21st day of menstrual cycle, while oxidant levels increased in PMS patients (19). Also, TAC levels in menstrual and luteal phases were lower than those in follicular phase in PMS cases (39). Today, we know that ovarian hormones perform a basic role in the pathogenesis of PMS and estrogen has a pro-oxidant property that leading to decrease of TAC in these patients (40, 41). However, this study did not check the effect of estrogen in PMS but perhaps this hormone cause to decrease TAC of our PMS group.
In agreement with the previous studies our study indicated that the serum levels of zinc are significantly lower in PMS patients (13, 42, 43). Studies also confirmed that serum zinc levels in luteal phase of a normal menstruation cycle are particularly lower than those in follicular phase (44). Some hypotheses suggested that the reduction in serum zinc during the luteal phase may be due to estrogen levels, enhancement of interleukin-1 or regulating progesterone and prolactin activity (45-47). Das and Chowdhury suggested that zinc is taken up by endometrial tissue during the luteal phase to regulate progesterone and estradiol binding receptors (48).
Conclusion
Serum TAC and zinc levels in young women with PMS were lower than those of healthy women. The improvement of body antioxidant status through the consumption of rich dietary sources like fresh fruit and vegetables with exercise can improve the syndrome. Hydrogenated oils increase the reactive oxygen species and accordingly, the consumption of the hydrogenated oils could decrease serum TAC levels and subsequently, amplify PMS (31). Finally, the role of healthy nutrition is emphasized in this study. It is suggested that other redox biomarkers and higher number of PMS cases are needed in the future studies.
Acknowledgements
The authors wish to thank the vice-chancellor for research, Jundishapur University of Medical Sciences for the financial support of this project (register no. B-9413).
Note
This article extracted from M.Sc. thesis. (Sanaz Fathizadeh)
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Pinar G, Colak M, Oksuz E. Premenstrual Syndrome in Turkish college students and its effects on life quality. Sex Reprod Healthcare. 2011;2:21–27. doi: 10.1016/j.srhc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Masoumi SZ, Ataollahi M, Oshvandi K. Effect of Combined Use of Calcium and Vitamin B6 on Premenstrual Syndrome Symptoms: a Randomized Clinical Trial. J Caring Sci. 2016;5:67–73. doi: 10.15171/jcs.2016.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nobles CJ, Thomas JJ, Valentine SE, Gerber MW, Vaewsorn AS, Marques L. Association of premenstrual syndrome and premenstrual dysphoric disorder with bulimia nervosa and binge-eating disorder in a nationally representative epidemiological sample. Int J Eat Disord. 2016;49:641–650. doi: 10.1002/eat.22539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozgoli G, Selselei EA, Mojab F, Majd HA. A randomized, placebo-controlled trial of Ginkgo biloba L in treatment of premenstrual syndrome. J Altern Complement Med. 2009;15:845–851. doi: 10.1089/acm.2008.0493. [DOI] [PubMed] [Google Scholar]
- 5.Darabi F, Rasaie N, Jafarirad S. The Relationship Between Premenstrual Syndrome and Food Patterns in University Student Girls. Jundishapur J Health Res. 2014;5:e26656. [Google Scholar]
- 6.Kleinstauber M, Witthoft M, Hiller W. Cognitive-behavioral and pharmacological interventions for premenstrual syndrome or premenstrual dysphoric disorder: a meta-analysis. J Clin Psychol Med Set. 2012;19:308–319. doi: 10.1007/s10880-012-9299-y. [DOI] [PubMed] [Google Scholar]
- 7.Farahani HN, Ashthiani AR, Masihi MS. Study on serum zinc and selenium levels in epileptic patients. Neurosciences (Riyadh) 2013;18:138–142. [PubMed] [Google Scholar]
- 8.Tabarroki SH, Amiri Z, Tabarroki E, Ozgoli G. Influence of premenstrual syndrome on energy and nutrient intake. J Shahid Beheshti School Nurs Midwif. 2012;21:37–44. [Google Scholar]
- 9.Lustyk MKB, Gerrish W. Premenstrual syndrome and premenstrual dysphoric disorder: issues of quality of life, stress and exercise. Handbook of Disease Burdens and Quality of Life Measures. 2010;22:1951–75. [Google Scholar]
- 10.Imai A, Ichigo S, Matsunami K, Takagi H. Premenstrual syndrome: management and pathophysiology. Clin Exp Obstet Gynecol. 2015;42:123–128. [PubMed] [Google Scholar]
- 11.Fanaei H, Khayat S, Kasaeian A, Javadimehr M. Effect of curcumin on serum brain-derived neurotrophic factor levels in women with premenstrual syndrome: A randomized, double-blind, placebo-controlled trial. Neuropeptides. 2016;56:25–31. doi: 10.1016/j.npep.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Amani R, Saeidi S, Nazari Z, Nematpour S. Correlation between dietary zinc intakes and its serum levels with depression scales in young female students. BiolTraceElem Res. 2010;137:150–158. doi: 10.1007/s12011-009-8572-x. [DOI] [PubMed] [Google Scholar]
- 13.Mitsuya H, Omata N, Kiyono Y, Mizuno T, Murata T, Mita K, et al. The co-occurrence of zinc deficiency and social isolation has the opposite effects on mood compared with either condition alone due to changes in the central norepinephrine system. Behav Brain Res. 2015;284:125–130. doi: 10.1016/j.bbr.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D, Ma G, Zhang X, He Y, Li M, Han X, et al. Zinc Finger Homeodomain Factor Zfhx3 is Essential for Mammary Lactogenic Differentiation by Maintaining Prolactin Signaling Activity. J Biol Chem. 2016;291:12809–12820. doi: 10.1074/jbc.M116.719377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teimoori B, Ghasemi M, Hoseini ZS, Razavi M. The Efficacy of Zinc Administration in the Treatment of Primary Dysmenorrhea. Oman Med J. 2016;31:107–111. doi: 10.5001/omj.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon Y, Yoon JD, Cai L, Hwang SU, Kim E, Zheng Z, et al. Supplementation of zinc on oocyte in vitro maturation improves preimplatation embryonic development in pigs. Theriogenology. 2014;82:866–874. doi: 10.1016/j.theriogenology.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Bertone-Johnson ER, Hankinson SE, Johnson SR, Manson JE. A simple method of assessing premenstrual syndrome in large prospective studies. J Reprod Med. 2007;52:779–786. [PubMed] [Google Scholar]
- 18.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Report. 2003;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 19.Duvan CI, Cumaoglu A, Turhan NO, Karasu C, Kafali H. Oxidant/antioxidant status in premenstrual syndrome. Arch Gynecol Obstet. 2011;283:299–304. doi: 10.1007/s00404-009-1347-y. [DOI] [PubMed] [Google Scholar]
- 20.Kalia G, Sudheendran S, Rao A. Antioxidant status and lipid peroxidation in premenstrual syndrome: a preliminary study. Clin Chim Acta. 2001;309:97–99. doi: 10.1016/s0009-8981(01)00498-3. [DOI] [PubMed] [Google Scholar]
- 21.Rossignol AM, Bonnlander H. Rossignol and Bonnlander Respond. Am JPublic HealthRes. 1991;81:1674. [Google Scholar]
- 22.Ryan KJ, khosro sobhanian. Kistner's gynecology and women's health. Vol. 4. Tehran: Golbaran; 2005. pp. 65–67. [Google Scholar]
- 23.Ahangari A, Backstrom T, Innala E, Andersson C, Turkmen S. Acute intermittent porphyria symptoms during the menstrual cycle. Intern Med J. 2015;45:725–731. doi: 10.1111/imj.12784. [DOI] [PubMed] [Google Scholar]
- 24.Veissi M, Anari R, Amani R, Shahbazian H, Latifi SM. Mediterranean diet and metabolic syndrome prevalence in type 2 diabetes patients in Ahvaz, southwest of Iran. Diabetes Metab Syndrome Clin Res Rev. 2016;10:26–29. doi: 10.1016/j.dsx.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Rakhshandehroo E, Razavi S, Nazifi S, Farzaneh M, Mobarraei N. Dynamics of the enzymatic antioxidants during experimental caprine coccidiosis. Parasitol Res. 2013;112:1437–1441. doi: 10.1007/s00436-013-3273-y. [DOI] [PubMed] [Google Scholar]
- 26.Burtis C, Ashwood E, Bruns O. Trace elements. Tietz Textbook of Clinical Chemistry. 3rd Ed. . Philadelphia: WB Saunders Co.; 1999. pp. 505–507. [Google Scholar]
- 27.Verma RK, Chellappan DK, Pandey AK. Review on treatment of premenstrual syndrome: from conventional to alternative approach. J Basic Clin Physiol Pharmaco. 2014;25:319–327. doi: 10.1515/jbcpp-2013-0072. [DOI] [PubMed] [Google Scholar]
- 28.Abdollahifard S, Rahmanian Koshkaki A, Moazamiyanfar R. The effects of vitamin B1 on ameliorating the premenstrual syndrome symptoms. Glob J Health Sci. 2014;6:144–153. doi: 10.5539/gjhs.v6n6p144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertone-Johnson ER, Hankinson SE, Forger NG, Powers SI, Willett WC, Johnson SR, et al. Plasma 25-hydroxyvitamin D and risk of premenstrual syndrome in a prospective cohort study. BMC Womens Health. 2014;14:56. doi: 10.1186/1472-6874-14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bilici M, Efe H, Köroğlu MA, Uydu HA, Bekaroğlu M, Değer O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord . 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 31.Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Report. 2013;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 32.Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short‐term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol Clin Exp. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 33.Ghodake SR, Suryakar AN, Kulhalli PM, Padalkar RK, Shaikh AK. A study of oxidative stress and influence of antioxidant vitamins supplementation in patients with major depression. Curr Neurobiol. 2012;3:107–111. [Google Scholar]
- 34.Longhi R, Almeida RF, Machado L, Duarte MM, Souza DG, Machado P, et al. Effect of a trans fatty acid-enriched diet on biochemical and inflammatory parameters in Wistar rats. Eur J Nutr. 2016;4:1–14. doi: 10.1007/s00394-015-1148-y. [DOI] [PubMed] [Google Scholar]
- 35.Bendsen NT, Stender S, Szecsi PB, Pedersen SB, Basu S, Hellgren LI, et al. Effect of industrially produced trans fat on markers of systemic inflammation: evidence from a randomized trial in women. J Lipid Res. 2011;52:1821–1828. doi: 10.1194/jlr.M014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haghighi ES, Jahromi MK, Daryano Osh F. Relationship between cardiorespiratory fitness, habitual physical activity, body mass index and premenstrual symptoms in collegiate students. J Sports Med Phys Fitness. 2015;55:663–667. [PubMed] [Google Scholar]
- 37.Samadi Z, Taghian F, Valiani M. The effects of 8 weeks of regular aerobic exercise on the symptoms of premenstrual syndrome in non-athlete girls. Iran J Nurs Midwif Res. 2013;18 [PMC free article] [PubMed] [Google Scholar]
- 38.Balat O, Dikensoy E, Ugur MG, Atmaca R, Cekmen M, Yurekli M. Malon dialdehyde, nitrite and adrenomedullin levels in patients with premenstrual syndrome. Arch Gynecol Obstet. 2007;275:361. doi: 10.1007/s00404-006-0269-1. [DOI] [PubMed] [Google Scholar]
- 39.ET Tuladhar AR. Plasma protein oxidation and total antioxidant power in premenstrual syndrome. Asian Pac J Trop Med. 2010;3:237–240. [Google Scholar]
- 40.Rosenfeld R, Livne D, Nevo O, Dayan L, Milloul V, Lavi S, et al. Hormonal and volume dysregulation in women with premenstrual syndrome. Hypertension. 2008;51:1225–1230. doi: 10.1161/HYPERTENSIONAHA.107.107136. [DOI] [PubMed] [Google Scholar]
- 41.Bäckström T, Andreen L, Birzniece V, Björn I, Johansson I-M, Nordenstam-Haghjo M, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. 2003;17:325–342. doi: 10.2165/00023210-200317050-00003. [DOI] [PubMed] [Google Scholar]
- 42.Chuong CJ, Dawson EB. Zinc and copper levels in premenstrual syndrome. Fertil Steril. 1994;62:313–320. doi: 10.1016/s0015-0282(16)56884-8. [DOI] [PubMed] [Google Scholar]
- 43.Posaci C, Erten O, Üren A, Acar B. Plasma copper, zinc and magnesium levels in patients with premenstrual tension syndrome. Acta Obstet Gynecol Scand. 1994;73:452–455. doi: 10.3109/00016349409013429. [DOI] [PubMed] [Google Scholar]
- 44.Oktem G, Sahin C, Dilsiz OY, Demiray SB, Goker EN, Tavmergen E. Altered Stem Cell Receptor Activity in the Ovarian Surface Epithelium by Exogenous Zinc and/or Progesterone. Drug Res (Stuttg) 2015;65:252–258. doi: 10.1055/s-0034-1376975. [DOI] [PubMed] [Google Scholar]
- 45.Du H, Sarno J, Taylor HS. HOXA10 inhibits Kruppel-like factor 9 expression in the human endometrial epithelium. Biol Reprod. 2010;83:205–211. doi: 10.1095/biolreprod.110.083980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, et al. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao HH, Hong HJ, Cheng TH, Shih NL, Loh SH, Liu JC, et al. Nicorandil Inhibits Cyclic Strain- Induced Interleukin-8 Expression in Human Umbilical Vein Endothelial Cells. Pharmacology. 2016;98:42–50. doi: 10.1159/000445075. [DOI] [PubMed] [Google Scholar]
- 48.Das K, Chowdhury AR. Metallic ion concentration during menstrual cycle in normally menstruating women. Indian J Med Sci. 1997;51:52–54. [PubMed] [Google Scholar]