Abstract
Metastatic spread of colorectal cancer (CRC) to the peritoneal cavity is common and difficult to treat, with many patients dying from malignant bowel obstruction. Chimeric antigen receptor T cell (CAR-T) immunotherapy has shown great promise, and we previously reported murine and phase I clinical studies on regional intrahepatic CAR-T infusion for CRC liver metastases. We are now studying intraperitoneal (IP) delivery of CAR-Ts for peritoneal carcinomatosis. Regional IP infusion of CAR-T resulted in superior protection against CEA+ peritoneal tumors, when compared to systemically infused CAR-Ts. IP CAR-Ts also provided prolonged protection against IP tumor re-challenges and demonstrated an increase in effector memory phenotype over time. IP CAR-Ts provided protection against tumor growth at distant subcutaneous (SC) sites in association with increases in serum IFNγ levels. Given the challenges posed by immunoinhibitory pathways in solid tumors, we combined IP CAR-T treatment with suppressor cell targeting. High frequencies of myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) were found within the IP tumors, with MDSC expressing high levels of immunosuppressive PD-L1. Combinatorial IP CAR-T treatment with depleting antibodies against MDSC and Treg further improved efficacy against peritoneal metastases. Our data support further development of combinatorial IP CAR-T immunotherapy for peritoneal malignancies.
Introduction
Peritoneal carcinomatosis (PC) is a devastating condition that affects 15% of all colorectal cancer patients at initial presentation 1. These patients typically have a very poor prognosis and suffer from numerous complications of their disease, including progressive bowel obstruction. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) has been used with modest success in highly selected patients with limited disease burdens. During CRS-HIPEC, all visible intraperitoneal tumor is debulked and residual microscopic disease is treated with regionally delivered chemotherapy. A randomized controlled trial demonstrated that CRS-HIPEC for patients with colorectal cancer PC resulted in significantly improved survival compared to systemic chemotherapy 2, 3. Unfortunately, most PC patients are not candidates for CRS-HIPEC and ultimately progress and die of disease 1, 4. Even so, results with CRS-HIPEC for PC suggest that regionally delivered therapeutics are a promising approach to address this large unmet clinical need.
We recently reported pre-clinical and clinical results for a regional immunotherapy approach for CRC liver metastases (LM) 5-7. In general, immunotherapy has gained considerable traction in recent years 8, 9. Cellular immunotherapy for solid tumors has advanced largely through application of chimeric antigen receptor T cells (CAR-Ts). Our interest in CAR-Ts is based on their broad applicability since they can be produced for almost any patient and are not restricted by major histocompatibility complex types 10-12. We have recently tested CAR-T targeting carcinoembryonic antigen (CEA) in Phase I Hepatic Immunotherapy for Metastases (HITM) clinical trials (NCT01373047, NCT02416466) examining the safety and clinical activity of these cells against colorectal cancer LM 5. As the peritoneal cavity is another common site of failure in stage IV CRC patients, we are interested in testing regional CAR-T delivery for PC.
While regional delivery may enhance the anti-tumor efficacy of CAR-Ts, intratumoral immunosuppression will likely present additional challenges 13. The metastatic solid tumor microenvironment contains many immunosuppressive cell types that inhibit CAR-Ts, including myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) 14. We have previously shown that MDSC suppress CAR-T cells, and inhibit the function of liver B cells 15. MDSC accomplish this immunosuppressive function through the PD-1/PD-L1 axis and IDO 7. Treg are also well studied in tumor microenvironments and have been shown to suppress CAR-Ts via PD-L1 and CTLA4 16. We speculate that effective IP CAR-T therapy for PC will be further enhanced through inhibition of immunosuppressive cell populations.
We have tested a novel pre-clinical strategy for regional intraperitoneal (IP) CAR-T delivery combined with the targeting of suppressor cell populations in a murine model of PC. Our data indicate that IP CAR-T infusion is superior to systemic tail vein (TV) infusion in treating PC. Targeting of immunoinhibitory cells and pathways enhanced anti-tumor effects of IP CAR-Ts. IP CAR-T infusions were able to effectively protect mice from tumor re-challenge in the abdomen and induce responses at extra-abdominal sites. These results support clinical development of our Immunotherapy for Peritoneal Carcinomatosis (IPC) program.
Results
IP delivery of CAR-Ts results in superior tumor killing compared to systemic infusions
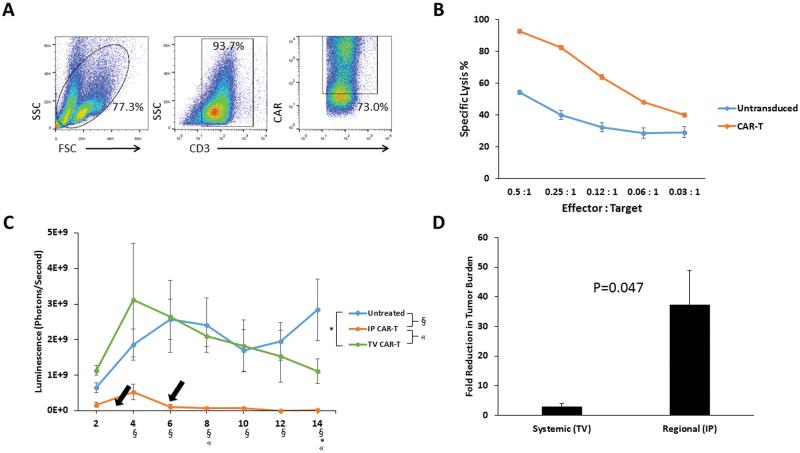
In preparation for our murine in vivo studies, we generated anti-CEA CAR-Ts. Transduction of murine splenocytes (Figure 1A) was confirmed by measuring CAR expression on CD3+ cells. Anti-CEA CAR-Ts mediated efficient killing in vitro, as demonstrated by lysis of CEA+ MC38 tumor cells (Figure 1B). At an Effector: Target ratio as low as 0.03:1, specific lysis was significantly higher than activated untransduced T cells (p=0.02).
Figure 1. Optimization of CAR-T delivery for treatment of peritoneal tumors.
CAR-Ts were generated from murine splenocytes and were characterized for expression of CD3 and the anti-CEA CAR (A). In vitro CAR-T tumor killing was tested with bioluminescence assays, in which CAR-Ts or untransduced T cells were co-cultured with MC38CEA-luc at various effector : target ratios (B). CAR-Ts were tested in vivo via IP or TV infusion, and peritoneal tumor killing was monitored by changes in bioluminescence. Arrows indicate the CAR-T injection time points. Each line on the plot is representative of the average of 4 mice. Symbols denoting significance (p<0.05) are found below the x-axis (C). Fold reduction in tumor luminescence was calculated between days 4 and 14 of the in vivo study, comparing TV to IP CAR-T delivery (D). Error bars are representative of SEM values. P values were calculated using Student’s t test.
To test our hypothesis that IP delivery will improve CAR-T efficacy in mice with PC compared to systemic TV infusion, we studied both infusion methods in mice with established IP tumors. On days 3 and 6, tumor-bearing mice were treated with CAR-Ts, either via IP or TV infusion, and received daily IL-2 injections. A single treatment of regionally delivered IP CAR-Ts resulted in significantly reduced tumor burden (p<0.01), and this remained significant compared to untreated animals at each subsequent time point. IP infusion of CAR-Ts remained more efficacious than systemic TV CAR-Ts for up to 8 days following the second CAR-T treatment (Figure 1C). In contrast to IP CAR-Ts, TV CAR-Ts did not have a significant impact on tumor growth until day 14 when compared to untreated animals (p=0.04). IP CAR-T treated mice exhibited a 37-fold reduction in tumor burden between days 4 and 14, whereas TV CAR-T treated mice exhibited only a 3-fold reduction in tumor burden over the same time period (p=0.05) (Figure 1D). In 4 mice treated with regionally delivered IP CAR-Ts, there was no detectable tumor upon necroscopy at day 14. Microscopic tumor was, however, still detectable by bioluminescence monitoring on the same day. In contrast, all of the TV treated animals had grossly visible IP tumor upon necroscopy.
Regionally delivered CAR-Ts provide durable protection against IP tumor growth
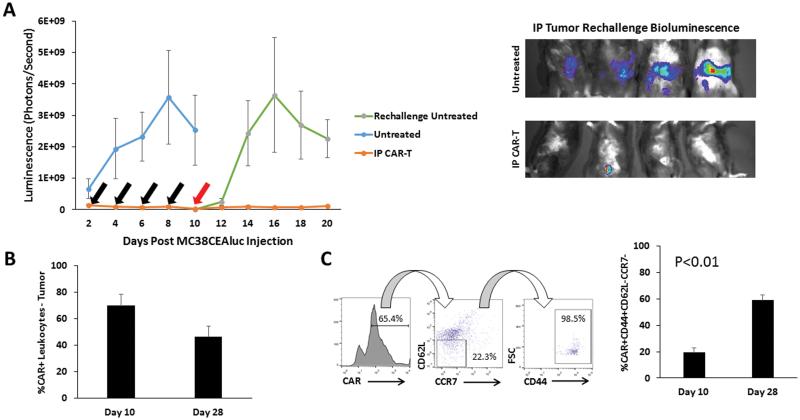
Having confirmed that IP CAR-T infusions are superior to systemic administration, we studied the durability of the protection against IP tumor challenge. Following IP CAR-T infusions, mice were re-challenged with IP tumor injections and tumor progression was monitored by bioluminescence. Mice that had received prior CAR-T IP infusions demonstrated a significant decrease in tumor growth compared to mice with no prior CAR-T treatment (p=0.02). Protection from IP tumor growth extended for up to 10 days following tumor re-challenge (p=0.01) (Figure 2A). Small amounts of visible tumor were harvested and CAR-Ts were found to comprise 69% of intratumoral leukocytes on day 10, and 47% on day 28 (Figure 2B). Intratumoral CAR-Ts were immunophenotyped and we detected an increase in the proportion of CAR-Ts with an effector memory phenotype (CAR+CD44+CD62L−CCR7−) (Figure 2C).
Figure 2. Durability of IP CAR-T tumor efficacy in mice with peritoneal tumors.
Duration of CAR-T anti-tumor efficacy in the peritoneal space was tested with IP tumor re-challenge, following response to initial tumor burden. Black arrows represent days that mice received CAR-T treatment and the red arrow represents the day of tumor re-challenge (A, left). Tumor growth was measured by bioluminescence. Representative bioluminescence images from day 20 of the study are depicted from mice that received tumor re-challenge (A, right). The frequencies of CAR+ lymphocytes recovered from IP tumor tissue at both day 10 (n=5) and day 28 (n=3) time points were compared (B). Memory phenotypes of CAR+ lymphocytes were examined at both the day 10 (n=5) and day 28 (n=3) time points. Dot plots show the gating for the effector memory phenotype (C). Error bars are representative of SEM values. P values were calculated using Student’s t test.
IP CAR-T infusions provide protection against extra-abdominal tumor growth
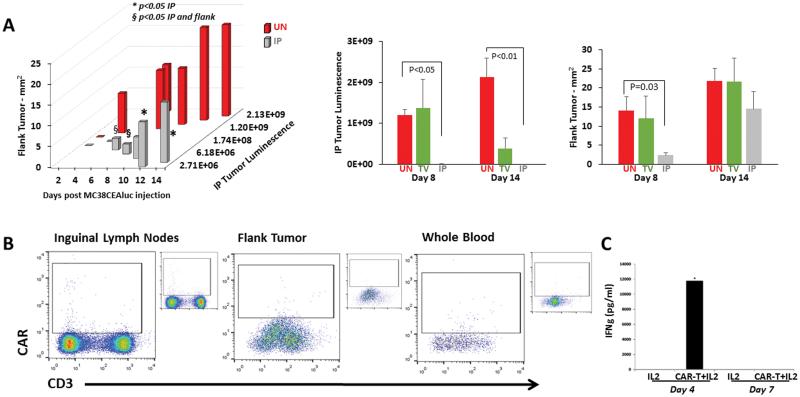
Considering that patients with IP tumors may have disease at other anatomic sites, we tested if IP CAR-T infusions protected against SC flank tumor growth. Mice were simultaneously injected with MC38CEA-luc cells IP and in the left flank. Following two treatments on days 3 & 6, IP CAR-Ts led to decreased IP and flank tumor burden compared to untreated animals (p<0.05), as well as animals receiving untransduced splenic T cells (data not shown). Tumor reduction also trended favorably when compared to mice that received CAR-Ts via TV and mice that received IL-2 support only. This corresponded with a significantly less flank tumor area in IP CAR-T treated mice when compared to untreated animals on the same day (p=0.03, Figure 3A). CAR-Ts were not detected by flow cytometry in whole blood, flank tumor tissue, or left inguinal lymph nodes, (Figure 3B). However, IP CAR-T infusions did lead to high levels of systemic IFNγ at 4 days following treatment (Figure 3C).
Figure 3. Systemic response to IP CAR-Ts.
The systemic anti-tumor effect of regionally delivered IP CAR-Ts was tested by injecting mice with MC38CEA-luc both IP and subcutaneously in the left flank. IP tumor burden and flank tumor sizes were measured for 14 days in untreated (UN), TV CAR-T and IP CAR-T groups. Bars are representative of 4 mice per group. (A). On day 14, left inguinal lymph nodes, flank tumors, and whole blood were harvested to determine if CAR-Ts were present at these sites. Fully stained, untreated controls are shown in insets next to the dot plots (B). Error bars are representative of SEM values. P values were calculated using Student’s t test. Bioluminescence is expressed as photons per second. (C) Serum IFNγ concentrations were determined on days 4 and 7 to detect systemic immune activity in response to IP CAR-T infusions.
Peritoneal tumors are infiltrated with immunosuppressive cells
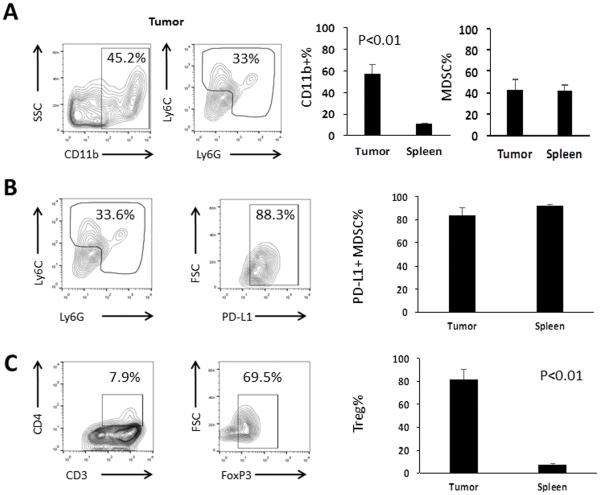
Although IP CAR-T infusions mediated durable responses in mice with PC, we speculate that immunosuppressive cells will limit CAR-T function. MDSC and Treg, which we have previously shown to suppress CAR-Ts in our colorectal cancer LM models 7, were detected within IP tumors. On average, CD11b+ cells represented 57% of leukocytes in IP tumors, compared to 11% from the spleens of the same animals (p<0.01). Both Ly6G+ granulocytic MDSC (gMDSC) and Ly6C+ monocytic MDSC (mMDSC) were found within IP tumor (43%) and spleen (41%) (Figure 4A). The immunosuppressive marker PD-L1 was expressed on both MDSC subsets, whether they were derived from the tumor or the spleen (Figure 4B). Treg (FoxP3+) were found to comprise 82% of CD4 T cells within the tumors, compared with 7% in spleens from the same animals (p<0.01) (Figure 4C).
Figure 4. Suppressor cell content of intraperitoneal tumors.
Tumor leukocyte contents were immunophenotyped to detect the presence of suppressive cell populations. MDSC were found in the tumors after staining for CD11b, Ly6C and Ly6G. Representative dot plots show MDSC from the IP tumors, along with bar graphs comparing MDSC populations from the tumors and spleens of the same untreated animals. The percentages of CD11b+ cells among all live cells and MDSC (Gr-1+) among CD11b+ cells are shown (A). MDSC were also immunophenotyped for the expression of the immunosuppressive marker PD-L1 (B). Representative tumor dot plots show that Treg, expressed as the percentage of FoxP3+ cells among CD3+CD4+ T cells, were also found within the IP tumors. Smaller populations were found within the spleens of the same animals (C). Bars are representative of 3 mice per group. Error bars are representative of SEM values. P values were calculated using Student’s t test.
Suppressor cell depletion combined with regional CAR-T delivery in vivo
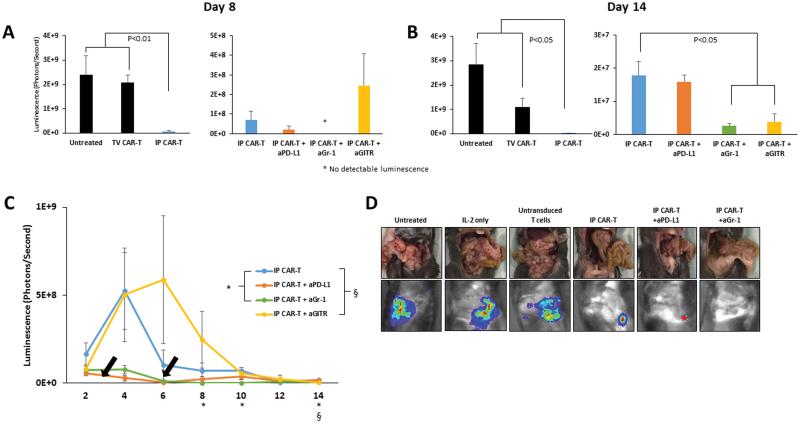
We performed in vivo testing of IP CAR-T infusions in combination with suppressor cell depletion or blockade of the PD-1/PD-L1 immunoinhibitory pathway. IP CAR-Ts alone, and when used in combination with anti-PD-L1, anti-Gr1, or anti-GITR, resulted in significant reductions in tumor burden compared to untreated animals on day 8 (Figure 5A). On day 14, CAR-Ts alone significantly diminished tumor burden when compared to untreated mice, mice that received untransduced T cells, and mice that received daily dose IL-2 alone (p<0.05). CAR-Ts combined with the depletion of Treg showed even further reduced burden from CAR-Ts alone (p<0.01), as did the combination of CAR-Ts and MDSC depletion (p=0.017) (Figure 5B). The combination of CAR-Ts and anti-Gr-1 was the most efficacious overall, showing no detectable bioluminescence on days 8 and 10 (Figure 5C). On day 14, there was no detectable tumor found in any mouse that received IP CAR-T upon gross inspection (Figure 5D).
Figure 5. IP CAR-Ts in combination with suppressor cell targeting for in vivo treatment.
IP CAR-Ts combined with depleting antibodies against MDSC and Treg, or blocking antibodies against the PD-L1 pathway, were administered to mice injected with MC38CEA-luc. Tumor reduction was monitored by bioluminescence over 14 days. Bar graphs compare the efficacy of regional IP CAR-Ts to systemic TV CAR-Ts, and IP CAR-Ts alone to IP CAR-Ts with antibodies on day 8 after the treatments (A) and at the end of the study on day 14 (B). Bars are representative of 4 animals per group. Black arrows represent CAR-T treatments. Symbols denoting significance appear for specific days below the x axis (C). Gross inspection images, as well as bioluminescence images, are shown for all groups on day 14 (D). Error bars are representative of SEM values. P values were calculated using Student’s t test.
Discussion
Adoptive cell therapy for solid tumors is presently limited by several challenges, including inefficient delivery of immune cells and influence of immunoinhibitory pathways. We believe that direct regional delivery of CAR-Ts into organs or spaces harboring metastatic solid tumors will enhance safety and efficacy. In addition, combining regional CAR-T delivery with inhibition of immunosuppressive pathways will likely enable further improvements in CAR-T performance in the treatment of solid tumors. In this report, we demonstrate the pre-clinical efficacy of IP CAR-T infusions for PC, a grave clinical condition. The ability of IP CAR-T infusions to control IP tumor growth in durable fashion was associated with systemic efficacy as well. Depleting suppressive immune cells and targeting immunosuppressive pathways known to limit CAR-T function further enhanced IP CAR-T efficacy. Our data provide the rational for development of phase I IP CAR-T trials for patients with PC.
Our findings that direct IP infusion of CAR-Ts in mice with PC was more effective at controlling tumor than systemic infusion resonate well with results from other groups. Adusumilli reported that regional intrapleural administration of CAR-Ts in a murine mesothelioma model led to superior results when compared to systemic injection 17. We were able to detect CAR-Ts within peritoneal tumors following IP infusion, whereas CAR-Ts were not present in peritoneal tumors following systemic injection. The benefits of regional CAR-T delivery are also consistent with our pre-clinical and clinical results with hepatic artery CAR-T infusions for LM 5, 7. The advantages of IP CAR-T infusion likely extend beyond anti-tumor efficacy and also include limitation of off-target adverse events, an issue that will require confirmation in phase I studies
Patients with PC are also at risk for progression of disease outside of the peritoneal cavity 18. As such, we determined if IP CAR-T infusions could impact the growth of SC flank tumors in mice with synchronous PC. IP CAR-T infusions were able to significantly limit the growth of distant flank tumors while inducing marked IP responses. As we were unable to detect CAR-Ts within the flank tumors, we speculate that the flank tumor responses were related to IFNγ surges which we detected 4 days following IP CAR-T treatment. IP infusion of CAR-Ts with profound destruction of peritoneal tumors may have induced a phenomenon similar to the abscopal effect seen with radiation therapy 19. Alternatively, CAR-Ts may have infiltrated the flank tumor at earlier time points, which we did not examine. Surprisingly, systemic infusion also did not lead to a meaningful flank tumor response, which may reflect inadequate CAR-T dosing by this route, as most cells likely traffic to nodes, lung, and spleen. Importantly, the response of distant SC tumors to IP therapy was less durable than the response of IP tumors in accordance with the brief surge in serum IFNγ levels. Sequential regional and systemic therapy may offer improvements in efficacy for co-existing PC and extra-abdominal disease. The mechanisms through which IP CAR-T infusions elicit systemic anti-tumor immunity require further investigation.
As PC can have a prolonged natural history, we examined the durability of protection from IP tumor growth following IP CAR-T infusion. Following IP CAR-T treatment, mice were protected from repeat IP tumor challenge for up to 10 additional days. We were able to detect CAR-Ts within the PC as late as 28 days. This finding suggests persistence of CAR-Ts in the peritoneal space, potentially with CAR-Ts acquiring effector memory features. CAR-Ts with an effector memory phenotype (CD44+CD62L−CCR7−) were detected within IP tumors in greater proportion at day 28 compared to day 10. These data suggest that following initial IP infusion, CAR-Ts undergo effector memory programming, which may have accounted for the prolonged anti-tumor protection in the peritoneal space.
Despite effective killing of IP tumors and durable anti-tumor protection, immunosuppressive cells will likely pose additional challenges when translating this approach into clinical use. Development of CAR-T therapies for solid tumors requires potent tumor killing strategies. It will likely be beneficial to couple these strategies with an efficient means for blocking the effects of immunoinhibitory pathways 20-22. In our model, we detected both MDSC and Treg within IP tumors. Both MDSC and Treg have been well described as inhibitors of endogenous T cell and CAR-T anti-tumor responses 23. IP MDSC also expressed high levels of PD-L1, which we previously demonstrated to be an important mediator of CAR-T suppression 7. Addition of MDSC depletion antibody or PD-L1 blocking antibody treatment enhanced IP CAR-T performance in terms of tumor killing. The encouraging additive effects of IP CAR-T and suppressor cell targeting provide justification for combinatorial strategies in developing solid tumor immunotherapy.
The present work sets forth several questions worthy of consideration. The mechanisms underlying the systemic response to IP CAR-T infusion remain to be fully elucidated. While we speculate that it is a systemic cytokine based response, consistent with what we have found in patients receiving hepatic artery CAR-T infusions 6, confirmation is needed. We have shown that the effects of IP CAR-Ts are durable and associated with an increase in effector memory marker expression. The genetic and molecular mechanisms underlying the longevity of the IP CAR-T effect requires deeper examination. Finally, a better understanding of the efficacy of IP CAR-T infusions in our model will benefit from confirmation with other tumor and CAR systems.
PC is a grave condition with dismal clinical outcomes despite aggressive treatments such as CRS-HIPEC 1. Given our group’s experience with regional immunotherapy for colorectal cancer LM 5, 7, we plan to further develop the IP CAR-T approach for translation into phase I testing. Through highly targeted regional IP delivery of CAR-Ts for patients with PC, we speculate that anti-tumor responses will be enhanced while limiting off-target toxicity. Further development of IP CAR-T therapy for PC will likely require combinations with agents capable of blocking immunoinhibitory pathways. Combinatorial strategies, inclusive of regional CAR-T delivery, are promising for overcoming barriers for development of effective solid tumor immunotherapy.
Materials and Methods
Animals
6-8 week old C57Bl/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were used in all in vivo models. 6-8 week old B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice were also purchased from Jackson for the purpose of generating distinguishable CAR-Ts when isolated from tissues ex vivo. Mice were housed in the animal facility at Roger Williams Medical Center in pathogen-free conditions under guidelines from the Institutional Animal Care and Use Committee.
Generation of anti-CEA CAR-Ts
CD45.1 spleens were harvested in sterile fashion then pulverized. Red blood cells were lysed and T cells were isolated using MACS immunomagnetic bead isolation (Miltenyi San Diego, CA, USA). T cells were cultured in complete media with IL-2 (500 IU/mL) and anti-CD3/CD28 T-activator Dynabeads (Life Technologies Franklin, MA, USA) for 48 hours to achieve activation. Phoenix Ecotropic cells harboring a hMN14 sFv-CD8α-CD28/CD3ζ CAR were used to produce supernatant for transduction. Activated T cells were cultured in the retroviral supernatant and underwent two spinfections. Transduced T cells were cultured and expanded in the presence of IL-2 (500 IU/mL), and CAR expression levels were checked 48 hours after transduction.
Cell lines and in vivo work
CEA+ PC was generated by the IP injection of luciferase expressing MC38CEA cells. MC38CEA-luc was generated by transfecting MC38CEA with pLenti-III-UbC-Luciferase (Applied Biological Materials Inc, Richmond, BC Canada). MC38 and MC38CEA cells were a generous gift from Dr. Jeffrey Schlom. Mice were injected with 2.5×106 MC38CEA-luc cells on day 0. Mice were treated IP or via TV with anti-CEA CAR-T (2.5×106 cells) alone or in combination with 10μg anti-PD-L1, anti-GITR, or anti-Gr-1 antibodies. Antibodies were administered along with the first CAR-T injections on day 3, and given every other day until the end of the study. IL-2 (1000IU/injection) was administered on a daily basis beginning with the first CAR-T injection on day 3. In vivo work was carried out over the span of 14 days, with CAR-T injections on days 3 and 6. Groups of control mice were treated with untransduced T cells on days 3 and 6 with IL-2, or treated with IL-2 or antibodies alone. For the rechallenge experiments, mice received CAR-Ts on days 2, 4, 6 and 8, and received a rechallenge dose of MC38CEA-luc on Day 10. For the systemic response experiments, in addition to IP tumors, mice received 1.0×106 MC38CEA-luc cells on the left flank. Flank tumor size was measured in two dimensions (mm2) with calipers. Mice were imaged on an IVIS 100 on even days during in vivo studies, after being injected with 200μL of 15mg/mL luciferin. Serum was isolated from cardiac whole blood on days 4 and 7 for IFNγ ELISA (eBioscience San Diego, CA, USA) to assess systemic immune activity.
CAR-T Killing Assays
Killing assays were performed with either CAR-Ts or untransduced splenic T cells as effectors and MC38CEA-luc as targets. Effectors were cultured in complete media with IL-2 (500 IU/mL) prior to the assays. Cells were plated in complete media in 96 well optical plates at varying E : T ratios and incubated overnight. After incubation, media was discarded and luciferin (150μg/mL) was added to the wells. Plates were analyzed in an IVIS 100. Specific Lysis % was calculated as 100 × [(experimental killing − spontaneous luminescence)/(maximal killing − spontaneous luminescence)].
Antibodies and Flow Cytometry
The Cyan ADP flow cytometer (Beckman Coulter, Indianapolis IN) was used for all flow samples. Antibodies for these surface markers were used for flow cytometry: CD3 (145-2C11, BD Bioscience Franklin Lakes, NJ, USA), CD4 (RM4-5, BD Bioscience), anti-CEA CAR (Wi2, Immunomedics Morris Plains, NJ, USA), CD11b (M1/17, BD Bioscience), Ly6C (AL-21, BD Bioscience), Ly6G (1AB, BD Bioscience), PD-L1 (MIH5, BD Bioscience), CD62L (MEL-14, BD Bioscience), CCR7 (4B12, BD Bioscience), CD44 (IM7, BD Bioscience). Intracellular FoxP3 staining was performed with Mouse FoxP3 Permeabilization Kit (BD Bioscience). Single stain and isotype controls were used for each experiment. Analysis of acquired flow samples was performed with FlowJo software (Tree Star Inc., Ashland OR).
Acknowledgements
This project was made possible by a grant from the National Organization for Rare Disorders (NORD). Additional support for this work was provided by the National Institutes of Health (1K08CA160662-01A1). The authors would like to thank Immunomedics, Inc. for generously providing the Wi2 anti-idiotype antibody to detect CAR expression. We would like to thank Dr. John Morgan and Roger Williams Medical Center Core Facility for providing us with the necessary equipment to carry out flow cytometry and in vivo bioluminescence experiments.
References
- 1.Coccolini F, Gheza F, Lotti M, Virzi S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19(41):6979–94. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 3.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–32. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 4.Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16(8):2152–65. doi: 10.1245/s10434-009-0487-4. [DOI] [PubMed] [Google Scholar]
- 5.Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, et al. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res. 2015;21(14):3149–59. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saied A, Licata L, Burga RA, Thorn M, McCormack E, Stainken BF, et al. Neutrophil:lymphocyte ratios and serum cytokine changes after hepatic artery chimeric antigen receptor-modified T-cell infusions for liver metastases. Cancer Gene Ther. 2014;21(11):457–62. doi: 10.1038/cgt.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer immunology, immunotherapy : CII. 2015;64(7):817–29. doi: 10.1007/s00262-015-1692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saied A, Pillarisetty VG, Katz SC. Immunotherapy for solid tumors--a review for surgeons. The Journal of surgical research. 2014;187(2):525–35. doi: 10.1016/j.jss.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo AS, Ma Q, Liu DL, Junghans RP. Anti-GD3 chimeric sFv-CD28/TCRzeta designer T cells for treatment of melanoma and other neuroectodermal tumors. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0043. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 11.Loskog A, Giandomenico V, Rossig C, Pule M, Dotti G, Brenner MK. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia. 2006;20(10):1819–28. doi: 10.1038/sj.leu.2404366. [DOI] [PubMed] [Google Scholar]
- 12.Eshhar Z. Adoptive cancer immunotherapy using genetically engineered designer T-cells: First steps into the clinic. Curr Opin Mol Ther. 2010;12(1):55–63. [PubMed] [Google Scholar]
- 13.Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer immunology, immunotherapy : CII. 2013;62(7):1137–48. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525–41. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 15.Thorn M, Point GR, Burga RA, Nguyen CT, Joseph Espat N, Katz SC. Liver metastases induce reversible hepatic B cell dysfunction mediated by Gr-1+CD11b+ myeloid cells. Journal of leukocyte biology. 2014;96(5):883–94. doi: 10.1189/jlb.3A0114-012RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JC, Hayman E, Pegram HJ, Santos E, Heller G, Sadelain M, et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 2011;71(8):2871–81. doi: 10.1158/0008-5472.CAN-10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12(1):65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 19.Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res. 2015;3(6):610–9. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer immunology, immunotherapy : CII. 2007;56(5):739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John LB, Devaud C, Duong CM, Yong C, Beavis PA, Haynes NM, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21(9):1065–77. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol. 2013;91(8):493–502. doi: 10.1038/icb.2013.29. [DOI] [PubMed] [Google Scholar]