Abstract
Mineral trioxide aggregate (MTA) is considered at the present time as the gold standard for root-end filling in endodontic surgery. However, this biocompatible material presents several drawbacks such as a long setting time and handling difficulties. The aim of this article is to present a new commercialized calcium silicate-based material named Biodentine with physical improved properties compared to MTA in a clinical application. Two endodontic microsurgeries were performed by using specific armamentarium (microsurgical instrumentation, ultrasonic tips) under high-power magnification with an operatory microscope. Biodentine was used as a root-end filling in order to seal the root canal system. The two cases were considered completely healed at 1 year and were followed for one more year. The 2-year follow-up consolidated the previous observation with absence of clinical symptoms and radiographic evidence of regeneration of the periapical tissues.
Keywords: Biodentin, endodontics, healing, periapical surgery, retrograde filling
Introduction
Among the many factors contributing to the success of endodontic surgery such as case selection and instrumentation technique (use of microscope, type of equipment, etc.), the obturation material plays a critical role. The properties of the ideal root-end filling material are as follows: biocompatibility, promotion of tissue regeneration without causing inflammation, ease of handling, low solubility in tissue fluids, bonding to dental tissue, non-absorbable, dimensional stability, radio-opacity and no staining of surrounding tissues. Numerous materials for root-end filling have been used including amalgam, gutta-percha, zinc oxide-eugenol based cements, glass-ionomer cements, composite resins and silicates. All of these materials have been shown to be compatible with tissue cicatrisation and the reconstitution of periradicular alveolar bone, but none of them is able to induce cementum formation and full periodontal ligament repair. Mineral trioxide aggregate (MTA), a calcium silicate-based material developed by the modification of Portland cement, has been introduced to address this problem and has shown good biocompatibility and sealing properties.1,2,3 Even if studies which evaluate success outcomes do not show statistical differences with the most commonly used root-end fillings, Super ethoxy benzoic acid (SuperEBA; Bosworth, Skokie, IL, USA) or intermediate restorative material (IRM; Denstply de Trey, Konstaz, Germany), this material permits a full regenerative healing which is not the case between Super EBA and IRM.4 For these reasons, MTA can be considered as the material of choice in endodontic surgery. In addition, the sealing properties of MTA are not affected by moisture during treatment.5
However, there are several drawbacks to its use such as its difficult handling properties6 and its long setting time,2,3 which explains why clinicians still favor IRM or Super EBA. Several new calcium silicate-based materials have recently been developed with the aim of improving clinical use and overcoming MTA limitations. One of these materials, Biodentine (Septodont, Saint Maur des Fossés, France) has shown reduced time setting with interesting physical and biological properties as a dentine restorative material7,8,9 and was recently suggested as a pulp-capping material.10
The aim of this article was to report the successful use of Biodentine as a root-end filling in endodontic surgery. Assessment of healing was checked clinically and radiographically for both study cases after 2 years.
Case reports
Indications for endodontic surgery
The two patients reported were referred by their general dental practitioner to the Dental Department of the Groupe Hospitalier Pitié-Salpêtrière (GHPS) in Paris for endodontic surgery. They underwent standard history taking, clinical and radiographic examination, and evaluation of risk/benefits which resulted in the decision to perform endodontic surgery. Patients were fully instructed about the surgical procedure, postoperative care, follow-up examinations and alternative treatment options. They gave written informed consent for the intervention and were included in an ongoing study evaluating the clinical behavior of Biodentine in different situations (#protocol: 2009-A00800-57). The protocol, its amendments and the informed consent form were approved by an Independent Ethics Committee (CPP Ile de France II, Paris).
Description of the cases
The first case concerned a 48-year-old female in good health (ASA 1) referred by her general practitioner after the discovery of a periapical lesion during routine follow-up.
Clinical examination revealed moderate horizontal bone loss (2 mm probing), no pain upon percussion and palpation. The radiographic examination revealed a periapical lesion on tooth #25 (Figure 1a). The tooth was restored with a well-fitting metallic crown and a large post with a metallic core. According to the shape of the root, the existence of an apical isthmus was suspected (Figure 1b).
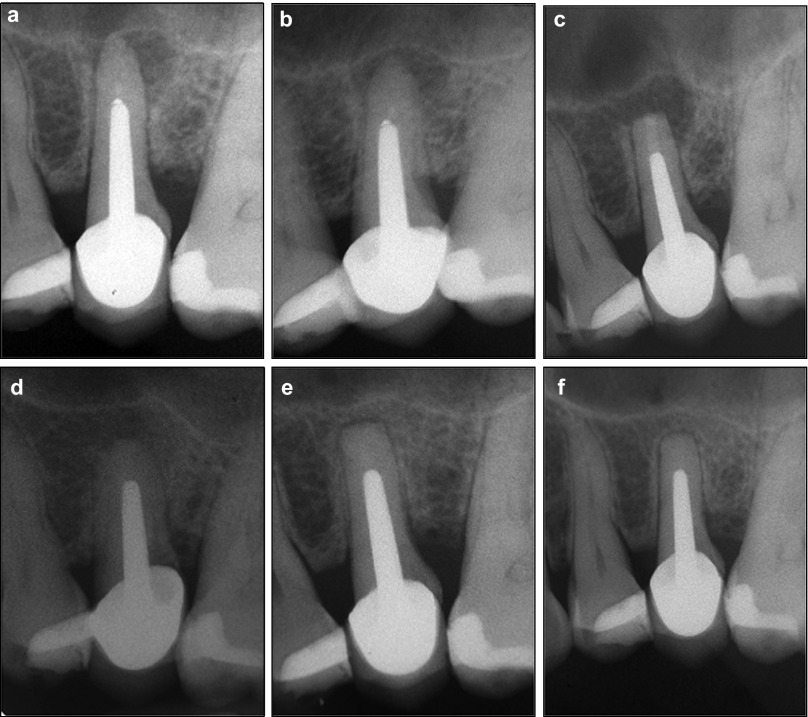
Figure 1.
Case 1. (a) Orthocentric preoperative view. (b) Mesiobuccal preoperative view. (c) Immediate postoperative view. (d) Three months after surgery. (e) One year after surgery. (f) Two years after surgery.
The second case was a 50-year-old female with Crohn's disease (ASA 2) who was being treated by mesalazine 2 g⋅d−1. This pathology was stabilized at the time of consultation and the scientific board of GHPS decided to include the patient in the protocol. Clinical examination showed no periodontal defect. However, pain on percussion, and especially on palpation, were noted. Dental history was recorded: after initial root canal treatment with extrusion of gutta-percha, the patient's general practitioner had re-instrumented the tooth # 25. Despite his attempt, the existing overfilling could not be removed during the orthograde treatment (Figure 2a) and discomfort persisted. A flap was then reflected and only part of the material was removed. A post/core and a temporary crown were fabricated. Due to persistent symptoms, the patient was referred to our department. The radiographic examination revealed a periapical lesion on tooth #25 with overfilling of material in the periapical area (Figure 2b).
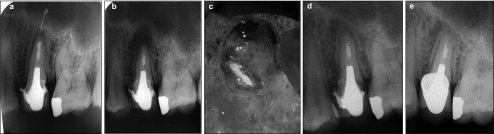
Figure 2.
Case 2. (a) Preoperative view. (b) Immediate postoperative view. (c) View of the retrofilling under optical magnification. (d) Six months after surgery. (e) Two years after surgery.
Surgeries
After disinfection of the teeth and mucosal surfaces by swabbing with an oral antiseptic (chlorhexidine digluconate 0.12%), local anesthetic was given via supraperiosteal infiltration (articaine hydrochloride 4% with epinephrine at 1/100.000, Septodont, France). Sulcular incisions with an additional releasing incision were performed and a full-thickness mucoperiostal flap was reflected. The exposed tissues were moistened with sterile saline throughout the surgical procedure to avoid dehydration of the bone and soft tissues. Apical positions were estimated and an osteotomy was performed at the apical zone of the targeted root. This osteotomy was performed under a continuous saline solution spray using a round tungsten carbide bur mounted on a surgical handpiece. Curettage of granulation tissue was done with a Lucas curette, an endodontic and/or a Gracey or CK6 curette (Hu Friedy, Chicago, IL, USA). Hemostasis was obtained with the assistance of sterile compresses impregnated with ferric sulfate (Astringedent; Ultradent Products, South Jordan, UT, USA). Resection of approximately 3 mm of the apical part of the root was performed perpendicular to the root axis. This resection was performed under a continuous saline solution spray using a fissured tungsten carbide bur. This resection was undertaken carefully with magnification and illumination using an operating microscope (OPMI Pico; Carl Zeiss Surgical GmbH, Oberkochen, Germany). A micro-mirror was used to examine the root-end which was examined for the presence of fissures/cracks. The use of a stain was considered unnecessary since there was no change of coloration. The retro preparation was performed in the long axis of the root using ultrasonic inserts (Apical Surgery set; Satelec, Mérignac, France) to a depth of 3–4 mm.
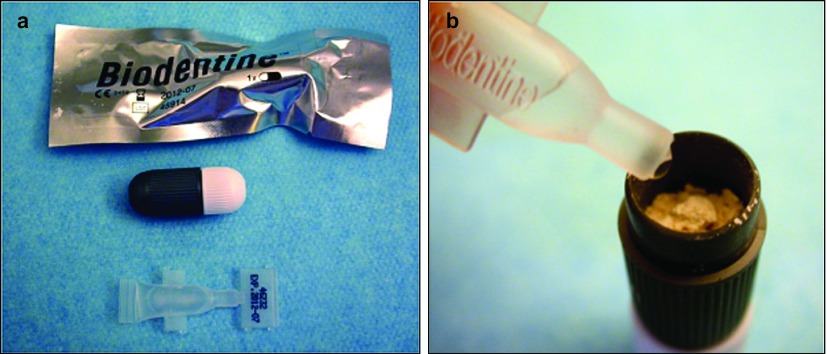
The prepared cavity was dried with sterile paper points and reinspected to verify that the canal walls were free of debris. An assistant prepared the Biodentine (Septodont, Saint Maur des Fossés, France) according to the manufacturer's instructions by pouring the mixing solution into the capsule with the powder (Figure 3). Then the capsule was inserted into a high velocity vibrator (4 000 r⋅min−1) for 30 s.
Figure 3.
Biodentine. (a) Biodentine is commercialized as predosed sets including a capsule containing the powder and a liquid. (b) After pouring the liquid, the capsule must be vibrated at 4 000 r⋅min−1 for 30 s.
The material was then inserted into the cavity using the Microapical Placement System (MAPS) (Produits Dentaires, Vevey, Switzerland) and compacted with a condenser. A series of increments were introduced into the canal and packed so as to fully fill the cavity. A radiograph was taken to double-check the coronal filling level, its adaptation to the root canal walls, and its density. Excess of material at the root canal section was removed with a sterile moistened cotton pellet before the material was allowed to set (<10 min) (Figure 2c). The osseous crypt was flushed with sterile saline solution to eliminate any material residues, and the walls of the osseous crypt were re-curetted for further cleaning and to trigger bleeding. The flap was repositioned and its margins were sutured (vertical mattress suture pattern at the interdental papilla, interrupted sutures at the releasing incision). The primary wound closure of the surgical site was accomplished with multiple interrupted sutures of sizes 5-0 (Ethicon; Johnson & Johnson, Somerville, NJ, USA).
Post-surgery
A postoperative periapical radiograph was taken as a basis for radiological review. The patients were given post-surgery guidelines for care, and prescriptions for antibiotics and analgesics. The posology was as follow: acetaminophen 1 g, every 6 h the first and second day, then if needed; amoxicillin 1 g, bis in die, 7 days; prednisolone 60 mg once daily, 2 days. Amoxicillin and prednisolone were also given 3 h before intervention.
Review appointments were scheduled after 1 week for suture removal and soft tissue healing assessment, then at 3, 6 and 12 months for bone healing assessment.
Results
Radiographic follow-up was done postoperatively, and at 1 month, 3 months, 6 months, 12 months and 24 months (Fig 1c–1f and 2d–2e). The dental radiographs were taken using a Rinn aiming device (Dentsply, Montigny le Bretonneaux, France) with photographic X-ray films (Kodak Ultraspeed, Carestream health).
Each follow-up visit included a patient interview and a clinical examination with an evaluation based on the following parameters: (i) clinically: signs or symptoms of apical periodontitis, in particular: mechanical allodynia, spontaneous pain or dysesthesia, periodontal or alveolar defect identified as early-stage or persistent fistula, periodontal attachment loss identified as early-stage or persistent probable depth >3 mm, root fracture, gingival discoloration, swelling and tooth mobility; (ii) radiographically: 1-year follow-up radiographs were compared with postoperative radiographs to define radiographic periapical healing as complete, incomplete (scar tissue formation), uncertain (some reduction of former radiolucency) or unsatisfactory (no reduction or enlargement of former radiolucency), according to the established criteria.11,12
Both cases were considered to be completely healed at the 1-year follow-up and this was confirmed at the 2-year follow-up evaluation. Clinical examination showed no pain, no dysesthesia, no periodontal defect and no gingival discoloration. Radiographic examination showed complete periapical resolution of the previous periapical radiolucency (Figures 1d–2f and 2d–2e) and reconstitution of an apparently normal-appearing periodontal ligament space. The two cases were then considered to be completely healed.
Discussion
To the best of our knowledge, this is the first report of the use of Biodentine as a material for root-end filling in surgical endodontics. The degree of clinical success observed in the two cases presented here after a 2-year follow-up indicate that this calcium silicate cement can be used successfully in this indication. These results confirm the biological observations of the lack of toxicity and genotoxicity (Ames' test, micronuclei test on human lymphocytes, single-cell gel (Comet assay), immuno-cytochemical detection of human pulp fibroblasts function, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay)8 according to the ISO 10993 standard. In addition to its lack of toxicity, Biodentine displayed bioactivity, i.e., activation of angiogenesis and activation of progenitor pulpal cells promoting healing and remineralization.10 From a biomechanical point of view, the sealing properties of Biodentine have been reported to be superior to MTA.13 The formation of mineral tags13 was similar to those observed with MTA.14,15 along with resistance to acid degradation, as observed in inflammatory sites. The main difference between Biodentine and commercially available MTA calcium silicates is the absence of calcium aluminates and calcium sulfate in the formulation which are known to bring decreased mechanical strength as well as longer setting time.16
The primary clinical advantage of Biodentine is its fast setting (between 12 and 15 min). This is an a advantage when compared to the 170 min of MTA17 since a delayed setting time leads to an increased risk of partial material loss and alteration of the interface during the finishing phases of the procedure (cleaning and rinsing the crypt).3 Another interesting feature of Biodentine is the product packaging in a capsule: Biodentine powder is mainly composed of tricalcium silicate, calcium carbonate and zirconium oxide as a radio-opacifier, while Biodentine liquid contains calcium chloride as the setting accelerator and water-reducing agent. The mixing is achieved by using a capsule mixing device and the ratio powder/liquid is set by the manufacturer. This allows the practitioner to achieve a reproducible material with optimum properties every time.
We found several drawbacks to the use of Biodentine. First, its handling is not as easy as claimed by the manufacturer. Another drawback, common to all silicate-based cements, is the impossibility to create small cones which would be easy to insert into the retro-prepared cavity, as for ZOE or Super EBA cements. It is possible to use the Lee block but the use of a specific carrier like the MAP system makes the procedure easier. The root-end filling was placed as for MTA with several pellets of Biodentine first inserted with the MAPS and then packed with a micro-plugger. We did not use paper points for this procedure in order to avoid modifying the ratio of powder/liquid.
The primary clinical limitation of Biodentine is its low radiopacity. Despite the presence of an X-ray opacifier (zirconium oxide), the radiopacity compares unfavorably with MTA which contains bismuth oxide. This poor radiopacity makes the visualization of the retrograde obturation difficult when small amounts of material are used. With more substantial amounts, the material is more detectable (Figure 1c). In addition, the risk of leaving some material in the bone cavity is enhanced. This drawback originated with the initial indication for Biodentine as a dentine substitute for coronal restoration.9 In the latter indication, the same radiopacity as dentin could be an advantage. On the contrary, the radiopacity is more important in case of retrofilling.
In conclusion, Biodentine is a promising material which is suitable for surgical endodontics, demonstrating excellent biological properties and fast clinical setting time, but with poor radiopacity. Further studies are needed to explore thoroughly its clinical behavior on a long term basis.
Conflicts of interest
Grégory Caron, Jean Azérad, Pierre Machtou and Yves Boucher declare no conflict of interest.
Marie-Odile Faure is an employee of Septodont which commercializes Biodentine.
Acknowledgments
We would like to thank Dr. Morton Sobel for his assistance with the English manuscript.
References
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—Part I: chemical, physical, and antibacterial properties. J Endod 2010; 36(1): 16–27. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review—Part II: leakage and biocompatibility investigations. J Endod 2010; 36(2): 190–202. [DOI] [PubMed] [Google Scholar]
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—Part III: clinical applications, drawbacks, and mechanism of action. J Endod 2010; 36(3): 400–413. [DOI] [PubMed] [Google Scholar]
- Baek SH, Lee WC, Setzer FC et al. Periapical bone regeneration after endodontic microsurgery with three different root-end filling materials: amalgam, SuperEBA, and mineral trioxide aggregate. J Endod 2010; 36(8): 1323–1325. [DOI] [PubMed] [Google Scholar]
- Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod 1999; 25(3): 197–205. [DOI] [PubMed] [Google Scholar]
- Johnson BR. Considerations in the selection of a root-end filling material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 87(4): 398–404. [DOI] [PubMed] [Google Scholar]
- Laurent P, Camps J, About I. BiodentineTM induces TGF-β1 release from human pulp cells and early dental pulp mineralization Int Endod J. 2012; 45(5): 439–448. [DOI] [PubMed] [Google Scholar]
- Laurent P, Camps J, de Méo M et al. Induction of specific cell responses to a Ca3SiO5-based posterior restorative material. Dent Mater 2008; 24(11): 1486–1494. [DOI] [PubMed] [Google Scholar]
- Koubi G, Colon P, Franquin JC et al. Clinical evaluation of the performance and safety of a new dentine substitute, Biodentine, in the restoration of posterior teeth—a prospective study. Clin Oral Investig 2013; 17(1): 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini M, Sautier JM, Berdal A et al. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod 2012; 38(9): 1220–1226. [DOI] [PubMed] [Google Scholar]
- Rud J, Andreasen JO, Jensen JE. Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg 1972; 1(4): 195–214. [DOI] [PubMed] [Google Scholar]
- Molven O, Halse A, Grung B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int J Oral Maxillofac Surg 1987; 16(4): 432–439. [DOI] [PubMed] [Google Scholar]
- Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J 2011; 44(12): 1081–1087. [DOI] [PubMed] [Google Scholar]
- Sarkar NK, Caicedo R, Ritwik P et al. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 2005; 31(2): 97–100. [DOI] [PubMed] [Google Scholar]
- Reyes-Carmona JF, Felippe MS, Felippe WT. Biomineralization ability and interaction of mineral trioxide aggregate and white portland cement with dentin in a phosphate-containing fluid. J Endod 2009; 35(5): 731–736. [DOI] [PubMed] [Google Scholar]
- Li Z. Advanced concrete technology. New York: John Wiley & Sons, 2011: 23–93. [Google Scholar]
- Gandolfi MG, Iacono F, Agee K et al. Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and ProRoot MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108(6): e39–e45. [DOI] [PubMed] [Google Scholar]