Abstract
Chlorogenic acid (5-caffeoylquinic acid, CGA) is a phenolic compound that is found ubiquitously in plants, fruits and vegetables and is formed via the esterification of caffeic acid and quinic acid. In addition to its notable biological functions against cardiovascular diseases, type-2 diabetes and inflammatory conditions, CGA was recently hypothesized to be an alternative for the treatment of neurological diseases such as Alzheimer's disease and neuropathic pain disorders. However, its mechanism of action is unclear. Voltage-gated potassium channel (Kv) is a crucial factor in the electro-physiological processes of sensory neurons. Kv has also been identified as a potential therapeutic target for inflammation and neuropathic pain disorders. In this study, we analysed the effects of CGA on the two main subtypes of Kv in trigeminal ganglion neurons, namely, the IK,A and IK,V channels. Trigeminal ganglion (TRG) neurons were acutely disassociated from the rat TRG, and two different doses of CGA (0.2 and 1 mmol⋅L−1) were applied to the cells. Whole-cell patch-clamp recordings were performed to observe alterations in the activation and inactivation properties of the IK,A and IK,V channels. The results demonstrated that 0.2 mmol⋅L−1 CGA decreased the peak current density of IK,A. Both 0.2 mmol⋅L−1 and 1 mmol⋅L−1 CGA also caused a significant reduction in the activation and inactivation thresholds of IK,A and IK,V. CGA exhibited a strong effect on the activation and inactivation velocities of IK,A and IK,V. These findings provide novel evidence explaining the biological effects of CGA, especially regarding its neurological effects.
Keywords: chlorogenic acid, trigeminal ganglion neuron, voltage-gated potassium channel, whole-cell patch clamp
Introduction
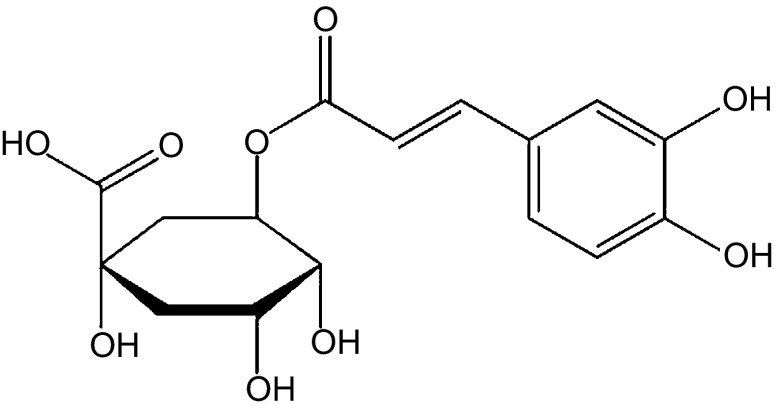
Chlorogenic acid (5-caffeoylquinic acid, CGA), the ester of caffeic acid and quinic acid, is primarily extracted from folium cortex eucommiae and the flower buds of lonicera confuse1 (Figure 1). As a natural organic phenolic compound, CGA is widely found in numerous botanic species.2 As one of the most abundant polyphenol compounds in the human diet, CGA has exhibited various biological effects and therapeutic potential, including antioxidant,3 anticarcinogenic4 and radioprotective5 effects. Novel CGA studies focused on its profound neuroprotective6 and neurotrophic activities.7 In the early 1970s, several groups suggested that CGA has a central-stimulating effect8 and significantly promotes human central nervous excitement.9 Recent studies demonstrated that CGA exhibited protective effects on dopaminergic neurons in neuro-inflammatory conditions associated with Alzheimer's disease.10 However, the mechanism underlying the favourable effects of CGA is largely unknown. One possible explanation linked to its oxidant function is that pure CGA suppresses the release of NO from LPS/IFN-γ-stimulated C6 astrocyte cells, which are crucial mediators in the physiological process of pain.1 These experiments were also performed in animal behaviour models to analyse the analgesic effects of CGA on the nervous system.
Figure 1.
Chemical structure of CGA. CGA, chlorogenic acid.
Voltage-gated potassium channels (Kvs) are the key physiological regulators of membrane potential in sensory neurons. Trigeminal ganglion (TRG) neurons express two distinct classes of Kv currents, including the dominant sustained K-current (IK,V) and the fast inactivating transient A-current (IK,A).11,12 The IK,A channels belong to the Kv 1.4 family13 and contribute to neuronal repolarisation and repetitive firing.14 The inhibition of this type of channel leads to hyper-excitability and hyperalgesia.15 Because Kv 1.4 channels are expressed in the small-diameter (Aδ-, C-fibres) neurons in the dorsal root ganglion,16 IK,A plays a significant role in regulating the activity of nociceptive neurons.13 By contrast, IK,V channels, which also regulate repetitive firing, are activated with a threshold potential that is more positive than that required for IK,A channels to exhibit delayed long-lasting activation.15 IK,A and IK,V can be pharmacologically isolated from whole-cell potassium channel currents due to their different sensitivities to 4-aminopyridine and tetraethylammonium.17 For example, 3 mmol⋅L−1 4-aminopyridine in the extracellular solution demonstrates a preferable inhibition of IK,A compared with IK,V, whereas 70 mmol⋅L−1 tetraethylammonium exhibits the opposite.11 Kv malfunctions contribute to neuronal excitability disorders in various pathologic conditions, such as epilepsy, chronic pain, autism, migraine and multiple sclerosis.11
Given that Kv opening leads to cell membrane hyperpolarisation and a subsequent decrease in cell excitability, several Kv subtypes have been proposed as potential target candidates for pain therapy.18 Additionally, CGA is a novel candidate for the treatment of neuropathic pain. In this study, we determined whether CGA could alter the electrophysiological characteristics of IK,A and IK,V in rat TRG neurons in vivo. Our results provide new evidence explaining the antihyperalgesic effects of CGA, especially in the oral and maxillofacial regions.
Materials and methods
Acute dissociation of TRG neurons
All animal procedures were reviewed and approved by the State Key Laboratory of Oral Diseases, Sichuan University. Rat TRG isolation and neurons dissociation were described in our previous reports.11,19 Briefly, bilateraltrigeminal ganglia were isolated from neonatal (3–5 days) Sprague–Dawley rats that were anaesthetized with ether. The extracted TRG were washed using ice-cold Hanks' balanced salt solution (pH=7.4; Sigma-Aldrich China, Shanghai, China), minced into small pieces under a dissecting microscope and incubated in Hanks' balanced salt solution containing 25 U⋅mL−1 papain (Sigma-Aldrich China, Shanghai, China) at 37 °C for 40 min. After appropriate digestion, the cells were washed thrice with DMEM/F12 culture medium (1∶1 volume; Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% foetal bovine serum (Gibco, Life Technologies, Carlsbad, CA, USA). Then, the cells were gently triturated using a series of fire-polished Pasteur pipettes and plated on poly-L-lysine (Sigma-Aldrich China, Shanghai, China)-coated glass coverslips placed in 35 mm dishes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The dishes were maintained in a humidified atmosphere of 5% CO2 at 37 °C for 2 h prior to the measurements. All TRG neurons could be distinguished by their distinct larger figures and surrounding halo. The neurons could also be further identified based on Nissl substance stain with cresyl echt violet and electrophysiological characteristics in patch-clamp recordings.
Whole-cell patch-clamp recordings
Prior to the patch-clamp recordings, the culture medium in the dishes was carefully removed, and the cells were washed thrice with an external solution. The external solution for the IK,A current recordings contained 5 mmol⋅L−1 KCl, 2 mmol⋅L−1 CaCl2, 1 mmol⋅L−1 MgCl2, 70 mmol⋅L−1 tetraethylammonium (Sigma-Aldrich China, Shanghai, China), 70 mmol⋅L−1 choline-Cl, 10 mmol⋅L−1 d-glucose, 10 mmol⋅L−1 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 0.1 mmol⋅L−1 CdCl2 (pH=7.4). For the IK,V recordings, the solution contained 5 mmol⋅L−1 KCl, 2 mmol⋅L−1 CaCl2, 1 mmol⋅L−1 MgCl2, 3 mmol⋅L−1 4-aminopyridine (Sigma-Aldrich China, Shanghai, China), 137 mmol⋅L−1 choline-Cl, 10 mmol⋅L−1 d-glucose, 10 mmol⋅L−1 HEPES and 0.1 mmol⋅L−1 CdCl2 (pH=7.4). Finally, the external solution volume in the dishes was adjusted to 2 mL. Patch-clamp pipettes were pulled from borosilicate glass and filled with an internal solution composed of 120 mmol⋅L−1 potassium methanesulphonate, 20 mmol⋅L−1 KCl, 7.5 mmol⋅L−1 HEPES and 2 mmol⋅L−1 ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA) (pH=7.3) for both the IK,A and IK,V recordings. The mean resistance of the electrodes was 2–4 MΩ. The whole cell recordings were conducted using an Axopatch 200B patch-clamp amplifier (Axon Instruments, Union City, CA, USA), and the output was digitized with the Digidata 1440A converter (Axon Instruments, Union City, CA, USA). Both the capacitance and series resistance were well compensated. All data were acquired using Clampex 10.0 software (Axon Instruments , Union City, CA, USA). All recordings above were performed at a conditioned temperature of 25–26 °C.
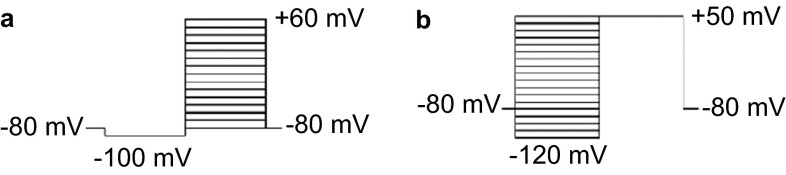
Activation and inactivation currents of Kv subtypes were generated using various stimulus protocols. After achieving a giga-ohm seal between the cell membrane and patch pipette, neurons were initially held at −80 mV followed by hyperpolarisation to −80 mV for 70 ms as a conditioning prepulse potential. The inward K+ activation currents were elicited using 100-ms pulses stepping from −80 mV to +70 mV in 10-mV increments (Figure 2a). The inactivation properties of the IK,A and IK,V were studied using another stimulus protocol. The neurons held at −80 mV were subject to a series of 250-ms prepulses stepping from −120 mV to +50 mV followed by a 250-ms test pulse depolarising to +50 mV (Figure 2b).
Figure 2.
The stimulus protocols of IK,A and IK,V. (a) The activation protocol, 100-ms pulses stepping from −80 to +70 mV in 10-mV increments. (b) The inactivation protocol, 250-ms pre-pulses stepping from −120 to +50 mV in 10-mV increments.
CGA delivery
Purified CGA was directly dissolved to a final concentration in the extracellular solution and then added to the 35-mm dishes via gravity through a bath perfusion apparatus integrated into the patch-clamp system. Bath solution perfusion was maintained at a rate of 1 mL⋅min−1. The perfusion pipette tip was extended as close as possible towards the target neuron without any unfavourable interference with the electrode sealing. All patch-clamp recordings were initiated 30 s after the perfusion began.
Data analysis
For IK,A and IK,V, current densities were obtained by dividing the peak currents with their own whole-cell capacitances. The channel conductance (G) at various membrane potentials was calculated using the following equation:
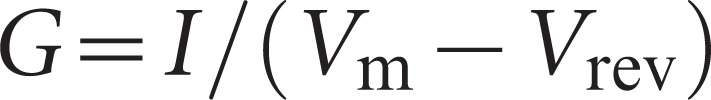 |
where I represents the current density, Vm represents the voltage command and Vrev represents the reversal potential. Normalized activation curves were plotted as G/Gmax against the voltage commands. The curves of all groups were fit to a Boltzmann equation,
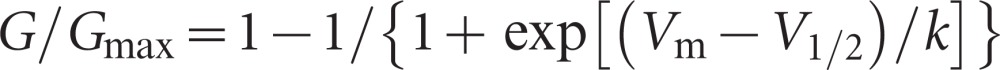 |
where Vm represents the voltage command, V1/2 represents the membrane potential at half activation and k represents the slope factor. Their inactivation curves were fit to another Boltzmann equation,
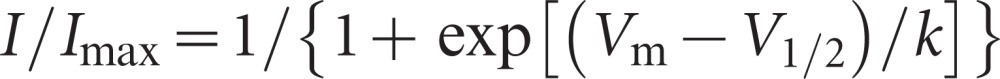 |
where V1/2 represents the membrane potential at half inactivation at this time.
The data were analysed using Clampfit 10.0 software (Axon Instruments, Union City, CA, USA). All curve fittings and statistical comparisons were performed with Origin 9.0 software (OriginLab, Northampton, MA, USA). Differences were considered to be significant at P<0.05.
Results
The small and medium ‘nociceptive' TRG and dorsal root ganglion neurons and associated Aδ- and C-fibre afferents are critical for detecting noxious stimuli and initiating pain sensation.20 Therefore, TRG neurons ranging from 15 to 45 µm in diameter were selected for further recordings. Finally, 22 and 18 TRG neurons were randomly selected to obtain the IK,A (11 cells each for the 0.2 and 1.0 mmol⋅L−1 CGA groups) and IK,V current recordings (9 cells each for the 0.2 and 1.0 mmol⋅L−1 CGA groups), respectively. For every TRG neuron, two complete Kv currents were recorded prior to and after treatment with CGA. In this study, we found that Kv on TRG neurons initiated activation when the membrane potential was depolarized to approximately −60 mV; the largest current densities were achieved for all four groups at approximately +70 mV.
Effects of the CGA on IK,A and IK,V activation
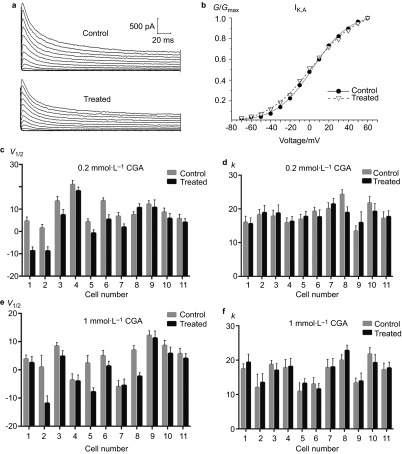
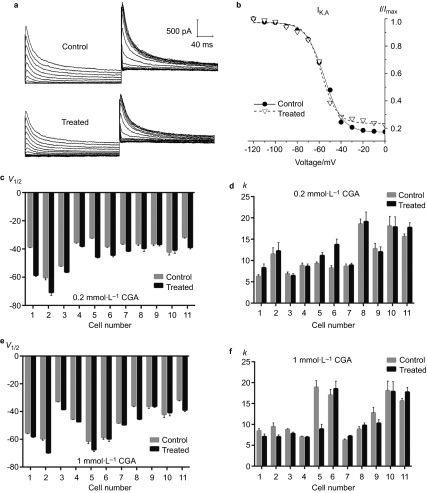
For IK,A, the Boltzmann fitting results demonstrated that both 0.2 and 1 mmol⋅L−1 CGA caused a significant reduction in the peak current densities and a shift of the activation curves towards a more re-polarisation direction compared with the control group (Figure 3a and 3b). However, no significant differences were noted regarding the change in V1/2 between the 0.2 and 1 mmol⋅L−1 CGA groups (Figure 3c and 3e). More diverse results were obtained for the k value. For the 0.2 mmol⋅L−1 CGA group, 63.4% of the TRG neurons exhibited an increase, whereas the remaining neurons exhibited a decrease. These findings led to an overall unchanged result. In the 1.0 mmol⋅L−1 CGA group, 72.7% of the cells exhibited an increased k value, which resulted in a similar overall effect (Figure 3d and 3f).
Figure 3.
The effects of 0.2 and 1 mmol⋅L−1 CGA on the activation of IK,A. (a) Typical examples of IK,A activation in the 0.2 mmol⋅L−1 CGA treatment and control groups. (b) Normalized IK,A activation curves in the 0.2 mmol⋅L−1 CGA treatment and control groups. (c, d) Comparison of the fitted results of IK,A activation currents between the 0.2 mmol⋅L−1 CGA and control groups. (e, f) Comparison of the fitted results of IK,A activation currents between the 1 mmol⋅L−1 CGA and control groups. CGA, chlorogenic acid.
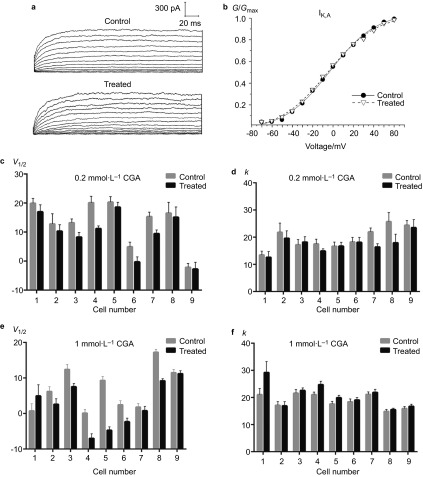
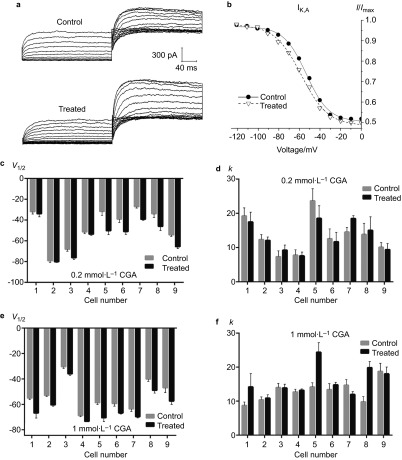
Although no obvious change on the peak current density of IK,V was noted, the effects of CGA on the V1/2 of IK,V were generally consistent with those noted for IK,A given that both the 0.2 and 1 mmol⋅L−1 CGA groups decreased significantly (Figure 4c and 4e). Nevertheless, the general effects of 0.2 and 1 mmol⋅L−1 CGA on the k value were in opposition. In the 0.2 CGA group, k values in 88.9% of the TRG neurons exhibited a decrease, whereas the changes caused by 1 mmol⋅L−1 CGA were not statistically significant (Figure 4d and 4f).
Figure 4.
The effects of 0.2 and 1 mmol⋅L−1 CGA on the activation of IK,V. (a) Typical examples of IK,V activation in the 0.2 mmol⋅L−1 CGA and control groups. (b) Normalized IK,V activation curves in the 0.2 mmol⋅L−1 CGA and control groups. (c, d) Comparison of the fitted results of IK,V activation currents between the 0.2 mmol⋅L−1 CGA and control groups. (e, f) Comparison of the fitted results of the IK,V activation currents between the 1 mmol⋅L−1 CGA and control groups. CGA, chlorogenic acid.
The above results imply that treatment with 0.2 mmol⋅L−1 and 1 mmol⋅L−1 CGA produce similar effects by activating both IK,A and IK,V channels at a lower threshold. Given that the k value indicates the activation velocity, 1 mmol⋅L−1 CGA enables a more rapid activation of the IK,A channels, whereas 0.2 mmol⋅L−1 CGA resulted in slower IK,V channel activation.
Effects of CGA on IK,A and IK,V inactivation
With regard to the inactivation characteristics of IK,A, both 0.2 and 1 mmol⋅L−1 CGA induced a significant reduction in V1/2 (Figure 5c and 5e). No significant differences were noted between the 0.2 and 1 mmol⋅L−1 CGA groups. However, the two CGA doses resulted in opposite effects for the k values (Figure 5d and 5f). With 0.2 mmol⋅L−1 CGA, approximately 63.4% of the TRG neurons exhibited an increase. With 1.0 mmol⋅L−1 CGA, only 36.4% demonstrated a similar outcome.
Figure 5.
The effects of 0.2 and 1 mmol⋅L−1 CGA on IK,A inactivation. (a) Typical examples of IK,A inactivation in the 0.2 mmol⋅L−1 CGA and control groups. (b) Normalized inactivation curves of IK,A in the 0.2 mmol⋅L−1 CGA and control groups. (c, d) Comparison of the fitted results of the IK,A inactivation currents between the 0.2 mmol⋅L−1 CGA and control groups. (e, f) Comparison of the fitted results of the IK,A inactivation currents between the 1 mmol⋅L−1 CGA and control groups. CGA, chlorogenic acid.
For IK,V, the V1/2 values also decreased with both 0.2 and 1 mmol⋅L−1 CGA (Figure 6c and 6e). However, no significant alteration in the k value was observed when the TRG neurons were treated with 0.2 or 1 mmol⋅L−1 CGA (Figure 6d and 6f).
Figure 6.
The effects of 0.2 and 1 mmol⋅L−1 CGA on IK,V inactivation. (a) Typical examples of IK,V inactivation in the 0.2 mmol⋅L−1 CGA and control groups. (b) Normalized inactivation curves of IK,V in the 0.2 mmol⋅L−1 CGA and control groups. (c, d) Comparison of the fitted results of the IK,V inactivation currents between the 0.2 mmol⋅L−1 CGA and control groups. (e, f) Comparison of the fitted results of the IK,V inactivation currents between the 1 mmol⋅L−1 CGA and control groups. CGA, chlorogenic acid.
The results above indicated that both 0.2 and 1 mmol⋅L−1 CGA decreased the inactivation threshold of IK,A and IK,V. However, the speed of inactivation appears to increase at a lower CGA concentration and decrease with a higher CGA concentration.
Discussion
Multiple types of voltage-gated ion channels constitute the structure and function basis of neuronal excitability in which Kv plays an important role in maintaining the membrane potential, regulating the action potential phase and controlling the firing capacity.18 At least two main subtypes of Kv are present on peripheral sensory neurons (such as dorsal root ganglion and TRG neurons): the fast-inactivating transient IK,A and dominant-sustained IK,V.11 IK,A and IK,V channels give rise to two distinct channel currents, respectively. Each Kv subtype is composed of distinct subunit combinations, and the Kv 1.4, 4.2 and 4.3 subunits are involved in the formation of IK,A and IK,V channels in sensory neurons.16,21 Among these, Kv 1.4 is the prominent candidate for a nociceptive interference given that it is highly expressed in small-diameter TRG neurons.22 Additionally, the Kv 1.4 family may also directly contribute to the regulation of C-fibre conduction and is particularly important in the treatment of pain.16 Reduced Kv 1.4 subunit expression in both myelinated (Aδ-fibre type) and unmyelinated (C-fibre type) neurons evokes the hyperactivity of small-diameter TRG neurons.23
Kv was recently considered to be the key factor involved in nociceptive signal transduction induced via inflammatory mediators and nerve damage in primary sensory neurons. Takeda et al.22 found that the excitability of rat TRG neurons was enhanced via the decrease of IK,A in temporomandibular joint inflammation. Harriott et al.24 also demonstrated that the excitability of masseter muscle afferents was increased by inhibiting Kv in inflammation conditions. The underlying mechanism implicated various inflammation-related cytokines and neurotransmitters that reduced Kv densities on neurons. Because IK,A and IK,V play different roles in regulating the firing frequency and duration of action potentials in the TRG neurons, alterations in the Kv subtypes cause an incremental spike discharge and prolonged duration of action potentials in neuropathic or inflammatory pain models. Secondary chain reactions are also elicited during such prolonged durations, including alterations in the amount of neurotransmitter release, the opening of voltage-gated Ca2+ channels and the triggering of the paracrine or autocrine mechanism of neighbouring TRG neurons. Therefore, recent studies have focused on IK,A and IK,V as potential therapeutic targets for trigeminal neuropathic and inflammatory pain.13,16,25,26,27,28
In our experiment, the activation and inactivation currents of both IK,A and IK,V were significantly shifted toward depolarisation upon CGA treatment, which implies that Kv is triggered at a lower threshold with a prolonged duration. Thus, the present study was the first to demonstrate that CGA enhances Kv activities both in IK,A and IK,V channels in rat TG neurons; this would gradually decrease the excitability of neurons in trigeminal hyperalgesic conditions in neuropathic and inflammatory pain.29,30,31
CGAs have been associated with multiple biological effects in recent years, including the reduction of the relative risks of cardiovascular disease and type-2 diabetes as well as antibacterial and anti-inflammatory functions.32 Recent CGA research focused on its application in neurology. In the central neural system, CGA has been considered as a potential drug to treat Alzheimer's disease33,34,35 given that CGA represses NO and TNF-α release in LPS-stimulated primary microglia, which subsequently prevented neurotoxicity caused by microglial activation.6 Additionally, CGA exhibits neuroprotective properties in Alzheimer's disease treatment by inhibiting acetylcholinesterase and butyrylcholinesterase activities, as well as preventing oxidative stress-induced neurodegeneration.10 CGA also exhibits inhibitory effects on peripheral synthesis and the release of certain inflammatory mediators such as TNF-α, NO and several interleukins.1,6,36
Interestingly, CGA is also implicated in the modulation of Kv activity;37,38,39,40 however, the underlying mechanism remains largely unknown. In our study, the activation and inactivation currents of both IK,A and IK,V were significantly shifted to the depolarisation direction upon CGA treatment, which implied that Kv is triggered at a lower threshold with a prolonged duration. Thus, the present study was the first to demonstrate that CGA enhances Kv activities both in IK,A and IK,V channels in rat TG neurons, thereby gradually decreasing the excitability of neurons in trigeminal hyperalgesic conditions in neuropathic and inflammatory pain.29,30,31
Acknowledgments
We thank Ms Da-Qing Liao, Ms Yan-Fang Chen and Ms Xiao-Yu Li for their technical assistance. This study was supported by the National Science Foundation of China (Grant No. 81000456) and the Science and Technology Department of Sichuan Province (Grant No. 2009SZ0171).
References
- dos Santos MD, Almeida MC, Lopes NP et al. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull 2006; 29(11): 2236–2240. [DOI] [PubMed] [Google Scholar]
- Bagdas D, Cinkilic N, Ozboluk HY et al. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J Nat Med 2013; 67(4): 698–704. [DOI] [PubMed] [Google Scholar]
- Feng R, Lu Y, Bowman LL et al. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J Biol Chem 2005; 280(30): 27888–27895. [DOI] [PubMed] [Google Scholar]
- Kang TY, Yang HR, Zhang J et al. The studies of chlorogenic acid antitumor mechanism by gene chip detection: the immune pathway gene expression. J Anal Methods Chem 2013; 2013: 617243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cinkilic N, Cetintas SK, Zorlu T et al. Radioprotection by two phenolic compounds: chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem Toxicol 2013; 53: 359–363. [DOI] [PubMed] [Google Scholar]
- Shen W, Qi R, Zhang J et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res Bull 2012; 88(5): 487–494. [DOI] [PubMed] [Google Scholar]
- Ito H, Sun XL, Watanabe M et al. Chlorogenic acid and its metabolite m-coumaric acid evoke neurite outgrowth in hippocampal neuronal cells. Biosci Biotechnol Biochem 2008; 72(3): 885–888. [DOI] [PubMed] [Google Scholar]
- Hach B, Heim F. [Comparative studieson the central stimulating effects of caffeie and chlorogenic acid in white mice.] Arzneimittelforschung 1971; 2: 23–25. German. [PubMed] [Google Scholar]
- Ammon HP, Künkel H. [Significance of chlorogenic acid in the centrally-stimulating effect of coffee.] Dtsch Med Wochenschr 1976; 101(12): 460–464. German. [DOI] [PubMed] [Google Scholar]
- Oboh G, Agunloye OM, Akinyemi AJ et al. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer's disease and some pro-oxidant induced oxidative stress in rats' brain-in vitro. Neurochem Res 2013; 38(2): 413–419. [DOI] [PubMed] [Google Scholar]
- Shen JF, Chao YL, Du L. Effects of static magnetic fields on the voltage-gated potassium channel currents in trigeminal root ganglion neurons. Neurosci Lett 2007; 415(2): 164–168. [DOI] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Matsumoto S. Inflammation enhanced brain-derived neurotrophic factor-induced suppression of the voltage-gated potassium currents in small-diameter trigeminal ganglion neurons projecting to the trigeminal nucleus interpolaris/caudalis transition zone. Neuroscience 2014; 261: 223–231. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M et al. Opioidergic modulation of excitability of rat trigeminal root ganglion neuron projections to the superficial layer of cervical dorsal horn. Neuroscience 2004; 125(4): 995–1008. [DOI] [PubMed] [Google Scholar]
- Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol Lond 2003; 552(Pt 3): 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RJ, Duchen MR. Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience 1994; 63(4): 1041–1056. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW et al. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 2001; 98(23): 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao L, Lee H, Li HY et al. Mechanosensitivity of voltage-gated K+ currents in rat trigeminal ganglion neurons. J Neurosci Res 2006; 83(7): 1373–1380. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tsuboi Y, Kitagawa J et al. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol Pain 2011; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Wang H, Ma Y et al. [Effects of intracellular osmolality changes on the voltage-gated sodium channels currents of trigeminal ganglion neuron.] Hua Xi Kou Qiang Yi Xue Za Zhi 2012; 30(4): 338–342. Chinese. [PubMed] [Google Scholar]
- Hampson RE, Evans GJ, Mu J et al. Role of cyclic AMP dependent protein kinase in cannabinoid receptor modulation of potassium “A-current” in cultured rat hippocampal neurons. Life Sci 1995; 56(23/24): 2081–2088. [DOI] [PubMed] [Google Scholar]
- Winkelman DL, Beck CL, Ypey DL et al. Inhibition of the A-type K+ channels of dorsal root ganglion neurons by the long-duration anesthetic butamben. J Pharmacol Exp Ther 2005; 314(3): 1177–1186. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Ikeda M et al. Temporomandibular joint inflammation potentiates the excitability of trigeminal root ganglion neurons innervating the facial skin in rats. J Neurophysiol 2005; 93(5): 2723–2738. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Nasu M et al. Temporomandibular joint inflammation decreases the voltage-gated K+ channel subtype 1.4-immunoreactivity of trigeminal ganglion neurons in rats. Eur J Pain 2008; 12(2): 189–195. [DOI] [PubMed] [Google Scholar]
- Harriott AM, Dessem D, Gold MS. Inflammation increases the excitability of masseter muscle afferents. Neuroscience 2006; 141(1): 433–442. [DOI] [PubMed] [Google Scholar]
- Takeda M, Kitagawa J, Nasu M et al. Glial cell line-derived neurotrophic factor acutely modulates the excitability of rat small-diameter trigeminal ganglion neurons innervating facial skin. Brain Behav Immun 2010; 24(1): 72–82. [DOI] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Nasu M et al. Peripheral inflammation suppresses inward rectifying potassium currents of satellite glial cells in the trigeminal ganglia. Pain 2011; 152(9): 2147–2156. [DOI] [PubMed] [Google Scholar]
- Takeda M, Kitagawa J, Takahashi M et al. Activation of interleukin-1beta receptor suppresses the voltage-gated potassium currents in the small-diameter trigeminal ganglion neurons following peripheral inflammation. Pain 2008; 139(3): 594–602. [DOI] [PubMed] [Google Scholar]
- Liu CY, Li N, Zhao YF et al. [BK(Ca) channel agonist NS1619 and Kv channel antagonist 4-AP on the facial mechanical pain threshold in a rat model of chronic constriction injury of the infraorbital nerve.] Sheng Li Xue Bao 2010; 62(5): 441–449. Chinese. [PubMed] [Google Scholar]
- Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol 1999; 82(2): 700–708. [DOI] [PubMed] [Google Scholar]
- Birinyi-Strachan LC, Gunning SJ, Lewis RJ et al. Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol Appl Pharmacol 2005; 204(2): 175–186. [DOI] [PubMed] [Google Scholar]
- Harriott AM, Gold MS. Contribution of primary afferent channels to neuropathic pain. Curr Pain Headache Rep 2009; 13(3): 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah A, Monteiro M, Donangelo CM et al. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J Nutr 2008; 138(12): 2309–2315. [DOI] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D et al. Inflammation in neurodegenerative diseases. Immunology 2010; 129(2): 154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 2007; 8(1): 57–69. [DOI] [PubMed] [Google Scholar]
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 2005; 76(2): 77–98. [DOI] [PubMed] [Google Scholar]
- Chauhan PS, Satti NK, Sharma P et al. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phytother Res 2012; 26(8): 1156–1165. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Erdjument-Bromage H, Ferris CD et al. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 2001; 3(2): 193–197. [DOI] [PubMed] [Google Scholar]
- Ahern GP, Klyachko VA, Jackson MB. cGMP and S-nitrosylation: two routes for modulation of neuronal excitability by NO. Trends Neurosci 2002; 25(10): 510-517. [DOI] [PubMed] [Google Scholar]
- Jiang N, Shi P, Desland F et al. Interleukin-10 inhibits angiotensin II-induced decrease in neuronal potassium current. Am J Physiol Cell Physiol 2013; 304(8): C801–C807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schäfers M et al. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett 2008; 434(3): 293–298. [DOI] [PubMed] [Google Scholar]