Abstract
ErbB signaling through extracellular signal-regulated kinase (ERK) has been implicated in regulating the expression of ErbB ligands in hyperproliferative skin disorders and wound healing. Here, we characterize the process of autocrine ERK activation in cultured normal human keratinocytes (NHKs) subjected to growth factor (GF) deprivation. Basal ERK phosphorylation was lower after 48 h than after 24 h of GF deprivation, and lowest at 30–60 min after an additional medium change. ERK phosphorylation was markedly increased by low concentrations of epidermal growth factor (EGF) (0.2–1 ng/ml) that provoked only a limited increase in ErbB1 tyrosine phosphorylation and internalization. Basal ErbB tyrosine phosphorylation and ERK phosphorylation were inhibited by two different ErbB receptor tyrosine kinase inhibitors, by the ErbB1-specific neutralizing monoclonal antibody 225 IgG, by two different metalloproteinase inhibitors, and by neutralizing antibodies against amphiregulin (AR). In contrast, these responses were unaffected by neutralizing antibodies against other ErbB1 ligands or the ErbB2 inhibitors geldanamycin and AG825. The time course of autocrine ERK phosphorylation correlated with the appearance of soluble AR, and two different metalloproteinase inhibitors blocked AR release. These results define an amphiregulin- and ErbB1-dependent mechanism by which autocrine ERK activation is maintained in NHKs, even when ErbB1 autophosphorylation and internalization are limited.
INTRODUCTION
The mammalian c-ErbB family is comprised of four closely related receptor tyrosine kinases (RTKs) that interact hierarchically in response to multiple ErbB receptor ligands (Klapper et al., 2000; Olayioye et al., 2000). Ligand binding to the extracellular domain promotes receptor homo- and heterodimerization, resulting in phosphorylation of specific tyrosine residues on the cytoplasmic domain. These events lead to activation of multiple signal transduction pathways via Src homology 2 (SH2)- and phosphotyrosine binding (PTB)-domain containing cytoplasmic proteins, ultimately affecting many cellular functions, including cell migration, proliferation, and differentiation (Hubbard et al., 1998; Hackel et al., 1999). We and others have demonstrated that human skin expresses ErbB1, ErbB2, and ErbB3, but little or no ErbB4 (Press et al., 1990; Prigent et al., 1992; De Potter et al., 2001; Stoll et al., 2001).
ErbB signaling plays a very important role in the reepithelialization of skin wounds, as evidenced by acceleration of burn or partial-thickness wound healing by epidermal growth factor (EGF) (Brown et al., 1989), transforming growth factor-α (TGF-α) (Schultz et al., 1987), heparin-binding EGF-like growth factor (HB-EGF) (Cribbs et al., 1998), and epiregulin (Draper et al., 2003). Corneal wound healing also is markedly inhibited after treatment with ErbB receptor tyrosine kinase inhibitors (RTKIs) (Nakamura et al., 2001). Organ cultures of skin display many features of early wounds, including rapid keratinocyte cytoskeletal alterations, an early migratory phase without proliferation, and a later proliferative phase (Hebda, 1988; Varani et al., 1995b; Stoll et al., 2003). We have demonstrated marked inhibition of keratinocyte outgrowth and the induction of apoptosis by ErbB-specific RTKIs in human skin organ culture (Stoll et al., 1997, 1998).
Multiple ErbB ligands are expressed by keratinocytes, including TGF-α, AR, HB-EGF, betacellulin, and epiregulin (Coffey et al., 1987; Barnard et al., 1994; Hashimoto et al., 1994; Piepkorn et al., 1998, 2003; Shirakata et al., 2000). Several of these ligands are up-regulated during wound healing (Grotendorst et al., 1989; McCarthy et al., 1996; Stoll et al., 1997), in hyperproliferative skin conditions such as psoriasis (Elder et al., 1989; Cook et al., 1992; Downing et al., 1997; Stoll and Elder, 1998) and retinoid-treated skin (Stoll and Elder, 1998; Varani et al., 2001), and during malignant transformation by chemical carcinogens (Kiguchi et al., 1998) or oncogenes (Dlugosz et al., 1995). Using the skin organ culture system, we have shown that the induction of HB-EGF and amphiregulin (AR) expression during wounding is strongly dependent on ErbB signaling (Stoll et al., 1997), and also on downstream signaling through the extracellular signal-regulated kinase (ERK) pathway (Stoll et al., 2002).
Cultured normal human keratinocytes (NHKs) display many features of reepithelializing skin, including high rates of proliferation, active migration, and the expression of keratins such as K6 and K16. Proliferation and migration of NHKs are strongly dependent on ErbB signaling (Cook et al., 1991b; Klein et al., 1992; McCawley et al., 1998). Conditioned medium (CM) produced by NHKs stimulates DNA synthesis in a way that can be partially blocked with antibodies against various ErbB ligands and strongly blocked by antibodies against ErbB1 (Coffey et al., 1987; Cook et al., 1991a; Pittelkow et al., 1993, 1994; Hashimoto et al., 1994; Shirakata et al., 2000). Moreover, we and others have observed that it is very difficult to obtain proliferative quiescence in NHKs by removal of growth factors (GFs) (Coffey et al., 1987; Klein et al., 1992; Praskova et al., 2002). Together, these findings are strongly suggestive of an autocrine mechanism of ErbB activation in NHKs. However, the exact ErbB species and downstream effectors being activated by this pathway, the ligands involved, and the mechanism(s) by which ligand(s) are made available for receptor stimulation remain incompletely defined.
Using the skin organ culture system, we have shown that broad-spectrum metalloproteinase (MP) inhibitors markedly reduce ERK phosphorylation (Stoll et al., 2002). These experiments suggest that the basal activity of ERK in human epidermis is controlled by release of preformed, membrane-associated ErbB ligand precursors, which are able to stimulate ErbB1 upon proteolytic release, leading to ERK activation. In this report, we further investigate the mechanisms responsible for the maintenance of autocrine ErbB signaling in NHKs. Our results demonstrate that AR released by one or more MPs acts exclusively over ErbB1 to maintain basal levels of ERK phosphorylation in GF-depleted NHK. Our findings illuminate a distinctive autocrine behavior of NHKs that is likely to be of importance in wound healing and hyperproliferative skin conditions.
MATERIALS AND METHODS
Reagents
Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal antihuman ErbB1 antibodies (mAbs) were purchased from BD Transduction Laboratories (Lexington, KYl clone 13) and from Labvision (Freemont, CA; Ab2, Ab-13, and Ab-14). mAbs to ErbB2 were from Labvision (Ab-17 and Ab-2) and BD Transduction Laboratories (clone 42). Anti-phosphotyrosine mAb 4G10 and horseradish peroxidase-conjugated secondary antibodies were from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal anti-ERK and anti-phospho-ERK antibodies, as well as mouse mAb specific for phosphorylated ERK, were from Cell Signaling Technologies (Beverly, MA). A rabbit polyclonal antibody directed against the phosphorylated form of Tyr 1148 of ErbB1 (ErbB1 pY 1148) was from Biosource International (Camarillo, CA). Recombinant human AR (rhAR) and neutralizing antibodies against TGF-α, AR, HB-EGF, epiregulin, and betacellulin were from R&D Systems (Minneapolis, MN). Recombinant human EGF (rhEGF) was from Preprotech (Rocky Hill, NJ). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG and protein A-agarose were from Santa Cruz Biotechnology (Santa Cruz, CA). PD158780, PD153035, AG825, GM6001, and MMP-2/MMP-9 inhibitor II [(2R)-[(4-biphenylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide] were from Calbiochem (San Diego, CA). Geldanamycin was a kind gift from Dr. William B. Pratt *Department of Pharmacology, University of Michigan Medical School).
Cell Culture and Stimulation of Keratinocytes
NHKs were obtained from sun-protected adult skin by trypsin flotation and propagated in modified MCDB 153 medium (complete M154; Cascade Biologics, Portland OR, containing EGF, insulin, hydrocortisone, and bovine pituitary extract) at a calcium concentration of 0.1 mM, as described previously (Stoll et al., 2001). NHKs were deprived of GFs for 24–48 h by maintenance in M154 containing 0.1 mM calcium but lacking growth supplements (basal M154 medium). For experiments involving inhibitors, the basal M154 was removed, the cells were washed twice with solution A (22.5 mM HEPES, 7.5 mM glucose, 2.25 mM KCl, 97.5 mM NaCl, 0.74 mM Na2HPO4 · 7H2O, pH 7.4), and the medium was replaced (“preincubated”) with fresh basal M154 containing various inhibitors or DMSO vehicle. The cells were then returned to the incubator for 5–240 min. Ligands (or phosphate-buffered saline [PBS] as a control) were then added directly to the preincubation medium for 5–120 additional minutes; therefore, inhibitors were present throughout the period of exposure to ligand. After stimulation, lysates were harvested by scraping into lysis buffer (50 mM Tris, pH 7.5, 5 mM EGTA, 120 mM NaCl, 20 mM β-glycerophosphate, 1% NP-40, 15 mM sodium pyrophosphate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 0.5 mM phenylmethylsulfonyl fluoride, 10 mM sodium orthovanadate, 50 mM sodium fluoride, 20% glycerol) with a rubber policeman. The lysate was clarified by centrifuging at 12,000 rpm for 15 min, and the supernatant was retained for further analysis. Protein concentrations were estimated using the Bio-Rad DC assay kit (Bio-Rad, Hercules, CA).
HaCaT cells (Boukamp et al., 1988) were obtained from Dr. Norbert Fusenig and propagated in DMEM as described previously (Stoll et al., 1998). HaCaT cells were rendered quiescent by 24 h of incubation in DMEM without serum, as described previously (Iordanov et al., 2002).
Western Blotting
Five to 20 μg of lysate protein was denatured by heating to 95°C for 5 min in 1× Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol), separated on 4–20% Tris-glycine gradient polyacrylamide gels (Invitrogen, Carlsbad, CA) and electrophoretically transferred onto polyvinylidene difluoride membranes (Invitrogen) according to the manufacturer's directions. Filters were washed with Dulbecco's PBS containing 0.1% Tween 20 (PBST), and then incubated in blocking buffer (0.1% Tween 20, 5% nonfat dry milk) with gentle rocking at room temperature for 30–60 min. Primary antibody incubations were performed in blocking buffer. Anti-ErbB1, anti-ErbB2, and anti-phospho-tyrosine were used at 1 μg/ml, and anti-ERK, anti-phospho-ERK, and anti-ErbB1 pY 1148 were used at a dilution of 1:1000. Primary antibody incubations were performed overnight at 4°C with gentle rocking. Subsequent to this, filters were rinsed three times for 10 min each at room temperature with PBST. After the third rinse, filters were incubated with the appropriate secondary antibody in blocking buffer for 1 h at room temperature, and then rinsed thrice for 10 min in PBST. Detection was performed using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) as directed by the manufacturer.
Immunoprecipitation
Immunoprecipitation was performed essentially as described previously (Stoll et al., 2001). Briefly, 200 μg of cell lysate in 500 μl of lysis buffer was incubated with 500 ng of anti-ErbB1 (Ab-13), anti-ErbB2 (Ab-2), or with 500 ng of the appropriate isotype control antibody overnight at 4°C with rotation. Immune complexes were captured using protein A-agarose beads according to the manufacturer's instructions (Santa Cruz Biotechnology). Bound proteins were eluted by boiling in Laemmli sample buffer, and the supernatant was analyzed by Western blotting as described above.
Enzyme-linked Immunosorbent Assay (ELISA)
AR was quantitated in unconcentrated NHK supernatants using an ELISA (R&D Systems) with microtiter plates precoated with goat anti-mouse IgG, according to the manufacturer's instructions. rhAR was used as the standard. Samples with optical density values >2.0 were diluted 1:8. Results of duplicate determinations were averaged; between-determination variation was <20%.
Immunofluorescence
NHKs were grown to 40% confluence on sterile glass coverslips, and then were depleted of GFs for 2 d, followed by two washes in solution A and a 20-min preincubation in fresh basal M154 medium. After stimulation, the coverslips were rinsed twice in solution A, immediately fixed in freshly prepared 4% paraformaldehyde, and immunostained as described previously (Stoll et al., 2001). Ab14 (Labvision), a mixture of three mAb specific for the extracellular domain of ErbB1, was used at 0.4 μg/ml. Equivalent molar amounts of the isotype control antibodies MOPC 31c (IgG1) and UPC 10 (IgG2a) yielded negative staining (unpublished data). The secondary antibody was FITC-conjugated goat anti-mouse IgG. PBS containing 1% bovine serum albumin was used as the diluent. Slides were visualized using an Axioskop microscope equipped for fluorescence (Carl Zeiss, Jena, Germany). Digital images were obtained using a 2.2 megapixel diode array camera (Optronics, Goleta, CA).
RESULTS
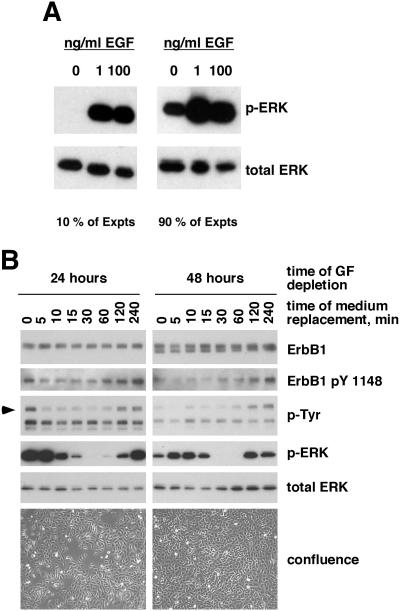
During analysis of an initial series of experiments involving GF-depleted NHKs, we encountered substantial variability in the basal level of ERK phosphorylation. Basal levels of ERK phosphorylation were detected in 71 of 78 (91%) of experiments performed (Figure 1A). However, the level of ERK phosphorylation varied widely. This variability was ultimately traced to the preincubation time (i.e., the time allowed to elapse between replacement of the basal M154 medium and subsequent treatment with EGF). After either 24 or 48 h of GF deprivation, preincubation in fresh basal M154 medium led to a rapid decline in ERK phosphorylation, reaching a minimum at 30–60 min after medium change and followed at 120 and 240 min by a return to levels near those observed before medium change (Figure 1B). Parallel but less obvious changes were detected in ErbB1 tyrosine phosphorylation. We also noted that levels of basal ErbB1 tyrosine phosphorylation and ERK phosphorylation decreased as the duration of GF deprivation increased from 24 to 48 h. These results explained the previously observed variability in basal ERK phosphorylation, because we had used preincubation times ranging from 10 to 90 min in the original series of experiments, and the precise state of confluence at the end of GF deprivation had not be precisely controlled.
Figure 1.
Variable levels of basal ERK phosphorylation in GF-deprived NHKs are correlated with preincubation time. (A) NHKs were depleted of GFs for 48 h and then preincubated in fresh basal M154 medium for various times (see text for details). Cells were then either left untreated or stimulated for 10 min at 37°C with EGF at 1 or 100 ng/ml. Equal amounts of nonionic detergent lysate (20 μg) were then assayed for phospho-ERK or total ERK by Western blotting as described in Materials and Methods. When observed, the basal level of ERK phosphorylation was variable and not always as intense as in the experiment shown in the right panel. (B) NHKs were seeded at 5000 cells/cm2 and grown until 40% confluent in complete M154 medium. Cells were then transferred to basal M154 medium for 24 or 48 h and photographed by phase contrast microscopy. Cells were then either lysed or the basal M154 was removed and replaced with fresh basal M154 medium for an additional 5–240 min before lysis, with the times indicated above the autoradiograms. Lysates (20 μg/lane) were subjected to Western blotting, and replicate blots were decorated with the antibodies indicated to the right of the autoradiographs. The bottom panels illustrate the confluence of the cultures after 24 or 48 h of GF deprivation. This result is one of two experiments producing very similar results.
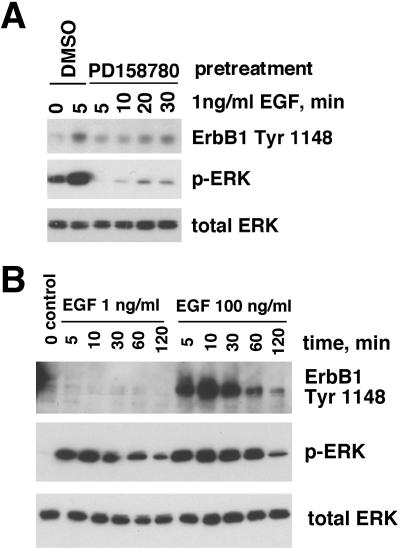
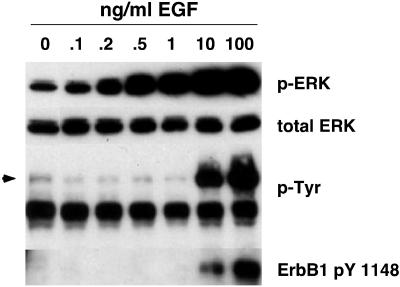
When 1 ng/ml EGF was used for stimulation of NHKs that had been depleted of GFs for 48 h, we observed a marked increase in EGF-stimulated ERK phosphorylation. This increase was reduced to below basal levels in the presence of 200 nM of the ErbB RTKI PD158780 (Figure 2A), which is highly selective for ErbB RTKs at this concentration (Rewcastle et al., 1998). Similar results were observed for PD153035, another pan-ErbB RTK inhibitor (Kansra et al., 2002; Stoll et al., 2002; and Kansra, Stoll., and Elder, unpublished data). However, as assessed using an antibody specific for ErbB1 pY 1148, a known ErbB1 autophosphorylation site (Margolis et al., 1989), there was little (Figure 2A) or no (Figure 2B) detectable increase in ErbB1 autophosphorylation in response to 1 ng/ml EGF. When the concentration of EGF was increased to 100 ng/ml, strong autophosphorylation of ErbB1 was observed (Figure 2B). Interestingly, despite these strong differences in ErbB1 autophosphorylation, the ERK phosphorylation responses to 1 ng/ml versus100 ng/ml EGF were very similar, being only slightly stronger and more prolonged in response to 100 ng/ml EGF (Figure 2B). To determine the lowest concentration of EGF that could stimulate ERK phosphorylation, we performed a more detailed dose-response curve. As shown in Figure 3, EGF concentrations as low as 0.2 ng/ml increased ERK phosphorylation above basal levels. In contrast, ErbB1 tyrosine phosphorylation was detectably increased only at 10 and 100 ng/ml EGF, as assessed either by antibodies against phosphotyrosine or ErbB1 pY 1148. From these data, we concluded ErbB RTK activity is required for maintaining basal levels of autocrine ERK phosphorylation in NHKs and that substantial ERK activation could be accomplished with minimal ErbB tyrosine phosphorylation.
Figure 2.
Kinetics of ERK phosphorylation and ErbB1 tyrosine phosphorylation in the presence or absence of ErbB RTK inhibitors. (A) NHKs were deprived of GFs for 48 h, preincubated with 200 nM PD158780 or DMSO vehicle in fresh basal M154 medium for 90 min at 37°C, and then treated with 1 ng/ml EGF or PBS vehicle for various times, as indicated above the autoradiograms. Equal amounts of cell lysate (20 μg) were subjected to Western blotting, and replicate blots were decorated with the antibodies indicated to the right of the autoradiograms. (B) NHKs were GF-deprived for 48 h, preincubated in fresh basal M154 for 30 min, and then treated with 1 or 100 ng/ml EGF for various times before nonionic detergent lysis. After SDS-PAGE (20 μg lysate/lane) and Western blotting, replicate blots were decorated with various antibodies as indicated to the right of the autoradiograms. The result shown is from a single experiment and is representative of two independent experiments yielding similar results.
Figure 3.
ERK phosphorylation and ErbB tyrosine phosphorylation in response to low concentrations of EGF. NHKs were GF– deprived in basal M154 for 48 h, preincubated for 15 min in fresh basal M154, and then treated with varying concentrations of EGF or PBS control (lane 0) as indicated above the autoradiograms. After lysate preparation and Western blotting, replicate filters were decorated with the antibodies indicated to the right of the autoradiograms. Arrow indicates electrophoretic mobility of ErbB1.
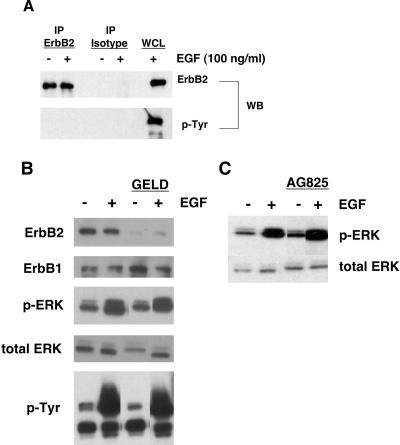
Because neither PD158780 nor PD153035 is selective for ErbB1, and because NHKs express substantial quantities of ErbB2 (Stoll et al., 2001), we next wanted to specifically examine the possible contribution of ErbB2 to signaling from ErbB to ERK. As shown in Figure 4A, there was no evidence for tyrosine phosphorylation of ErbB2 under basal or EGF-stimulated conditions, as determined by immunoprecipitation and Western blotting. Pretreatment with geldanamycin, which causes proteolytic degradation of ErbB2 (Tikhomirov and Carpenter, 2000), did not inhibit either basal or EGF-stimulated 180-kDa tyrosine phosphorylation or phospho-ERK, despite causing near-complete loss of ErbB2 (Figure 4B), and near-complete inhibition of heregulin-stimulated tyrosine phosphorylation of ErbB2 in MD-MBA-453 cells (unpublished data). Finally, the ErbB2-selective tyrosine kinase inhibitor AG825 (Tsai et al., 1996) had no effect on basal or EGF-stimulated ERK phosphorylation (Figure 4C). Together, these results suggested that ErbB2 is not involved in the regulation of autocrine or EGF-stimulated ERK phosphorylation in NHKs.
Figure 4.
Basal and EGF-stimulated ERK phosphorylation in NHKs is not dependent upon ErbB2. (A) GF-deprived NHKs were preincubated in fresh basal M154 for 20 min and then stimulated with 100 ng/ml EGF or PBS vehicle control for an additional 10 min at 37°C. Cell lysates were prepared and immunoprecipitated with ErbB2-specific antibody or isotype control antibody as described in Materials and Methods, followed by Western blotting and decoration with the antibodies indicated to the right of the autoradiograms. EGF (20 μg)-stimulated whole cell lysate (WCL) was run in parallel as a positive control. The result shown is representative of three independent experiments yielding similar results, one of which has been published previously by us (Stoll et al., 2002). (B) GF-deprived NHKs were pretreated for 4 h at 37°C with 3 μM geldanamycin or DMSO vehicle and then stimulated with EGF (100 ng/ml, 10 min at 37°C). Western blots were decorated using the antibodies indicated to the left of the panels. Results shown are from a single experiment and are representative of three independent experiments yielding similar results. (C) GF-deprived NHKs were either pretreated with AG825 (25 μM, 1 h at 37°C) or left untreated and then stimulated with EGF (100 ng/ml, 10 min at 37°C) or left untreated. Western blots of nonionic detergent lysates were decorated with mAb specific for phosphorylated ERK (top) or total ERK (bottom). Results shown are from a single experiment and are representative of three independent experiments yielding similar results.
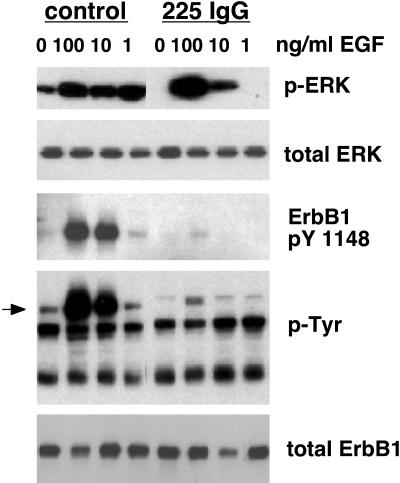
Given that NHKs express only very low levels of ErbB3 and no detectable ErbB4 (Stoll et al., 2001), the foregoing results suggested that ErbB1 might be responsible for the observed basal levels of ERK phosphorylation. To more specifically demonstrate this, we used the ErbB1-neutralizing mAb 225 IgG (Gill et al., 1984). When NHKs were incubated with 225 IgG, basal ERK phosphorylation was abolished. This antibody also completely blocked ERK phosphorylation stimulated by 1 ng/ml EGF. However, it yielded progressively less inhibition as the EGF concentration was increased to 10 and 100 ng/ml (Figure 5). Noting that the 225 IgG antibody was present at only twofold molar excess >100 ng/ml EGF, it is likely that 225 IgG was unable to effectively compete with EGF for access to ErbB1 at high EGF concentrations. This resulted in ineffective inhibition of ERK phosphorylation, because we have shown that minor increases in ErbB1 tyrosine phosphorylation provoke large increases in ERK phosphorylation in this system (Figures 2, 3, and 5).
Figure 5.
Basal EGF-stimulated ERK phosphorylation in NHK is dependent upon ErbB1. Growth factor-deprived NHKs were pretreated for 90 min at 37°C in fresh basal M154 with either the ErbB1 blocking antibody 225 IgG (5 μg/ml), or PBS (control), and then stimulated with 100, 10, or 1 ng/ml EGF for 10 min at 37°C. After lysate preparation and Western blotting, replicate blots were decorated with the antibodies indicated to the right of the autoradiograms. The arrowhead indicates the mobility of ErbB1 (170–180 kDa). The result shown is from a single experiment, and is representative of three independent experiments yielding similar results.
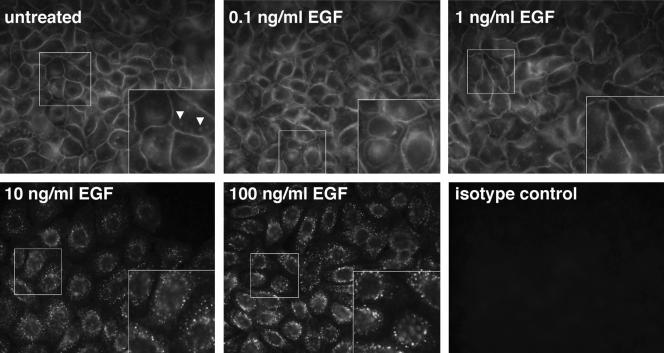
We next characterized the subcellular localization of ErB1 in response to autocrine stimulation as well as various concentrations of EGF by immunofluorescent staining of NHKs with a specific anti-ErbB1 antibody. As shown in Figure 6, under basal conditions (48 h of GF deprivation followed by 20-min preincubation in fresh medium), most of the ErbB1 is found in a “chicken-wire” pattern indicative of plasma membrane localization. When preincubation in fresh medium was omitted to observe the possible effect of autocrine ligands accumulating over the 48-h period of GF deprivation, substantial membrane localization of ErbB1 was still observed (unpublished data). Recalling that substantial ERK phosphorylation is observed under basal conditions (Figure 1B), these results indicate that ErbB1 is signaling to ERK largely at the plasma membrane in response to autocrine ErbB1 ligands. On stimulation with 0.1 ng/ml EGF, ErbB1 was still detected primarily in a plasma membrane pattern, but the apposing membranes of adjacent cells became separated from each other. The mechanism underlying this phenomenon remains to be elucidated. At 1 ng/ml EGF, the staining pattern once again resembled that observed under basal conditions. However, upon stimulation with larger amounts of EGF (10 and 100 ng/ml) membrane staining was lost, and staining became more punctate and located closer to the nucleus, indicative of receptor internalization. We also observed some ErbB1 staining in small, ill-defined patches (Figure 6A, arrowheads). Whereas the precise nature and subcellular localization of these patches remain to be defined, they could represent lipid rafts (Miljan and Bremer, 2002).
Figure 6.
Localization of ErbB1 receptors in response to different concentrations of EGF. NHKs were deprived of GFs for 48 h and then incubated for 20 min in fresh basal M154 medium. At that point, they were either left untreated or stimulated with 0.1, 1, 10, and 100 ng/ml EGF for 10 min at 37°C, as indicated on each panel. ErbB1 receptor localization was then assessed by immunofluorescence by using a mixture of three mAbs recognizing the extracellular domain of ErbB1. Insets show 2× magnifications of the segment of each image enclosed in the smaller white box. Arrowheads indicate the patchy ErbB1 staining referred to in the text. The results shown are from a single experiment, and are representative of three independent experiments yielding similar results.
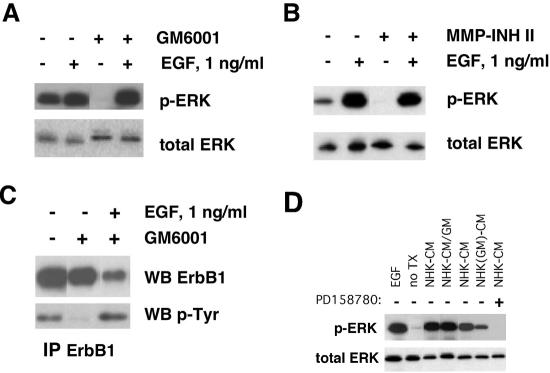
Because many receptor ligands, including ErbB ligands, are released from transmembrane precursors by MPs (Gschwind et al., 2001), we asked whether basal levels of ERK phosphorylation in NHKs could be reduced by the broad-spectrum MP inhibitors GM6001 (Holleran et al., 1997) and MMP-2/MMP-9 inhibitor II (Pikul et al., 1998; Tamura et al., 1998). As shown in Figure 7, A and B, basal ERK phosphorylation was markedly reduced by both compounds, and inhibition could be overcome by 1 ng/ml EGF. To demonstrate that release of membrane-bound GF is responsible for the basal activation state of ErbB1, we investigated the tyrosine phosphorylation status of ErbB1 by immunoprecipitation and Western blotting. As shown in Figure 7C, autocrine ErbB1 tyrosine phosphorylation was markedly reduced by GM6001 treatment, and inhibition could be overcome by 1 ng/ml EGF. To determine whether the observed effects of GM6001 were due to inhibition of MP-mediated release of soluble factors, we collected NHK-conditioned medium (NHK-CM) for 24 h in the presence or absence of GM6001, then used these CM to treat quiescent HaCaT cells followed by assessment of ERK phosphorylation (Figure 7D). HaCaT cells were used because they are keratinocyte-derived, but unlike NHKs, they have a low basal level of ERK phosphorylation at quiescence (Iordanov et al., 2002). As shown in Figure 7D, GM6001 treatment during the medium conditioning period markedly decreased the ability of NHK-CM to stimulate ERK phosphorylation in the HaCaT indicator cells. This decrease was not reproduced by addition of GM6001 to NHK-CM before addition to the HaCaT indicator cells, indicating that GM6001 is not acting directly on ErbB1 or between ErbB1 and ERK. Finally, ERK phosphorylation in response to NHK-CM was completely blocked by the ErbB RTK inhibitor PD158780. Together, these observations demonstrate that GM6001 causes a reduction in soluble ErbB ligand production by NHKs, resulting in lower levels of ErbB1 signaling to ERK.
Figure 7.
Basal ERK activity in NHKs is dependent upon proteolytic release of ErbB ligands. (A and B) GF-deprived NHKs were preincubated in fresh basal M154 for 90 min at 37°C in fresh basal M154 medium containing DMSO control (indicated by minus signs above the autoradiographs), 40 μM GM6001 (A), or 50 μM MMP-2/MMP-9 inhibitor II (B) and then stimulated with 1 ng/ml EGF for 10 min at 37°C, or left untreated. After lysate preparation and Western blotting (20 μg protein/lane), replicate blots were decorated with the antibodies indicated to the right of the autoradiographs. The results shown are from a single experiment and are representative of at least four independent experiments for A and two independent experiments for B. (C) GF-deprived NHKs were preincubated in fresh basal M154 containing 40 μM GM6001 or DMSO control for 90 min at 37°C, followed by stimulation with 1 ng/ml EGF or PBS control for 10 min. Conditions are indicated above the autoradiographs. Cell lysate (150 μg) was subjected to immunoprecipitation for ErbB1 as described in Materials and Methods. An isotype control was negative (unpublished data). Eluted proteins were detected by Western blotting with the primary antibodies indicated to the right of the autoradiographs. The results shown are representative of two independent experiments yielding similar results. (D) GF-deprived NHKs were grown to 40% confluence in complete M154 medium and then preincubated for 24 h in basal M154 containing 0.1% DMSO (NHK-CM) or 40 μM GM6001 [NHK(GM)-CM]. The CM was then collected and 4 ml was applied without concentration for 10 min to 60-mm dishes of HaCaT cells grown to 40% confluence, then maintained in serum-free DMEM for 24 h to induce quiescence (Iordanov et al., 2002). Controls included untreated quiescent HaCaT cells (no Tx), treatment with 100 ng/ml for 10 min (EGF), NHK-CM to which 40 μM GM6001 was added just before addition to HaCaT cells (NHK-CM/GM), and preincubation of HaCaT cells with 1 μM PD158780 for 1 h before addition of CM (denoted by the plus sign above the autoradiogram). Application of fresh basal M154 medium, rather than conditioned medium, to the HaCaT cells produced no increase in ERK phosphorylation (unpublished data). Note the reduction in ERK phosphorylation when GM6001 is added to NHKs during the 24-h medium-conditioning period, but not when it is added to the NHK-CM just before exposure to HaCaT cells. Lanes 1–4 are from one experiment and lanes 5–7 are from another. The no treatment controls from the first and second experiments both revealed similarly low levels of ERK phosphorylation. Only the no treatment control from the first experiment is shown in the figure.
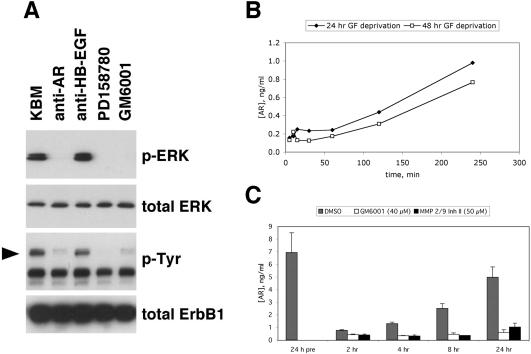
In an effort to define the GF(s) that might be involved in this pattern of autocrine stimulation, we treated GF-deprived NHKs with a panel of neutralizing mAbs directed against various EGF-like growth factors. As shown in Figure 8A, a neutralizing mAb directed against AR abrogated basal ERK phosphorylation and reduced basal tyrosine phosphorylation of ErbB1, whereas a neutralizing antibody directed against HB-EGF was ineffective against both responses. Antibodies directed against TGF-α, betacellulin, and epiregulin also were ineffective (unpublished data). Using an ELISA specific for AR, we measured AR levels in CM harvested from three independent experiments, one of which also is depicted in Figure 1B. As shown in Figure 8B, AR reappeared in the CM with a time course consistent with the reappearance of ERK phosphorylation (Figure 1B), becoming detectable over background levels at 120 min and reaching ∼1 ng/ml by 240 min. AR continued to accumulate over a 24-h period, reaching a level of ∼5 ng/ml (Figure 8C). Elaboration of AR into the CM was markedly and significantly reduced by either GM6001 or MMP-2/MMP-9 inhibitor II, demonstrating a dependence upon MP activity (Figure 8C).
Figure 8.
Autocrine ERK phosphorylation in NHKs is dependent upon AR. (A) Neutralization experiments. GF-depleted NHKs were preincubated in fresh M154 medium for 120 min at 37°C with neutralizing antibodies against AR (5 μg/ml) or HB-EGF (5 μg/ml), or with PD158780 (1 μM) or GM6001 (40 μM). After lysate preparation and Western blotting (20 μg protein/lane), replicate blots were decorated with the antibodies indicated to the right of the autoradiograms. The result shown is from a single experiment and is representative of six experiments. The arrowhead indicates the mobility of ErbB1 (170–180 kDa). (B) Time course of AR accumulation. After GF deprivation in basal M154 for 24 h (closed diamonds) or 48 h (open squares), the medium was changed to fresh basal M154 and CM was collected and assayed for AR by ELISA. Data points represent the average of the two experiments, each of which was performed in duplicate. Note that accumulation of AR in the CM parallels the reappearance of ERK phosphorylation shown in Figure 1B. (C) MP inhibitors block production of soluble AR. NHKs (∼50% confluent) were deprived of growth factors in for 24 h in basal M154 medium, and aliquots of CM were collected. The medium was then changed to fresh basal M154 containing 40 μM GM6001, 50 μM MMP-2/9 inhibitor II, or 1:1000 (vol/vol) DMSO control. After time intervals varying from 2–24 h, aliquots of unconcentrated CM were assayed by ELISA as described in Materials and Methods. Error bars indicate SEM, n = 3–5, for all conditions except MMP-2/9 inhibitor II at 8 h, for which n = 2. p values were determined using a one-sided t test with unequal variances, with the null hypothesis being no reduction in AR by inhibitor at each time point. The nominal p value for the effect of this inhibitor versus DMSO control at this time point was 0.0056, all other nominal p values were <0.005. All p values remained significant at the p = 0.05 level after Bonferroni correction for eight tests.
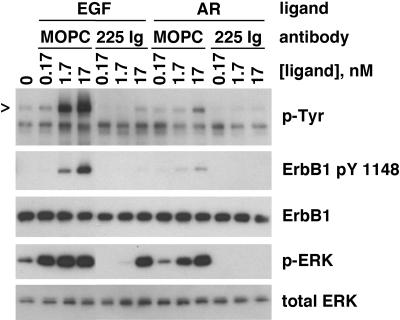
To determine whether EGF and AR exert similar effects on NHK signal transduction, we treated NHK with equimolar concentrations of rhEGF or rhAR, in the presence or absence of 225IgG. As shown in Figure 9, both rhEGF rhAR stimulated ErbB1 autophosphorylation and ERK phosphorylation. rhEGF was more potent and effective than rhAR in eliciting these responses (see Discussion). The effects of AR were completely blocked by 5 μg/ml 225 IgG, indicating that its effects were elicited by binding to and activation of ErbB1.
Figure 9.
Stimulation of ERK phosphorylation by AR. NHKs were depleted of GFs in basal M154 medium for 48 h and then incubated for 20 min with fresh basal M154 containing 5 μg/ml 225 IgG or 5 μg/ml MOPC 21. Then, the indicated concentrations of AR or EGF were added, and incubation was continued for another 10 min. PBS was added rather than AR or EGF in the lane labeled 0. Nonionic detergent lysates were then prepared and subjected to western blotting (20 μg protein/lane). Replicate blots were prepared and decorated with the antibodies indicated to the right of the autoradiographs. Arrowhead indicates mobility of ErbB1. For comparison with other figures, 0.17 nM EGF is 1 ng/ml. Note the decreased potency and effect of rhAR, compared with rhEGF.
DISCUSSION
High levels of ErbB-dependent autocrine ERK activation have been observed previously in NHKs, but the mechanism of activation has not been fully elucidated (Cai et al., 2002; Iordanov et al., 2002; Kansra et al., 2002; Stoll et al., 2002). We became interested in this phenomenon while trying to understand the variable levels of ERK phosphorylation we observed under basal conditions (Figure 1A). Additional experiments revealed a biphasic change in levels of ERK phosphorylation and ErbB1 tyrosine phosphorylation as a function of preincubation time, with a rapid and marked decline >10–15 min of preincubation followed by recovery to near-original levels at 120–240 min (Figure 1B). These experiments also revealed a decrease in ERK phosphorylation at 48 h of GF deprivation compared with 24 h, which may be attributable to the approach of confluence (Figure 1B, bottom).
Subsequent experiments demonstrated that this “basal” level of ERK phosphorylation was due to MP-mediated release of one or more soluble ErbB ligands (Figure 7). To determine which ErbB ligand(s) might be responsible for this behavior, we treated GF-depleted NHKs with neutralizing antibodies against various ErbB ligands. Basal levels of ERK phosphorylation could be blocked by pretreatment with neutralizing antibodies against AR, but not against HB-EGF (Figure 8A). Antibodies against TGF-α, betacellulin, and epiregulin also were ineffective (unpublished data). Moreover, the time course of accumulation of soluble AR in NHK-CM (Figure 8B) correlated with the reappearance of ERK phosphorylation after medium change (Figure 1B). Together, these results demonstrated that the predominant ErbB ligand responsible for autocrine ERK activation in NHK is AR.
Both the autocrine ERK activation response (Figure 7) and AR elaboration into the culture medium (Figure 8C) could be blocked by either GM6001 or MMP-2/MMP-9 inhibitor II, demonstrating that AR release is dependent upon MP activity. Other than this, the nature of the proteolytic activity responsible for cleavage of AR in NHKs is currently unknown. Ullrich and colleagues have identified an AR-driven ErbB1 activation circuit in oral squamous cell carcinoma cells, in which ADAM17 (a disintegrin and a metalloproteinase-17), also known as TACE, was identified as a major source of pro-AR proteolytic activity (Gschwind et al., 2003). Also, ADAM-10 has been implicated as a major source of pro-HB-EGF proteolytic activity in cardiac hypertrophy (Lemjabbar and Basbaum, 2002).
Our laboratory is currently investigating the nature of the MP(s) responsible for regulating AR release in NHKs. Our studies are being guided by available knowledge of MP expression in NHKs and skin. The MPs can be subdivided into the matrix metalloproteinases (MMPs) and the ADAMs. Although many MMPs are released into the extracellular space, they are still candidates for AR cleavage because certain MMPs (including tolloid, tolloid-like-1, MMP-2, and MMP-9) are known to bind to the surface of keratinocytes (Sternlicht and Werb, 2001; Franzke et al., 2002; Rattenholl et al., 2002; Veitch et al., 2003), and MT1-MMP (MMP-14) is membrane anchored. In resting adult skin, most MMPs are not constitutively expressed in keratinocytes, with the exception of MMP-7 (matrilysin) in eccrine sweat gland epithelium, MMP-2 (gelatinase A) in occasional basal keratin-ocytes, MMP-19, tolloid, and tolloid-like 1. However, several MMPs are induced or up-regulated in keratinocytes in the context of inflammation, embryogenesis, and tissue repair/remodeling, including MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, and MMP-19 (Kahari and Saarialho-Kere, 1997; Rattenholl et al., 2002; Impola et al., 2003; Kerkela and Saarialho-Kere, 2003; Sadowski et al., 2003; Veitch et al., 2003). Unstimulated NHKs in culture produce modest amounts of MMP-1, MMP-2, and MMP-9, but little MMP-10 or MMP19 (Varani et al., 1995a; Kahari and Saarialho-Kere, 1997; Impola et al., 2003; Kerkela and Saarialho-Kere, 2003; Sadowski et al., 2003). In contrast, NHKs produce substantial amounts of tolloid and tolloid-like 1 MPs (Rattenholl et al., 2002; Veitch et al., 2003). Compared with the MMPs, fewer studies have addressed the expression of the various ADAMs in NHKs. However, ADAM-9, ADAM-10, and ADAM-17 have been detected in NHKs and skin by immunocytochemical techniques, as well as by reverse transcription-polymerase chain reaction (Franzke et al., 2002; Kawaguchi et al., 2004). We have been able to detect low levels of ADAM-10 and ADAM-17 in NHK by Western blotting (unpublished data).
We also were interested in identifying the membrane receptor(s) through which the autocrine AR signal is passed to ERK. We have previously shown that NHKs express large amounts of ErbB1 and ErbB2, but very little ErbB3 and no detectable ErbB4 (Stoll et al., 2001). Because the kinase activity of ErbB3 is severely impaired (Guy et al., 1994), the most likely carriers of the ErbB signal are ErbB1 and ErbB2. Basal ERK phosphorylation was greatly reduced by pretreatment with two different ErbB-specific RTKIs: PD158780 (Figure 2A) and PD153035 (Kansra et al., 2002; Stoll et al., 2002) (unpublished data), demonstrating the dependence of basal ERK phosphorylation upon ErbB activation. However, neither of these agents are specific for ErbB1 (Rewcastle et al., 1998; Stoll et al., 2001). AR and EGF are known to activate ErbB1-ErbB2 heterodimers even more effectively than they activate ErbB1 homodimers (Klapper et al., 2000; Olayioye et al., 2000). However, tyrosine phosphorylation of ErbB2 was undetectable under basal or EGF-stimulated conditions (Figure 4A). This result is strongly suggestive of a lack of ErbB1-ErbB2 heterodimer formation and is consistent with our previous observation that ErbB1 and ErbB2 are segregated to different subcellular compartments in NHKs, with ErbB1 on the cell surface and ErbB2 on intracellular vesicles (Stoll et al., 2001). To explore the possible involvement of ErbB2 as homodimers or in conjunction with ErbB3, we treated NHKs with geldanamycin or AG825. Geldanamycin selectively increases the degradation of ErbB2 by caspase-dependent cleavage at ErbB2-specific residues within the kinase domain (Tikhomirov and Carpenter, 2001, 2003). AG825 is an RTKI that is selective for ErbB2 in vitro (Osherov et al., 1993). As shown in Figure 4, B and C, neither agent reduced basal or EGF-stimulated ERK phosphorylation. Although it remains possible that some residual ErbB2 RTK activity remained under our experimental conditions, when taken together the results shown in Figure 4 strongly suggest that neither ErbB2 homodimers nor ErbB2-ErbB3 heterodimers plays a significant role in maintaining basal ERK phosphorylation in NHKs. Corroborating the above-mentioned results, Figures 5 and 9 demonstrate that basal ERK phosphorylation was markedly diminished in the presence of the ErbB1-specific neutralizing antibody 225 IgG (Gill et al., 1984).
Having identified ErbB1 as the sole player in the AR-driven autocrine circuit driving ERK activation in NHKs, we also were interested in defining its subcellular distribution under conditions of autocrine stimulation. Immunofluorescence experiments revealed that the bulk of ErbB1 was located at the plasma membrane under conditions of GF deprivation and in the presence of low levels (≤1 ng/ml) of EGF (Figure 6). Receptor internalization was markedly increased at 10 and 100 ng/ml EGF, in concert with increased ErbB1 tyrosine phosphorylation (Figures 2, 3, 5, and 9).
ErbB1 tyrosine phosphorylation is known to create docking sites for c-Cbl, which is directly involved in internalization and ubiquitin-dependent lysosomal degradation of ErbB1 (Levkowitz et al., 1999; Thien and Langdon, 2001; Soubeyran et al., 2002). The very limited phosphorylation and internalization of ErbB1 at EGF concentrations ≤1 ng/ml suggest that c-Cbl is not engaged with ErbB1 after treatment with low concentrations of EGF, due to insufficient tyrosine phosphorylation of ErbB1. We would speculate that one or more tyrosine phosphatases may rapidly dephosphorylate ErbB1 after it is stimulated with autocrine AR or with low concentrations of EGF, but not before ErbB1 has established a signaling connection with ERK. This could explain the substantial signaling from ErbB1 to ERK that takes place in the relative absence of detectable ErbB1 tyrosine phosphorylation or internalization.
We found that the concentration of AR in NHK-CM is ∼5 ng/ml after 24 h of GF deprivation (Figure 8C), which translates into ∼2.5 ng/ml EGF. Assuming approximately equal biological potency of native AR and recombinant EGF (Cook et al., 1991a), this concentration of AR would be consistent with the limited internalization of ErbB1 that we observed under autocrine conditions (Figure 6), and with the pronounced autocrine phosphorylation of ERK demonstrated in Figure 1B. Curiously, however, the amount of AR produced by NHKs (∼1 ng/ml after 4 h and ∼5 ng/ml after 24 h; Figure 8) was less than the amount of purified rhAR needed to stimulate these responses when added to NHKs (1.7 nM ≈18.7 ng/ml; Figure 9). According to data provided by the manufacturers, the rhAR preparation we used (which is synthesized in Escherichia coli) is substantially less potent than rhEGF. Thus, the ED for stimulation of proliferation in Balb/3T3 cells was 5–15 50ng/ml for rhAR, compared with <0.1 ng/ml for EGF. In contrast, Cook et al. (1991a) have shown that native AR purified from NHKs is as potent or even more potent as a mitogen than EGF, when tested on mouse keratinocytes. We show in Figure 8B that the amount of AR that accumulates in NHK-CM after 2 h of GF deprivation (∼0.4 ng/ml) is adequate to stimulate ERK phosphorylation (Figure 1B) to an extent similar to 0.5 ng/ml rhEGF (Figure 3). Thus, in our hands, NHK-derived AR seems to be comparable with rhEGF in potency. AR is naturally secreted as a prepropeptide of 252 amino acids, and >100 of the N-terminal-most residues are not present in rhAR. Moreover, natural AR is subjected to extensive posttranslational glycosylation (Piepkorn et al., 1998) that is not possible in E. coli. Based on these findings, we suspect that native AR produced by NHKs is substantially more potent than rhAR, due to the presence of additional N-terminal amino acids and/or appropriate posttranslational modifications.
In aggregate, our findings indicate that AR is the major ligand responsible for autocrine ERK activation in NHKs and are consistent with previous reports that AR is a major autocrine mitogen in this system (Cook et al., 1991a; Piepkorn et al., 1994). Our results also demonstrate that one or more MPs are responsible for generation of soluble AR in NHKs. We believe that this autocrine, AR-driven ERK activation circuit is likely to be involved in the control of human keratinocyte proliferation in vivo, as suggested by the hyperplastic phenotype of mice engineered to overexpress AR in basal layer of the skin (Cook et al., 1997). Indeed, our findings could help explain a long-puzzling feature of psoriasis: it has not been possible to demonstrate increased levels of ErbB1 tyrosine phosphorylation in lesional psoriatic skin (Krane et al., 1992) despite reports of robust Ras activation (Lin et al., 1999) and ERK phosphorylation (Haase et al., 2001). Our findings also may have important implications for ErbB-directed cancer therapy, because it may be important to completely block ErbB autophosphorylation to shut off signaling via the ERK pathway. Clearly, an improved understanding of the expression of autocrine ErbB ligands and the context-dependent proteolytic milieu responsible for release of AR and other ErbB ligands in skin will be of major importance to cutaneous biologists in the coming years.
Acknowledgments
We thank Yong Li for expert technical assistance. This work was supported by an award (R21 AR48405) from the National Institute for Arthritis, Musculoskeletal and Skin Diseases, National Institute of Health. S.K. was supported by a National Research Service Award from the National Institute for Arthritis, Musculoskeletal and Skin Diseases, National Institute of Health (T32-AR07197). S.W.S. was supported by a Chesebrough Pond's Lever Brothers Dermatology Foundation Research Career Development Award and a Dermatology Foundation Research Grant. J.T.E. is supported by the Ann Arbor Veterans Affairs Hospital.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–03–0233. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–03–0233.
References
- Barnard, J.A., Graves Deal, R., Pittelkow, M.R., DuBois, R., Cook, P., Ramsey, G.W., Bishop, P.R., Damstrup, L., and Coffey, R.J. (1994). Autoand cross-induction within the mammalian epidermal growth factor-related peptide family. J. Biol. Chem. 269, 22817–22822. [PubMed] [Google Scholar]
- Boukamp, P., Petrussevska, R.T., Breitkreutz, D., Hornung, J., Markham, A., and Fusenig, N.E. (1988). Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 106, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, G.L., Nanney, L.B., Griffen, J., Cramer, A.B., Yancey, J.M., Curtsinger, L.D., Holtzin, L., Schultz, G.S., Jurkiewicz, M.J., and Lynch, J.B. (1989). Enhancement of wound healing by topical treatment with epidermal growth factor. N. Engl. J. Med. 321, 76–79. [DOI] [PubMed] [Google Scholar]
- Cai, T., Nishida, K., Hirano, T., and Khavari, P.A. (2002). Gab1 and SHP-2 promote Ras/MAPK regulation of epidermal growth and differentiation. J. Cell Biol. 159, 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey, R.J., Jr., Derynck, R., Wilcox, J.N., Bringman, T.S., Goustin, A.S., Moses, H.L., and Pittelkow, M.R. (1987). Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature 328, 817–820. [DOI] [PubMed] [Google Scholar]
- Cook, P.W., Mattox, P.A., Keeble, W.W., Pittelkow, M.R., Plowman, G.D., Shoyab, M., Adelman, J.P., and Shipley, G.D. (1991a). A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol. Cell. Biol. 11, 2547–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, P.W., Piepkorn, M., Clegg, C.H., Plowman, G.D., DeMay, J.M., Brown, J.R., and Pittelkow, M.R. (1997). Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J. Clin. Investig. 100, 2286–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook, P.W., Pittelkow, M.R., Keeble, W.W., Graves-Deal, R., Coffey, R., Jr., and Shipley, G.D. (1992). Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res. 52, 3224–3227. [PubMed] [Google Scholar]
- Cook, P.W., Pittelkow, M.R., and Shipley, G.D. (1991b). Growth factor-independent proliferation of normal human neonatal keratinocytes: production of autocrine- and paracrine-acting mitogenic factors. J. Cell. Physiol. 146, 277–289. [DOI] [PubMed] [Google Scholar]
- Cribbs, R.K., Luquette, M.H., and Besner, G.E. (1998). Acceleration of partial-thickness burn wound healing with topical application of heparin-binding EGF-like growth factor (HB-EGF). J. Burn Care Rehabil. 19, 95–101. [DOI] [PubMed] [Google Scholar]
- De Potter, I.Y., Poumay, Y., Squillace, K.A., and Pittelkow, M.R. (2001). Human EGF receptor (HER) family and heregulin members are differentially expressed in epidermal keratinocytes and modulate differentiation. Exp. Cell Res. 271, 315–328. [DOI] [PubMed] [Google Scholar]
- Dlugosz, A.A., Cheng, C., Williams, E.K., Darwiche, N., Dempsey, P.J., Mann, B., Dunn, A.R., Coffey, R.J., Jr., and Yuspa, S.H. (1995). Autocrine transforming growth factor alpha is dispensible for v-rasHa-induced epidermal neoplasia: potential involvement of alternate epidermal growth factor receptor ligands. Cancer Res. 55, 1883–1893. [PubMed] [Google Scholar]
- Downing, M.T., Brigstock, D.R., Luquette, M.H., Crissman-Combs, M., and Besner, G.E. (1997). Immunohistochemical localization of heparin-binding epidermal growth factor-like growth factor in normal skin and skin cancers. Histochem. J. 29, 735–744. [DOI] [PubMed] [Google Scholar]
- Draper, B.K., Komurasaki, T., Davidson, M.K., and Nanney, L.B. (2003). Epiregulin is more potent than EGF or TGF-alpha in promoting in vitro wound closure due to enhanced ERK/MAPK activation. J. Cell Biochem. 89, 1126–1137. [DOI] [PubMed] [Google Scholar]
- Elder, J.T., Fisher, G.J., Lindquist, P.B., Bennett, G.L., Pittelkow, M.R., Coffey, R., Jr., Ellingsworth, L., Derynck, R., and Voorhees, J.J. (1989). Overexpression of transforming growth factor alpha in psoriatic epidermis. Science 243, 811–814. [DOI] [PubMed] [Google Scholar]
- Franzke, C.W., et al. (2002). Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMs. EMBO J. 21, 5026–5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, G.N., Kawamoto, T., Cochet, C., Le, A., Sato, J.D., Masui, H., McLeod, C., and Mendelsohn, J. (1984). Monoclonal anti-epidermal growth factor receptor antibodies which are inhibitors of epidermal growth factor binding and antagonists of epidermal growth factor binding and antagonists of epidermal growth factor-stimulated tyrosine protein kinase activity. J. Biol. Chem. 259, 7755–7760. [PubMed] [Google Scholar]
- Grotendorst, G.R., Soma, Y., Takehara, K., and Charette, M. (1989). EGF and TGF-alpha are potent chemoattractants for endothelial cells and EGF-like peptides are present at sites of tissue regeneration. J. Cell. Physiol. 139, 617–623. [DOI] [PubMed] [Google Scholar]
- Gschwind, A., Hart, S., Fischer, O.M., and Ullrich, A. (2003). TACE cleavage of proamphiregulin regulates GPCR-induced proliferation and motility of cancer cells. EMBO J. 22, 2411–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind, A., Zwick, E., Prenzel, N., Leserer, M., and Ullrich, A. (2001). Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene 20, 1594–1600. [DOI] [PubMed] [Google Scholar]
- Guy, P.M., Platko, J.V., Cantley, L.C., Cerione, R.A., and Carraway, K.L. (1994). Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 91, 8132–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase, I., Hobbs, R.M., Romero, M.R., Broad, S., and Watt, F.M. (2001). A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J. Clin. Investig. 108, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel, P.O., Zwick, E., Prenzel, N., and Ullrich, A. (1999). Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr. Opin. Cell Biol. 11, 184–189. [DOI] [PubMed] [Google Scholar]
- Hashimoto, K., Higashiyama, S., Asada, H., Hashimura, E., Kobayashi, T., Sudo, K., Nakagawa, T., Damm, D., Yoshikawa, K., and Taniguchi, N. (1994). Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J. Biol. Chem. 269, 20060–20066. [PubMed] [Google Scholar]
- Hebda, P.A. (1988). Stimulatory effects of transforming growth factor-beta and epidermal growth factor on epidermal cell outgrowth from porcine skin explant cultures. J. Investig. Dermatol. 91, 440–445. [DOI] [PubMed] [Google Scholar]
- Holleran, W.M., Galardy, R.E., Gao, W.N., Levy, D., Tang, P.C., and Elias, P.M. (1997). Matrix metalloproteinase inhibitors reduce phorbol ester-induced cutaneous inflammation and hyperplasia. Arch. Dermatol. Res. 289, 138–144. [DOI] [PubMed] [Google Scholar]
- Hubbard, S.R., Mohammadi, M., and Schlessinger, J. (1998). Autoregulatory mechanisms in protein-tyrosine kinases. J. Biol. Chem. 273, 11987–11990. [DOI] [PubMed] [Google Scholar]
- Impola, U., Toriseva, M., Suomela, S., Jeskanen, L., Hieta, N., Jahkola, T., Grenman, R., Kahari, V.M., and Saarialho-Kere, U. (2003). Matrix metalloproteinase-19 is expressed by proliferating epithelium but disappears with neoplastic dedifferentiation. Int. J. Cancer 103, 709–716. [DOI] [PubMed] [Google Scholar]
- Iordanov, M.S., Choi, R.J., Ryabinina, O.P., Dinh, T.H., Bright, R.K., and Magun, B.E. (2002). The UV (Ribotoxic) stress response of human keratinocytes involves the unexpected uncoupling of the Ras-extracellular signal-regulated kinase signaling cascade from the activated epidermal growth factor receptor. Mol. Cell. Biol. 22, 5380–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahari, V.M., and Saarialho-Kere, U. (1997). Matrix metalloproteinases in skin. Exp. Dermatol. 6, 199–213. [DOI] [PubMed] [Google Scholar]
- Kansra, S., Stoll, S.W., and Elder, J.T. (2002). Differential cytoskeletal association of ErbB1 and ErbB2 during keratinocyte differentiation. Biochem. Biophys. Res. Commun. 295, 1108–1117. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, M., Mitsuhashi, Y., and Kondo, S. (2004). Localization of tumour necrosis factor-alpha converting enzyme in normal human skin. Clin. Exp. Dermatol. 29, 185–187. [DOI] [PubMed] [Google Scholar]
- Kerkela, E., and Saarialho-Kere, U. (2003). Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp. Dermatol. 12, 109–125. [DOI] [PubMed] [Google Scholar]
- Kiguchi, K., Beltran, L., Rupp, T., and DiGiovanni, J. (1998). Altered expression of epidermal growth factor receptor ligands in tumor promoter-treated mouse epidermis and in primary mouse skin tumors induced by an initiation-promotion protocol. Mol. Carcinog. 22, 73–83. [PubMed] [Google Scholar]
- Klapper, L.N., Kirschbaum, M.H., Sela, M., and Yarden, Y. (2000). Biochemical and clinical implications of the ErbB/HER signaling network of growth factor receptors. Adv. Cancer Res. 77, 25–79. [PubMed] [Google Scholar]
- Klein, S.B., Fisher, G.J., Jensen, T.C., Mendelsohn, J., Voorhees, J.J., and Elder, J.T. (1992). Regulation of TGF-alpha expression in human keratinocytes: PKC-dependent and -independent pathways. J. Cell. Physiol. 151, 326–336. [DOI] [PubMed] [Google Scholar]
- Krane, J.F., Gottlieb, A.B., Carter, D.M., and Krueger, J.G. (1992). The insulin-like growth factor I receptor is overexpressed in psoriatic epidermis, but is differentially regulated from the epidermal growth factor receptor. J. Exp. Med. 175, 1081–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemjabbar, H., and Basbaum, C. (2002). Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8, 41–46. [DOI] [PubMed] [Google Scholar]
- Levkowitz, G., et al. (1999). Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4, 1029–1040. [DOI] [PubMed] [Google Scholar]
- Lin, P., Baldassare, J.J., Voorhees, J.J., and Fisher, G.J. (1999). Increased activation of Ras in psoriatic lesions. Skin Pharmacol. Appl. Skin Physiol. 12, 90–97. [DOI] [PubMed] [Google Scholar]
- Margolis, B.L., Lax, I., Kris, R., Dombalagian, M., Honegger, A.M., Howk, R., Givol, D., Ullrich, A., and Schlessinger, J. (1989). All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails. Identification of a novel site in EGF receptor. J. Biol. Chem. 264, 10667–10671. [PubMed] [Google Scholar]
- McCarthy, D.W., Downing, M.T., Brigstock, D.R., Luquette, M.H., Brown, K.D., Abad, M.S., and Besner, G.E. (1996). Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. J. Investig. Dermatol. 106, 49–56. [DOI] [PubMed] [Google Scholar]
- McCawley, L.J., O'Brien, P., and Hudson, L.G. (1998). Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)-mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J. Cell Physiol. 176, 255–265. [DOI] [PubMed] [Google Scholar]
- Miljan, E.A., and Bremer, E.G. (2002). Regulation of growth factor receptors by gangliosides. Sci STKE 2002, RE15. [DOI] [PubMed] [Google Scholar]
- Nakamura, Y., Sotozono, C., and Kinoshita, S. (2001). The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp. Eye Res. 72, 511–517. [DOI] [PubMed] [Google Scholar]
- Olayioye, M.A., Neve, R.M., Lane, H.A., and Hynes, N.E. (2000). The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19, 3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osherov, N., Gazit, A., Gilon, C., Levitzki, A., Tikhomirov, O., and Carpenter, G. (1993). Selective inhibition of the epidermal growth factor and HER2/neu receptors by tyrphostins. J. Biol. Chem. 268, 11134–11142. [PubMed] [Google Scholar]
- Piepkorn, M., Lo, C., and Plowman, G. (1994). Amphiregulin-dependent proliferation of cultured human keratinocytes: autocrine growth, the effects of exogenous recombinant cytokine, and apparent requirement for heparin-like glycosaminoglycans. J. Cell. Physiol. 159, 114–120. [DOI] [PubMed] [Google Scholar]
- Piepkorn, M., Pittelkow, M.R., and Cook, P.W. (1998). Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factor-related growth factors. J. Investig. Dermatol. 111, 715–721. [DOI] [PubMed] [Google Scholar]
- Piepkorn, M., Predd, H., Underwood, R., and Cook, P. (2003). Proliferation-differentiation relationships in the expression of heparin-binding epidermal growth factor-related factors and erbB receptors by normal and psoriatic human keratinocytes. Archg Dermatol. Res. 295, 93–101. [DOI] [PubMed] [Google Scholar]
- Pikul, S., et al. (1998). Discovery of potent, achiral matrix metalloproteinase inhibitors. J. Med. Chem. 41, 3568–3571. [DOI] [PubMed] [Google Scholar]
- Pittelkow, M.R., Cook, P.W., Shipley, G.D., Derynck, R., and Coffey, R.J., Jr. (1993). Autonomous growth of human keratinocytes requires epidermal growth factor receptor occupancy. Cell Growth Differ. 4, 513–521. [PubMed] [Google Scholar]
- Praskova, M., Kalenderova, S., Miteva, L., Poumay, Y., and Mitev, V. (2002). Dual role of protein kinase C on mitogen-activated protein kinase activation and human keratinocyte proliferation. Exp. Dermatol. 11, 344–348. [DOI] [PubMed] [Google Scholar]
- Press, M.F., Cordon-Cardo, C., and Slamon, D.J. (1990). Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 5, 953–962. [PubMed] [Google Scholar]
- Prigent, S.A., Lemoine, N.R., Hughes, C.M., Plowman, G.D., Selden, C., and Gullick, W.J. (1992). Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene 7, 1273–1278. [PubMed] [Google Scholar]
- Rattenholl, A., Pappano, W.N., Koch, M., Keene, D.R., Kadler, K.E., Sasaki, T., Timpl, R., Burgeson, R.E., Greenspan, D.S., and Bruckner-Tuderman, L. (2002). Proteinases of the bone morphogenetic protein-1 family convert procollagen VII to mature anchoring fibril collagen. J. Biol. Chem. 277, 26372–26378. [DOI] [PubMed] [Google Scholar]
- Rewcastle, G.W., et al. (1998). Tyrosine kinase inhibitors. 14. Structure-activity relationships for methylamino-substituted derivatives of 4-[(3-bromophenyl)amino]-6-(methylamino)-pyrido[3,4-d]pyrimidine (PD 158780), a potent and specific inhibitor of the tyrosine kinase activity of receptors for the EGF family of growth factors. J. Med. Chem. 41, 742–751. [DOI] [PubMed] [Google Scholar]
- Sadowski, T., Dietrich, S., Muller, M., Havlickova, B., Schunck, M., Proksch, E., Muller, M.S., and Sedlacek, R. (2003). Matrix metalloproteinase-19 expression in normal and diseased skin: dysregulation by epidermal proliferation. J. Investig. Dermatol. 121, 989–996. [DOI] [PubMed] [Google Scholar]
- Schultz, G.S., White, M., Mitchell, R., Brown, G., Lynch, J., Twardzik, D.R., and Todaro, G.J. (1987). Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science 235, 350–352. [DOI] [PubMed] [Google Scholar]
- Shirakata, Y., Komurasaki, T., Toyoda, H., Hanakawa, Y., Yamasaki, K., Tokumaru, S., Sayama, K., and Hashimoto, K. (2000). Epiregulin, a novel member of the epidermal growth factor family, is an autocrine growth factor in normal human keratinocytes. J. Biol. Chem. 275, 5748–5753. [DOI] [PubMed] [Google Scholar]
- Soubeyran, P., Kowanetz, K., Szymkiewicz, I., Langdon, W.Y., and Dikic, I. (2002). Cbl-CIN85-endophilin complex mediates ligand-induced downregulation of EGF receptors. Nature 416, 183–187. [DOI] [PubMed] [Google Scholar]
- Sternlicht, M.D., and Werb, Z. (2001). How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17, 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll, S., Garner, W., and Elder, J. (1997). Heparin-binding ligands mediate autocrine EGF receptor activation in skin organ culture. J. Clin. Investig. 100, 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll, S.W., Benedict, M., Mitra, R., Hiniker, A., Elder, J.T., and Nuñez, G. (1998). EGF receptor signaling inhibits keratinocyte apoptosis: evidence for mediation by Bcl-XL. Oncogene 16, 1493–1499. [DOI] [PubMed] [Google Scholar]
- Stoll, S.W., and Elder, J.T. (1998). Retinoid regulation of heparin-binding EGF-like growth factor expression in human keratinocytes and skin. Exp. Dermatol. 7, 391–397. [DOI] [PubMed] [Google Scholar]
- Stoll, S.W., Kansra, S., and Elder, J.T. (2002). Metalloproteinases stimulate ErbB-dependent ERK signaling in human skin organ culture. J. Biol. Chem. 277, 26839–26845. [DOI] [PubMed] [Google Scholar]
- Stoll, S.W., Kansra, S., and Elder, J.T. (2003). Keratinocyte outgrowth from human skin explant cultures is dependent upon p38 signaling. Wound Repair Regen. 11, 346–353. [DOI] [PubMed] [Google Scholar]
- Stoll, S.W., et al. (2001). Differential utilization and localization of ErbB receptor tyrosine kinase activities in intact skin compared to normal and malignant keratinocytes. Neoplasia 3, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, Y., et al. (1998). Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J. Med. Chem. 41, 640–649. [DOI] [PubMed] [Google Scholar]
- Thien, C.B., and Langdon, W.Y. (2001). Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell. Biol. 2, 294–307. [DOI] [PubMed] [Google Scholar]
- Tikhomirov, O., and Carpenter, G. (2000). Geldanamycin induces ErbB-2 degradation by proteolytic fragmentation. J. Biol. Chem. 275, 26625–26631. [DOI] [PubMed] [Google Scholar]
- Tikhomirov, O., and Carpenter, G. (2001). Caspase-dependent cleavage of ErbB-2 by geldanamycin and staurosporin. J. Biol. Chem. 276, 33675–33680. [DOI] [PubMed] [Google Scholar]
- Tikhomirov, O., and Carpenter, G. (2003). Identification of ErbB-2 kinase domain motifs required for geldanamycin-induced degradation. Cancer Res. 63, 39–43. [PubMed] [Google Scholar]
- Tsai, C.M., Levitzki, A., Wu, L.H., Chang, K.T., Cheng, C.C., Gazit, A., and Perng, R.P. (1996). Enhancement of chemosensitivity by tyrphostin AG825 in high-p185(neu) expressing non-small cell lung cancer cells. Cancer Res. 56, 1068–1074. [PubMed] [Google Scholar]
- Varani, J., Perone, P., Inman, D.R., Burmeister, W., Schollenberger, S.B., Fligiel, S.E., Sitrin, R.G., and Johnson, K.J. (1995a). Human skin in organ culture. Elaboration of proteolytic enzymes in the presence and absence of exogenous growth factors. Am. J. Pathol. 146, 210–217. [PMC free article] [PubMed] [Google Scholar]
- Varani, J., Trinh, D., Carey, T.E., Liebert, M., and Wheelock, M.J. (1995b). Expression of cell surface adhesion molecules on the epithelium of organ-cultured skin. Invasion Metastasis 15, 189–196. [PubMed] [Google Scholar]
- Varani, J., Zeigler, M., Dame, M.K., Kang, S., Fisher, G.J., Voorhees, J.J., Stoll, S.W., and Elder, J.T. (2001). Heparin-binding-epidermal-growth factor-like growth factor activation of keratinocyte erbB receptors mediates epidermal hyperplasia, a prominent side effect of retinoid therapy. J. Investig. Dermatol. 117, 1335–1341. [DOI] [PubMed] [Google Scholar]
- Veitch, D.P., et al. (2003). Mammalian tolloid metalloproteinase, and not matrix metalloprotease 2 or membrane type 1 metalloprotease, processes laminin-5 in keratinocytes and skin. J. Biol. Chem. 278, 15661–15668. [DOI] [PubMed] [Google Scholar]