Abstract
Background
White matter (WM) integrity may represent a shared biomarker for emotional disorders (ED). Aims: To identify transdiagnostic biomarkers of reduced WM by meta-analysis of findings across multiple EDs.
Method
Web of Science was searched systematically for studies of whole brain analysis of fractional anisotropy (FA) in adults with major depressive disorder, bipolar disorder, social anxiety disorder, obsessive-compulsive disorder or posttraumatic stress disorder compared with a healthy control (HC) group. Peak MNI coordinates were extracted from 37 studies of voxel-based analysis (892 HC and 962 with ED) and meta-analyzed using seed-based d Mapping (SDM) Version 4.31. Separate meta-analyses were also conducted for each disorder.
Results
In the transdiagnostic meta-analysis, reduced FA was identified in ED studies compared to HCs in the left inferior fronto-occipital fasciculus, forceps minor, uncinate fasciculus, anterior thalamic radiation, superior corona radiata, bilateral superior longitudinal fasciculi, and cerebellum. Disorder-specific meta-analyses revealed the OCD group had the most similarities in reduced FA to other EDs, with every cluster of reduced FA overlapping with at least one other diagnosis. The PTSD group was the most distinct, with no clusters of reduced FA overlapping with any other diagnosis. The BD group were the only disorder to show increased FA in any region, and showed a more bilateral pattern of WM changes, compared to the other groups which tended to demonstrate a left lateralized pattern of FA reductions.
Conclusions
Distinct diagnostic categories of ED show commonalities in WM tracts with reduced FA when compared to HC, which links brain networks involved in cognitive and affective processing. This meta-analysis facilitates an increased understanding of the biological markers that are shared by these ED.
Keywords: Diffusion tensor imaging, Fractional anisotropy, Major depressive disorder, Bipolar disorder, Anxiety disorders, Meta-analysis
Highlights
-
•
A meta-analysis of FA in MDD, bipolar, social anxiety disorder, OCD and PTSD
-
•
Reduced FA in left superior longitudinal and inferior fronto-occipital fasciculi
-
•
Distinct diagnostic categories show commonalities of white matter changes.
-
•
Differences among diagnostic categories also found, PTSD most distinct
-
•
White matter integrity may be a shared biomarker for emotional disorders.
1. Introduction
White matter (WM) alterations have been identified in numerous emotional disorders (ED), including mood and anxiety disorders (AD) (Ayling et al., 2012, Nortje et al., 2013, Murphy and Frodl, 2011). These alterations are important because disruption to WM pathways could reveal a disconnection syndrome within neural circuits (Schmahmann et al., 2008, Thomason and Thompson, 2011), potentially modifying behavior and cognitive control of emotion. Although EDs have distinct mechanisms, they share many phenotypic (Clark and Watson, 1991) and genotypic commonalities (Kendler et al., 2003). Furthermore, they are often comorbid with one another (Brown et al., 2001, Kessler et al., 2005, Yerevanian et al., 2001). For example, 50–60% of individuals with major depressive disorder (MDD) have a comorbid anxiety disorder (Fava et al., 2000, Kessler et al., 1996), such as social anxiety disorder (SAD), panic disorder (PD), generalized anxiety disorder (GAD), obsessive-compulsive disorder (OCD) or posttraumatic stress disorder (PTSD). There is a high rate of comorbidity of these AD in bipolar disorders (BD) (Freeman et al., 2002, Henry et al., 2003, Pini et al., 1997, McElroy et al., 2001). Furthermore, the ADs are also highly comorbid within themselves (Goisman et al., 1995, Kessler et al., 1995, Pietrzrak et al., 2011). This study therefore sought to conduct a meta-analysis of studies of WM in mood and AD (hereafter emotion disorders, ED), to determine which WM alterations are common or distinct to these classes of psychopathologies. Given the call for transdiagnostic mechanisms put forth by NIMH's Research Domain Criteria (RDoC) initiative (Insel et al., 2010), it is particularly timely to identify shared neural circuitry abnormalities across multiple classes of EDs.
Numerous structural models of psychopathology have reliably reported commonalities among MDD and ADs, with both loading on a broad ‘internalizing disorders’ factor (a factor which appears to be distinct from ‘externalizing’ conditions such as substance use disorders (Vollebergh et al., 2001)). It is somewhat unclear where BD falls in this hierarchy, with some studies showing that it loads on a separate factor from MDD and ADs (Watson, 2005, Kotov et al., 2015). Given the high prevalence of comorbid externalizing disorders such as substance abuse in BD (Cassidy et al., 2001), it is possible that BD includes both internalizing and externalizing features. Furthermore, although most studies of WM abnormalities in BD have reported decreases in integrity similar to studies of MDD, increased measures of integrity in the left uncinate fasciculus and optic radiation have been reported (Versace et al., 2008). By aggregating data from multiple studies of ED, we hypothesize that this meta-analysis will identify WM alterations that are shared across ED. This knowledge will provide important insights into potential mechanisms that are shared across these classes of disorders. To our knowledge, no previous meta-analysis has attempted to examine WM abnormalities compared to healthy controls across MDD, BD and ADs. Therefore the results of this study, which followed the guidelines for Meta-analyses of Observational Studies in Epidemiology, will be important for identifying transdiagnostic biomarkers of WM pathology in ED.
Diffusion tensor imaging (DTI) measures the degree to which randomly diffusing water molecules move in one direction, rather than all directions of tissues (Thomason and Thompson, 2011), providing an indication of the orientation and integrity of WM fibers (Beaulieu, 2002, Kumar et al., 2015). The diffusion tensor is estimated at each voxel, and eigenvalues are calculated which indicate the extent of diffusion. Fractional anisotropy (FA) is a ratio of eigenvalues ranging from 0 (isotropic) to 1 (unidirectional, anisotropic movement) that reflects the degree to which diffusion is confined to a particular direction (Kumar et al., 2015). FA usually decreases in damaged, disorganized or atrophied WM (Thomason and Thompson, 2011), however it is only an indirect marker of WM microstructure (see discussion). Nevertheless, given it is the most widely reported DTI measure, FA was selected as the variable of interest for the present meta-analysis.
2. Materials and methods
2.1. Study selection
The Web of Science database was systematically searched by a postdoctoral researcher (LMJ) on the 16th of September 2014 using search terms (“DTI” OR “diffusion tensor”) AND (“anxiety” OR “depress*” OR “bipolar” OR “mania”). Reference lists were also manually searched for suitable papers, resulting in 695 results. A subsequent editorial decision to add OCD and PTSD resulted in a new Web of Science search on the 12th of April 2016, and yielded 228 results. This search included the terms (“OCD” OR “obsessive compulsive” OR “PTSD” OR “post traumatic stress disorder”) AND (“DTI” OR “diffusion tensor” OR “fractional anisotropy”). In total, 923 studies were identified by these two systematic searches (Fig. 1). Borderline personality disorder was considered for inclusion in the meta-analysis since this diagnosis also involves emotion dysregulation, however we were unable to locate any studies that met our inclusion criteria. Specifically, we conducted a literature search in Web of Science on April 5th, 2016, using the search terms “Borderline personality” and “DTI or diffusion tensor or fractional anisotropy”. This search yielded 25 results. Of these 25 results, none met our inclusion/exclusion criteria for entry into the study. There were 6 reviews, 3 abstracts, 2 studies that did not involve DTI, 4 that did not involve Borderline personality disorder, 5 were not whole-brain studies, 2 that only included adolescents. The remaining 3 studies involved TBSS. Since the main analysis used VBA and the TBSS analysis was used to support the findings of the VBA analysis, we decided not to include borderline personality disorder. As this was a meta-analysis of de-identified published results, institutional ethical approval was not required for this study.
Fig. 1.
Flow chart of exclusion of studies that were identified in the literature search.
Criteria for inclusion were: Whole brain (using voxel-based analysis, VBA) or whole WM (using tract-based spatial statistics, TBSS) analysis of FA in human participants with either MDD, BD or AD, reported in standardized 3D space, constituting original data from participants aged 18–65 years, compared with a healthy control (HC) group, reported in English in a peer-reviewed journal. Given the common comorbidity of PTSD with Traumatic Brain Injury (TBI), studies of PTSD that did not exclude patients with TBI or provide separate results for a non-TBI sub group were excluded. Fig. 1 shows a flowchart of the selection of studies for inclusion. For the purposes of this meta-analysis, AD were limited to generalized anxiety disorder (GAD), social anxiety disorder (SAD), panic disorder (PD), obsessive compulsive disorder (OCD) or post traumatic stress disorder (PTSD). Our literature search identified only one study of GAD that met our inclusion criteria, so GAD was excluded, since one study is not sufficient to establish within-disorder reliability. All studies of PD used TBSS, which was analyzed separately, thus the meta-analysis combined two classes of mood disorder (BD and MDD), and three classes of AD (SAD, OCD and PTSD) to examine transdiagnostic WM alterations for EDs.
The meta-analysis was conducted using seed-based d Mapping (SDM) Version 4.31 (described below). Unlike some other methods, SDM incorporates both positive and negative peaks, and null studies, thus whilst the majority of studies of FA in ED report reduced FA compared to HCs, we were able to include studies in the meta-analyses that reported null results or increased FA. SDM cannot simply or directly meta-analyze statistical parametric maps from diffusion-weighted studies that utilized whole brain WM as well as studies that used TBSS. This is due to the fact that the TBSS method restricts its analysis to mean FA tract skeletons, which do not completely overlap across studies. We chose the approach taken by previous researchers who used SDM (Nortje et al., 2013, Radua et al., 2014), and only meta-analyzed the VBA results. However for a supplementary analysis we also extracted data from the TBSS studies and conducted a separate meta-analysis in an attempt to support our results (see Supplement).
2.2. Data extraction
In total, there were 892 healthy controls (HCs) and 962 participants with ED. These included 415 participants with MDD, 273 with BD, 73 with SAD, 110 with OCD and 91 with PTSD. Only whole-brain WM results were reported; furthermore, we only included the whole-brain corrected results, unless the only significant results were uncorrected, then these were included (e.g. Arnold et al., 2012). SDM requires that included studies have the same correction across the whole brain (although it may differ between studies). This is to avoid biasing results towards regions more liberally thresholded (Radua and Matrix-Cols, 2009). When studies reported separate results by illness subtype, these were included as separate studies, i.e. two studies with non-overlapping contrasts for BD I and BD II (Liu et al., 2010, Ha et al., 2011), one study with separate results for suicide attempters and non-attempters with MDD (Jia et al., 2010), and one study with separate results for OCD and treatment-resistant OCD (TROCD) (Li et al., 2014). The 37 VBA studies and 41 contrasts included in the meta-analysis are reported in Table 1.
Table 1.
Characteristics of VBA studies included in meta-analysis.
| Paper | Patients male/female | HCs male/female | Contrast | Illness length (months) | Patient age mean (SD) | HC age mean (SD) | Meds | HRSD score mean (SD) # items | Dx tool | Excluded psychiatric comorbidities | B0 | Vox. size (mm) | Directions | Highest b (s/mm2) | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDD | |||||||||||||||
| Arnold et al. (2012) | 4 M/13 F | 7 M/14 F | rMDD vs HC | N/A | 30.41 (11.35) | 26.90 (7.82) | No | 9.18 (6.82) 17 item | SCID-I mood sect. | Other psychiatric Dx as per the MINI, SUD | 1.5 | 2.5 × 2.5 × 2.5 | 30 | 900 | Cluster differences of p < 0.001, uncorrected |
| Blood et al. (2010) | 10 M/12 F | 10 M/12 F | MDD vs HC | N/A | 36.3 (12.1) | 35.3 (11.6) | Yes | NA, 31 items | SCID | Psychotic, BD, eating, OCD, SUD, GAD, PD, PTSD | 3 | 2 × 2 × 2 | 6 | 700 | p < 0.00005, whole-brain Bonferroni corrected |
| Choi et al. (2014) | 64 M/70 F | 28 M/26 F | MDD vs HC | 112.08 | 38.49 (11.09) | 34.42 (10.06) | Med. free | 19.28 (3.47) – 17 item | SCID | Psychotic, BD, OCD, SUD | 3 | 2 × 2 × 2 | 60 | 1000 | Permutation based TFCE, and FWE correction (p < 0.05, 10,000 permutations) used with estimation of cluster size |
| Jia et al. (2010) | Suicide attempt = 5 M/11 F; non-attempt = 20 M/16 F | 24 M/28 F | 1. suicide attempt vs HC; 2. non-attempt vs HC | attempt = 80.00; non-attempt = 21.00 | attempt = 34.2 (13.7); non-attempt = 34.7 (12.5) | 37.1 (16.0) | Med.- free for at least 2 weeks prior to study | attempt = 24.6 (3.8); non-attempt = 22.3 (4.3)- 17 item | SCID | Any DSM-IV axis I comorbidities, SUD | 3 | 1.9 × 1.9 × 3 | 15 | 1000 | Voxel-level p < 0.05 (FWE corrected), extent threshold p < 0.05 (uncorrected), k ≥ 50 |
| Ma et al. (2007) | 2 M/12 F | 2 M/12 F | 1st ep. MDD vs HC | 10.30 | 28.9 (8.0) | 27.1 (6.7) | Med. naïve | N/A (BDI = 37.5; 6.9) | SCID | “Any lifetime psychiatric disorder” | 1.5 | 1.9 × 1.9 × 4 | 13 | 1000 | Voxelwise p < 0.001 (uncorrected) & k ≥ 50 |
| Osoba et al. (2013) | 8 M/12 F | 8 M/12 F | MDD vs HC | N/A | 38.3 (11.6) | 33.8 (7.2) | All patient antidep | 15.1 (6.1) – 21 item | ICD-10 | Major medical Dx, seizures, ECT, psychiatric Dx, SUD | 3 | 2 × 2 × 2 | 12 | 1000 | Voxelwise p < 0.05 (corrected) & whole brain αSim correction height p < 0.001 (uncorrected) & k ≥ 256 |
| Ouyang et al. (2011) | 9 M/9 F | 9 M/9 F | 1st ep. MDD vs HC | 15.10 | 27.4 (6.4) | 27.0 (6.8) | Naïve | 24.2 (4.3) 17 item | SCID | Substance abuse Hx | 1.5 | 1.9 × 1.9 × 4 | 13 | 1000 | p < 0.001 (uncorrected), k ≥ 30 |
| Peng et al. (2013) | 19 M/11 F | 14 M/11 F | TRD vs HC | 56.16 | 26.77 (5.28) | 28.24 (4.98) | N/A, resistant to 2 + classes of antidep > 4 weeks at max. dose |
N/A BDI: 20.47 (4.45) | DSM-IV | DSM axis I or II Dx | 3 | 1.3 × 1.3 × 3 | 30 | 1000 | Voxelwise p < 0.05 (FDR corrected) & k ≥ 50, cluster level p < 0.05 (FDR corrected) |
| Tha et al. (2013) | 12 M/7 F | 13 M/6 F | MDD vs HC | 18.37 | 38.6 (13.5) | 36.5 (12.5) | Antidep free > 6 months | 19.0 (4.0) 17 item | DSM-IV-TR and MINI | DSM axis I or II Dx | 1.5 | 1.9 × 1.9 × 5 | 12 | 1000 | p < 0.001 (uncorrected), k ≥ 50 |
| Wang et al. (2013) | 5 M/16 F | 8 M/14 F | MDD pre-treatment vs HC | 14.20 | 29.6 (12.6) | 30.2 (10.2) | Yes | 20.4 (6.3) -17 item | DSM-IV | Other psychiatric Dx or Hx of symptoms | 3 | 1 × 1 × 5 | 14 | 1000 | p < 0.001, k ≥ 50, & αSim correction at p < 0.05 (height threshold p < 0.001, k ≥ 23) |
| Wu et al. (2011) | 10 M/13 F | 9 M/12 F | 1st ep., treatment-naïve MDD vs HC | 2.17 | 31.4 (8.8) | 30.4 (8.2) | Naïve | 21.8 (3.8) 17 item | SCID | DSM axis I or II Dx | 1.5 | 1 × 1 × 4 | 13 | 1000 | p < 0.001 (uncorrected), k ≥ 30 |
| Zou et al. (2008) | 15 M/30 F | 15 M/30 F | MDD vs HC | 20.00 | 33.2 (8.9) | 31.0 (10.3) | Yes | 23.8 (3.3) 17 item | DSM-IV | Major psychiatric Dx including schizophrenia, BD, affective disorder, personality disorder, substance abuse, SUD | 3 | 1.9 × 1.9 × 3 | 15 | 1000 | Cluster threshold p < 0.001 (uncorrected), p < 0.05 at voxel-level after FDR correction |
| BD | |||||||||||||||
| Bruno et al. (2008) | 13 M/23 F | Total N = 28, # M/F not reported | BD (I&II) vs HC | 165.60 | 39 (no SD) | N/A other than matchBD group (range 21–63) | Yes | N/A | SCID | “Comorbid psychiatric conditions” | 1.5 | 2.5 × 2.5 × 5 | 7 | 4 values between 0 and 700 | p < 0.05 at cluster level after FWE correction for multiple comparisons |
| Chaddock et al. (2009) | 9 M/10 F | 10 M/8 F | Psychotic BD I in remission vs HC | N/A | 43.3 (10.2) | 41.7 (12.2) | Yes | N/A but BDI mean = 7.9 (7.0) | SADS-LV | SUD | 1.5 | 2.5 × 2.5 × 2.5 | 64 | 1300 | Voxelwise p < 0.05, the permutation tested with expected number of false positives < 1 per analysis |
| Chen et al. (2012) | 18 M/0 F | 27 M/0 F | BD (I & II 1st ep. Mania) vs HC | Median 50.40 | 32.0 (7.6) | 31.3 (6.8) | Yes but not > 5 days prior | 3.2 (1.1) – 17 item | SCID | Axis I Dx, Hx of drug or alcohol abuse | 3 | 1.9 × 1.9 × 3 | 15 | 1000 | p < 0.05 (FDR corrected) and k ≥ 50 |
| Cui et al. (2011) | 10 M/8 F | 18 M/12 F | BD I (current manic ep.) vs HC | 57.60 | 27.9 (5.7) | 23.9 (10.2) | Yes | 3.1 (2.2) | SCID | Hx substance abuse | 3 | 1.9 × 1.9 × 3 | 15 | 1000 | Voxelwise p < 0.001 (uncorrected) and k ≥ 50 |
| Ha et al. (2011) | BD I = 3 M/9 F; BD II = 2 M/10 F | 5 M/17 F | 1. BD I vs HC; 2. BD II vs HC | BD I = 159.60; BD II = 159.60 | BD I = 37.3 (10.59); BD II = 35.6 (7.56) | 34.7 (7.12) | Yes | BP I = 5.5 (6.9); BP II = 4.2 (4.43) - 17 item | SCID | Hx substance abuse, current “severe mood episode” | 1.5 | 2 × 2 × 3 | 15 | 600 | p < 0.001 (uncorrected), k ≥ 40 |
| Liu et al. (2010) | BD I = 7 M/7 F; BD II = 2 M/11 F | 8 M/13 F | 1. BD I vs HC; 2. BD II vs HC |
BD I = 87.60; BD II = 112.8 | BD I = 35.6 (10.9); BD II = 35.1 (9.8) | 38.3 (11.9) | Yes | BP I = 6.7 (5.8); BP II = 9.5 (6.6) 17 item | SCID | N/A | 1.5 | 2 × 2 × 2.2 | 13 | 900 | p < 0.001 (uncorrected, k ≥ 50), then applied SVC to significant clusters using 8 mm radius at peak with FDR p < 0.001 |
| Mahon et al. (2009) | 15 M/15 F | 22 M/16 F | BD (I, II & NOS) vs HC | N/A | 33.4 (8.7) | 31.9 (8.6) | Yes | N/A | SCID | N/A | 1.5 | 1.7 × 1.7 × 5 | 25 | 1000 | Voxelwise p < 0.001, k ≥ 50 & FDR corrected p < 0.015 |
| Sussmann et al. (2009) | 22 M/20 F | 19 M/19 F | BD I vs HC | 219.60 | 39.6 (10.1) | 37.2 (11.9) | Yes | 2.3 (5.8) | SCID | N/A | 1.5 | 1.7 × 1.7 × 2.8 | 51 | 1000 | p < 0.001 (uncorrected) |
| Wessa et al. (2009) | 11 M/11 F | 12 M/9 F | BD I & BD II (remitted) vs HC | 264 | 45.41 (12.60) | 42.95 (13.17) | Yes | 1.55 (1.53) | DIGS | N/A | 1.5 | 1.9 × 1.9 × 2 | 41 | 700 | For VBA: p < 0.05 (FDR corrected), k ≥ 70 |
| Zanetti et al. (2009) | 13 M/24 F | 12 M/14 F | BD I (active & remitted) vs HC | 139.20 | 34.1 (9.0) | 28.8 (9.5) | Yes | 9.4 (9.4) 25 item | SCID | Borderline personality disorder, current alcohol or substance abuse or dependence | 3 | 1.6 × 1.6 × 3 | 6 | 850 | Voxelwise p < 0.001 (uncorrected), k ≥ 50 |
| SAD | |||||||||||||||
| Baur et al. (2011) | 18 M/7 F | 18 M/7 F | SAD vs HC | 192 | 32 (10.4) | 32 (10.1) | Yes | N/A BDI = 15 (10.8) | MINI | N/A | 3 | 0.9 × 0.9 × 3.2 | 21 | 1000 | Voxelwise p < 0.00001 (uncorrected), cluster-extent FWE correction p < 0.05, k ≥ 48 |
| Phan et al. (2009) | 15 M/15 F | 10 M/20 F | SAD vs HC | N/A | 27.20 (7.80) | 29.90 (8.13) | Free for < 8 weeks (except n = 1) | N/A BDI = 10.7 (6.51) | SCID | OCD, PTSD, BD, psychotic disorder | 3 | 0.9 × 0.9 × 3 | 12 | 900 | p < 0.01 (uncorrected) volume ≥ 100 mm3 |
| Qiu et al. (2014) | 12 M/6 F | 12 M/6 F | SAD vs HC | 49.22 | 22.72 (3.85) | 21.78 (3.90) | Naïve | N/A | SCID | DSM axis I or II disorders, drug abuse or dependence | 3 | 1.9 × 1.9 × 3 | 15 | 1000 | p < 0.01, uncorrected |
| OCD | |||||||||||||||
| Admon et al. (2012) | 10 M/3 F | 10 M/3 F | OCD vs HC | 96 | 25.5 (1.0) | 27.0 (0.5) | All taking | N/A | SCID | Substance abuse | 1.5 | 1.6 × 1.6 × 3 | 6 | 1000 | Cluster p < 0.005, k ≥ 10, uncorrected |
| Cannistraro et al. (2007) | 3 M/5 F | 4 M/6 F | OCD vs HC | N/A | 26.4 (5.9) | 23.3 (1.5) | Med-free 4 + weeks | N/A | SCID | Current or past axis I Dx | 1.5 | 2 × 2 × 2 | 6 | 600 | Voxelwise p < 0.05, k ≥ 20, uncorrected, then cluster p < 0.005, uncorrected |
| Garibotto et al. (2010) | 15 M/0 F | 16 M/0 F | OCD vs HC | 121.56 | 31.87 (7.92) | 29.67 (6.3) | Yes | N/A | DSM-IV | Hoarding behavior, motor tics, Tourette's, axis I or II Dx | 1.5 | 1.9 × 1.9 × 2.3 | 35 | 1000 | p < 0.005, uncorrected, k ≥ 20 |
| Li et al. (2014) | Both OCD N = 11 (M/F N/A) | N = 11 (M/F N/A) | 1. TROCD vs HC; 2. OCD vs HC | TROCD = 23.4; OCD = 18.6 | N/A | N/A | All | TROCD = 15.36 (4.30); OCD = 13.55 (2.94) | SCID | Current axis I Dx or psychiatric Dx in past year | 3 | 2 × 2 × 2 | 30 | 1000 | p < 0.05 FDR corrected, k ≥ 50 |
| Menzies et al. (2008) | 9 M/21 F | 10 M/20 F | OCD vs HC | N/A | 32.2 (11.1) | 33.7 (11.2) | Yes | N/A | DSM-IV, MINI | Hoarding or motor tics, Hx of substance abuse, DSM-IV axis I Dx (except PD) | 1.5 | 2.3 × 1.9 × 4 | 25 | 1000 | Cluster p < 0.017, uncorrected |
| Nakamae et al. (2008) | 9 M/6 F | 9 M/6 F | OCD vs HC | 121.2 | 29.7 (6.9) | 29.1 (6.0) | All currently taking | N/A | SCID | SUD, schizophrenia, schizoaffective disorder, delusional disorder, brief reactive psychosis, psychotic disorder NOS | 1.5 | 1.8 × 2.2 × 3 | 15 | 1000 | p < 0.001 (uncorrected), k ≥ 100 |
| Szeszko et al. (2005) | 10 M/5 F | 10 M/5 F | OCD vs HC | 259.2 | 38.5 (10.9) | 38.5 (11.8) | Yes | 13.9 (4.9) | SCID | SUD, schizophrenia, schizoaffective disorder, delusional disorder, brief reactive psychosis, psychotic disorder NOS | 1.5 | 1.7 × 1.7 × 5 | 25 | 1000 | p < 0.005, k ≥ 20 (uncorrected) |
| Yoo et al. (2007) | 8 M/5 F | 8 M/5 F | OCD pre-treatment vs HC | 86.4 | 27.8 (7.3) | 26.9 (7.0) | Naïve | 12.5 (7.6) | SCID | Psychosis, BD, SUD, Tourette's or other tic-related conditions | 1.5 | 1.7 × 1.7 × 4 | 6 | 600 | p > 0.001 (uncorrected), k ≥ 20 |
| PTSD | |||||||||||||||
| Kennis et al. (2015) | rPTSD = 15 M/0 F; PTSD = 18 M/0 F | 24 M/0 F | Non-blast PTSD vs combat controls prior to PTSD treatment | N/A | rPTSD = 34.38 (9.58); PTSD = 36.61 (8.74) | 37.64 (10.97) | Yes | N/A | DSM-IV | Alcohol or substance dependence | 3 | 1.9 × 1.9 × 2 | 30 | 1000 | TFCE p < 0.05, corrected |
| Kim et al. (2005) | 8 M/12 F | 9 M/11 F | PTSD vs HC | N/A | 28.4 (8.9) | 30.6 (8.9) | N/A | N/A | SCID and CAPS | Premorbid axis I Dx, antisocial or borderline personality disorder, ADHD or lifetime exposure to illicit substances | 3 | 0.9 × 0.9 × 3.5 | 25 | 1000 | p < 0.05, corrected |
| Sun et al. (2013) | 8 M/13 F | 9 M/13 F | Trauma exposed with PTSD vs trauma exposed without PTSD | All scans within 2 days of trauma, Dx made at 1 or 6 months | 40.86 (12.26) | 40.23 (12.54) | Med-free for 4 weeks prior to MRI | N/A | MINI and CAPS | Axis I Dx, drug or alcohol abuse or dependence within 6 months | 3 | 2 × 2 × 2 | 20 | 1000 | αSim p < 0.05 corrected, with voxel p < 0.001, cluster k ≥ 76 |
| Zhang et al. (2011) | 17 M/0 F | 14 M/14 F | First episode PTSD vs HC | N/A | 34.06 (4.97) | 28.96 (6.22) | Naive | N/A | SCID | Any current or past comorbid psychiatric Dx | 1.5 | 1.9 × 1.9 × 4 | 13 | 1000 | p < 0.001, uncorrected, k ≥ 100 |
Note. Abbreviations: αSim = AlphaSim, antidep = antidepressant = BD = bipolar, CAPS = Structured Clinician-Administered PTSD Scale for DSM-IV, DIGS = Diagnostic Interview for Genetic Studies, DSM = Diagnostic and Statistical Manual, Dx = diagnosis, eating = eating disorder, ECT = electroconvulsive therapy, ep. = episode, FDR = False Discovery Rate, FWE = family-wise error, GAD = generalized anxiety disorder, HC = healthy control, HRSD = Hamilton Rating Scale for Depression, Hx = history, k = cluster size threshold, max = maximum, MDD = major depressive disorder, med = medication, MINI = Mini-International Neuropsychiatric Interview, NOS = not otherwise specified, OCD = obsessive compulsive disorder, PD = panic disorder, psychotic = psychotic disorder, PTSD = post-traumatic stress disorder, rMDD = remitted major depressive disorder, rPTSD = remitted post-traumatic stress disorder, SAD = Social Anxiety Disorder, SADS-LV = Schedule for Affective Disorders – Lifetime Version, SCID = Structured clinical interview for DSM disorders, SD = standard deviation, sect. = section, SVC = small volume correction, SUD = substance use disorder, TCFE = threshold-free cluster enhancement, TRD = treatment resistant depression, TROCD = treatment resistant obsessive compulsive disorder, VBA = voxel based analysis, vox size (mm) = voxel size in millimeters.
Data were extracted independently by two researchers (LMJ, AB), including the number of males and females in each group, age, illness duration, diagnostic tool, handedness, medication status, psychiatric comorbidities, number of diffusion directions, B0, voxel size, imaging sequence, b value and statistical thresholds. All imaging was performed as spin-echo EPIs. Where necessary authors were contacted for further information. For the meta-analysis, from each contrast we extracted peak MNI coordinates and t values of FA differences between ED and HC groups. Peak coordinates were double-checked by both researchers. Foci reported in Talairach space were converted to MNI space with Brett's transform (http://eeg.sourceforge.net/doc_m2html/bioelectromagnetism/mni2tal_matrix.html). When only z or p values were available, these were converted to t values using the SDM online conversion utilities.
2.3. Meta-analysis
Seed-based d Mapping (SDM), described in more detail elsewhere (Radua and Matrix-Cols, 2009, Radua et al., 2012), is a statistical technique for meta-analysis of neuroimaging data, including diffusion tensor imaging (DTI). SDM uses a random effects model that takes into account sample size, intra-study variability, and between-study heterogeneity, and statistical significance is assessed using a distribution-free permutation test (Radua et al., 2012). A main reason we chose SDM was because it has a specific mask correlation template for FA (Radua et al., 2011). This specific template accounts for the anisotropy in the spatial covariance of the brain, which increases the accuracy of the effect size maps, and has been successful in prior mood disorder studies (Nortje et al., 2013). While other meta-analyses of mood disorders have used different techniques, such as Activation Likelihood Estimation, these techniques are not optimal for use with DTI studies as they do not include a white-matter mask, and they also cannot include clusters of both increased and decreased FA in patients compared to HC.
Similar to Nortje et al. (2013), we employed threshold values of p < 0.005, Z > 1 and k = 10. This threshold has been shown to optimize sensitivity at the same time as correctly controlling the false positive rate, and was approximately equivalent to a corrected p value of .025 (Radua et al., 2012). Peaks of significant clusters were labeled automatically by the SDM software (Rojkova et al., 2016, Thiebaut de Schotten et al., 2011a, Thiebaut de Schotten et al., 2011b). A jack-knife analysis was performed to establish reliability of the results (Radua and Matrix-Cols, 2009). Publication bias was assessed with funnel plots (Supplement) and Egger tests. As per a previous SMD meta-analyses (Murphy and Frodl, 2011), a linear meta-regression assessed the influence of illness duration. An additional linear meta-regression assessed the influence of depression severity, as measured with the Hamilton Depression Rating Scale (HDRS).
3. Results
3.1. Transdiagnostic (shared) meta-analysis
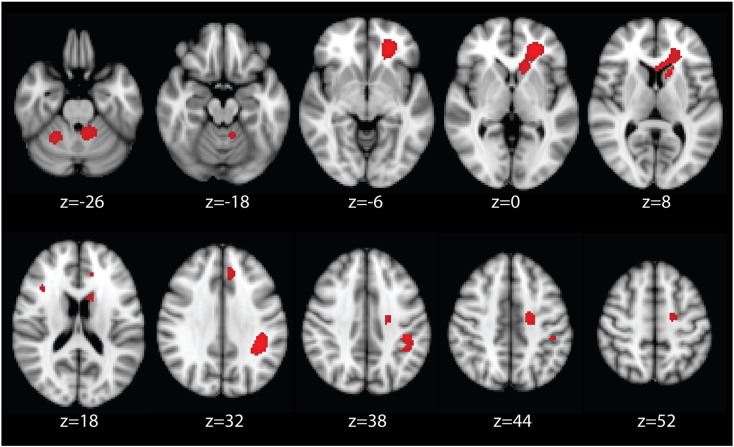
The results of the ED meta-analysis are presented in Fig. 2. For all significant clusters, the ED studies reported reduced FA compared to HCs. Cluster peaks are reported in Table 2.
Fig. 2.
Regions of decreased fractional anisotropy in emotional disorders compared to healthy controls, identified from the meta-analysis of VBA studies, p < 0.005.
Note. Figure is in radiological convention, i.e. left = right.
Table 2.
Cluster peaks of meta-analysis of VBA studies of emotional disorders vs HC.
| Peak region | x | y | z | Z | p | Voxels |
|---|---|---|---|---|---|---|
| Left forceps minor/ATR/IFOF/UF | − 24 | 40 | − 6 | − 1.59 | < 0.0001 | 1228 |
| Left SLF | − 40 | − 40 | 32 | − 1.82 | < 0.0001 | 389 |
| Left cerebellum (hemispheric lobule I–IV) | − 10 | − 48 | − 24 | − 1.16 | 0.0006 | 237 |
| Left superior corona radiata/corticospinal tract | − 18 | − 12 | 44 | − 1.27 | 0.0002 | 201 |
| Right cerebellum (hemispheric lobule VI) | 26 | − 52 | − 26 | − 1.12 | 0.0008 | 109 |
| Right SLF | 36 | 20 | 20 | − 1.11 | 0.0008 | 69 |
Note. SLF = Superior longitudinal fasciculus, ATR = anterior thalamic radiation, IFOF = inferior fronto-occipital fasciculus, UF = uncinate fasciculus.
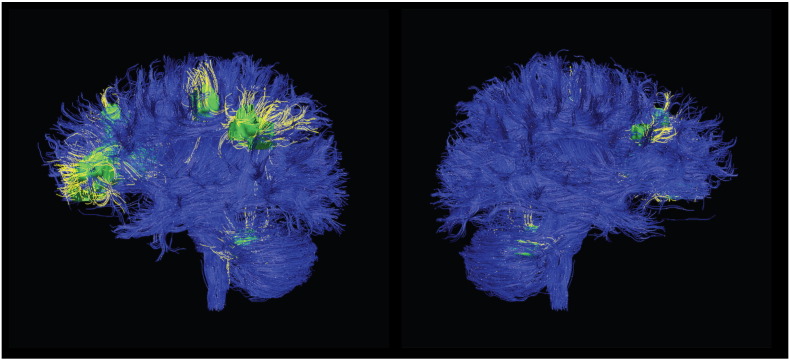
The largest cluster incorporated several white matter tracts, including the left forceps minor of the corpus callosum (CC), anterior thalamic radiations (ATR), inferior fronto-occipital fasciculus (IFOF) and the uncinate fasciculus. The second largest cluster peaked in the left superior longitudinal fasciculus (SLF), and also involved white matter in the parietal lobe. The third largest cluster peaked in the left cerebellum (hemispheric lobule I-IV), and there was a smaller cluster in the right cerebellum (hemispheric lobule VI). Another cluster peaked in the left superior corona radiata and incorporated the corticospinal tract. The smallest cluster peaked in the right SLF. For illustrative purposes, Fig. 3 shows the location of these clusters of reduced FA on the affected fiber tracts, which are otherwise intact. It should be noted that we did not perform tractography, rather Fig. 3 shows the significant clusters found in our meta-analysis superimposed on a standard tractography image. Significant clusters are shown in green, and affected WM tracts in yellow, for illustrative purposes. A three dimensional video is available in the online Supplement.
Fig. 3.
White matter tracts implicated in VBA meta-analysis of emotional disorders.
Note. Significant clusters are shown in green, and tracts implicated in yellow. The left hemisphere is shown in the left panel and the right hemisphere in the right panel.
To support these main findings from the VBA studies, we also extracted data from the TBSS studies and conducted a separate meta-analysis. These results are reported in the Supplement.
3.2. Illness duration analysis
A linear meta-regression was conducted in SDM to assess the influence of illness duration (years). Illness duration information was not available for 12 studies (three MDD, two BD, one SAD, two OCD and four, i.e. all PTSD). A univariate ANOVA found a significant effect of group for illness duration, F (3, 25) = 4.87, p = 0.008. The BD contrasts (n = 10) had the longest mean illness duration = 11.80, (SD = 5.65); SAD (n = 2), mean illness duration = 10.05, (SD = 8.41); OCD (n = 7), mean illness duration = 8.65, (SD = 6.71); the MDD contrasts (n = 10) included patients with the shortest mean illness duration = 2.91 (SD = 3.00). Post hoc tests found a significant difference in illness duration between the MDD and BD groups (p = 0.005).
The linear meta-regression revealed longer illness duration was associated with increased FA in nine diffuse brain regions, including several posterior clusters located in the forceps major, and decreased FA in seven brain regions, of which six were left lateralised. These results are reported in Table 3. The finding of increased FA associated with longer illness duration in several regions is difficult to interpret because of the significant difference in illness duration between the MDD and BD group, which may reflect an interaction.
Table 3.
Results of linear meta-regression of VBA studies of illness duration vs HCs.
| Peak region | Change of FA | x | y | z | Z | p | Voxels |
|---|---|---|---|---|---|---|---|
| Superior longitudinal fasciculus | + | − 30 | − 48 | 50 | 1.43 | 0.0001 | 138 |
| Corticospinal tract | + | 18 | − 26 | 64 | 1.33 | 0.0003 | 134 |
| Forceps minor | + | − 20 | 54 | 18 | 1.42 | 0.0002 | 128 |
| Forceps major | + | − 4 | − 80 | 22 | 1.45 | 0.0001 | 99 |
| Anterior thalamic radiation | + | − 14 | 18 | 8 | 1.15 | 0.0012 | 109 |
| Forceps major | + | 24 | − 80 | 30 | 1.26 | 0.0006 | 92 |
| Corticospinal tract | + | 6 | − 46 | 64 | 1.45 | < 0.0001 | 75 |
| Forceps major | + | − 34 | − 80 | 30 | 1.25 | 0.0007 | 82 |
| Forceps major | + | 4 | − 80 | 24 | 1.32 | 0.0004 | 59 |
| Superior corona radiata | − | − 20 | − 10 | 50 | − 2.06 | 0.0001 | 285 |
| Uncinate fasciculus | − | − 24 | 34 | − 8 | − 1.26 | 0.0021 | 113 |
| Inferior frontooccipital fasciculus | − | − 32 | − 30 | 14 | − 1.30 | 0.0018 | 72 |
| Cingulum | − | − 8 | 12 | 36 | − 1.48 | 0.0010 | 66 |
| Superior longitudinal fasciculus | − | − 48 | − 40 | 40 | − 1.30 | 0.0018 | 53 |
| Cingulum | − | 8 | 18 | 36 | − 1.20 | 0.0025 | 35 |
| Forceps major | − | − 18 | − 80 | 4 | − 1.10 | 0.0035 | 17 |
Note. FA is increased or decreased with longer illness duration.
3.3. Depression severity analysis
A linear meta-regression was also conducted to assess the influence of current depression severity, as measured by the Hamilton Rating Scale for Depression (HDRS). HDRS score was not available for 18 studies (three each of MDD and BD, five of OCD, and three and four, i.e. all, of SAD and PTSD, respectively). An ANOVA found a significant main effect of group for HDRS, F (2, 21) = 126.65, p < 0.001. Post hoc tests revealed that the MDD contrasts (M = 21.93, SD = 2.20) included patients with significantly higher HDRS scores than the BD contrasts (M = 4.72, SD = 2.53), p < 0.001, and the OCD contrasts (M = 13.83, SD = 1.18), p < 0.001. The BD and OCD contrasts also differed significantly, p < 0.001. Table 4 reports that higher HDRS score (indicating greater current depression severity) predicted decreased FA in four regions, with the largest clusters peaking in the left ATR and superior longitudinal fasciculus. Comparison of these results to those of Fig. 4 reveals that as expected, given the significantly higher HDRS scores in the MDD group, the regions significantly associated with depression severity were all regions that showed significantly reduced FA in MDD in the disorder-specific meta-analysis, reported below.
Table 4.
Regions of decreased FA associated with greater Hamilton Depression Rating Scale score.
| peak region | x | y | z | Z | p | voxels |
|---|---|---|---|---|---|---|
| Anterior thalamic radiation | − 14 | 20 | 4 | − 3.00 | < 0.0001 | 730 |
| Superior longitudinal fasciculus | − 38 | − 42 | 32 | − 2.88 | < 0.0001 | 354 |
| Cerebellum (right VI) | 28 | − 54 | − 24 | − 2.26 | < 0.0001 | 247 |
| Corticospinal tract | 16 | − 18 | 54 | − 1.48 | 0.0025 | 53 |
Note. FA is decreased with greater Hamilton Depression Rating Scale score.
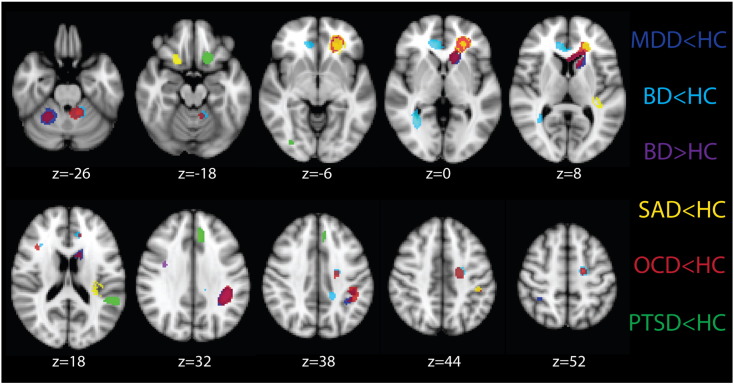
Fig. 4.
Regions of differences in fractional anisotropy between MDD, BD, SAD, OCD, PTSD and HC identified from separate meta-analyses of VBA studies for each disorder.
Note. Figure is in radiological convention, i.e. left = right. MDD = major depressive disorder, BD = bipolar disorder, SAD = social anxiety disorder, OCD = obsessive-compulsive disorder, PTSD = post-traumatic stress disorder, HC = healthy controls.
3.4. Assessments of reliability and publication bias
The Jack-knife analysis found that the left SLF cluster (− 40, − 40, 32) remained significant across all 41 sub-analyses, indicating that this was the most robust finding. The largest cluster, located in the left IFOF (− 24, 40, − 6), remained significant in all sub-analyses except for in five sub-analyses for which the peak of this cluster shifted to a different location within this large cluster (− 12, 18, 6). The cluster in the left superior corona radiata (− 18, − 12, 44) was quite robust, remaining significant in 40 sub-analyses (excluding Sussmann et al., 2009). The clusters in the left (− 10, − 48, − 24) and right cerebellum (26, − 52, − 26) were both significant in 39 sub analyses, and the right SLF (36, 20, 20) was significant in 38 sub-analyses.
Publication bias was assessed by extracting values from each of the significant peaks in the ED meta-analysis. Funnel plots were created to allow for visual inspection of the inter-study heterogeneity (see Supplement). Egger tests were calculated and revealed no evidence for publication bias for any of the clusters, p > 0.05.
3.5. Disorder-specific meta-analyses
We also conducted separate meta-analyses for each ED. These results are presented in Fig. 4. Peak coordinates of these results are reported in the Supplement. The two largest peaks of reduced FA in MDD contrasts were in left hemisphere regions of the ATR and SLF, which were two of the most robust regions identified from the Jackknife analysis. The BD contrasts revealed the only increase in FA (relative to HC), in the right SLF. They also had decreased FA in this same tract more anteriorly. Other regions of decreased FA in the right hemisphere were the forceps minor and IFOF, however the BD group also had decreased FA in several left hemisphere regions, including the SLF, cingulum, forceps minor and cerebellum. The SAD studies showed a primarily left lateralized pattern of decreased FA, which occurred in the SLF and corticospinal tract, and the UF bilaterally (more ventrally on the right). Two of the clusters for SAD overlapped with OCD and/or MDD. The OCD group showed reduced FA in several regions, all of which overlapped with at least one other diagnosis. These included the left SLF, IFOF, cerebellum and right ILF, shared with the BD contrasts, the left superior corona radiata shared with the SAD contrasts, the left IFOF/UF/ATR shared with the MDD and SAD contrasts, and the right cerebellum shared with the MDD group. Finally, like the OCD, SAD and to a lesser extent MDD contrasts, the PTSD contrasts revealed reduced FA in primarily left hemisphere regions. Unlike OCD and to a lesser extent SAD, however, these regions did not overlap with those of other disorders. Reduced FA was found in the left UF, however this was inferior to the region identified in MDD, SAD and OCD. Similarly, the region of the left SLF identified in the PTSD contrasts was inferior to that of the other diagnoses. There was also reduced FA in the left cingulum and the right ILF in PTSD.
4. Discussion
The present meta-analysis revealed that overall, emotional disorders exhibit a shared pattern of reduced FA in fronto-temporal and fronto-parietal white matter tracts compared to HCs. The most robust and replicable finding (determined by Jack-knife analysis), peaked in the left superior longitudinal fasciculus (SLF). The largest cluster identified peaked in the left forceps minor/inferior fronto-occipital fasciculus, and extended to include the uncinate fasciculus (UF) and anterior thalamic radiations (ATR). There was also a significant peak in the region of the left superior corona radiata/corticospinal tract, bilateral cerebellum and right SLF. These tracts link important networks involved in cognition, affect, and the regulation thereof. The discussion therefore focuses on the SLF, IFOF, UF, anterior corpus callosum and tracts within the cerebellum, including evidence for their potential functional roles that may be relevant to emotional disorders. We also present converging evidence for alterations in FA in these tracts in ED from the TBSS studies listed in the Supplement. Due to space limitation, relatively less discussion of ED differences is provided.
4.1. Superior longitudinal fasciculus
The left SLF was the most replicable tract identified in the transdiagnostic meta-analysis. The SLF is a major association fiber tract connecting parieto-temporal association areas and the frontal lobe, and links aspects of default mode (medially) and cognitive control (laterally) networks. The SLF II terminates in the dorsolateral PFC (Makris et al., 2005), which is closely associated with executive functioning (Tekin and Cummings, 2002). Thus the SLF II may offer a means for the PFC to control the focusing of spatial attention by the parietal lobe (Schmahmann et al., 2008, Makris et al., 2005). The SLF III is located in the parietal and frontal opercula and reaches from the supramarginal gyrus to ventral prefrontal and premotor areas (Makris et al., 2005). It provides higher order somatosensory input to the ventral premotor region and pars opercularis. It may also be important for monitoring hand and facial action, as well as articulatory and phonemic aspects of language (Schmahmann et al., 2008, Makris et al., 2005). These functions are consistent with the impairments in perception of and attention to emotional information in ED, including prosody and facial expressions of emotion. Our findings of reduced FA in the SLF are also supported by the TBSS studies reported in the Supplement, including findings from studies of reduced FA in the left SLF in OCD (Spalletta et al., 2014) and PTSD (Fani et al., 2012), as well of studies of BD (Ambrosi et al., 2013), particularly in the left SLF (Versace et al., 2010), as well as in the right hemisphere in MDD (Murphy et al., 2012) and OCD (Benedetti et al., 2013), and bilaterally in treatment-resistant depression (de Diego-Adelino et al., 2014).
We also found evidence for increased FA in BD in the right SLF. Increased FA in BD has been reported previously (Versace et al., 2008). Whilst this may seem counter-intuitive, it is important to note that higher FA reflects more numerous, myelinated, dense, and coherently orientated fibers in many circumstances, but it does not always signify healthier WM or beneficial outcomes. On the contrary, higher FA could suggest, for example, compensatory mechanisms, as WM structure is shaped by experiences (Thomason and Thompson, 2011). Therefore, when evaluating results of FA studies, the interpretation that decreased FA is maladaptive and increased FA is adaptive may be too simplistic an explanation.
4.2. Inferior fronto-occipital fasciculus
The largest cluster peaked in the left IFOF, connecting the dorsolateral and inferolateral frontal cortex with the occipital lobe and posterior temporal cortex (Catani et al., 2002). As such, the IFOF is an intra- and cross-network connection of cognitive control and salience networks often implicated in EDs. The IFOF traverses the sagittal striatum, and progresses posteriorly through the medial temporal lobe, passing through the anterior floor of the external capsule where it is parallel to the inferiorly located UF fibers (Catani et al., 2002). The fronto-occipital fasciculus comprises part of the dorsal visual stream and is implicated in peripheral vision and processing visuospatial information (Schmahmann et al., 2008) and the IFOF is located near a region important for processing of facial emotions (Nakamura et al., 1999). The finding of reduced FA in the left IFOF in the VBA studies is supported by the TBSS studies listed in the Supplement, which have reported reduced FA in this region in patients with OCD (Benedetti et al., 2013), MDD (Kieseppa et al., 2010), melancholic MDD (Korgaonkar et al., 2011), and in patients with MDD who were homozygous for the A allele of NTRK2 (Murphy et al., 2012), a neurotrophic factor hypothesized to be a risk factor for MDD. Reduced FA bilaterally has also been reported in the IFOF in patients with TRD (de Diego-Adelino et al., 2014) in BD (Ambrosi et al., 2013, Chan et al., 2010), PD (Lai and Wu, 2013) and OCD (Fan et al., 2016).
4.3. Uncinate fasciculus
The UF joins the orbital and polar frontal cortex to the anterior temporal lobe, as it reaches out from the amgydala, hippocampal gyrus, uncus and temporal pole (Catani et al., 2002). Considered part of the limbic system (emotion and salience networks), the UF may be a pathway via which the PFC exerts control over limbic regions involved in emotion processing, an ability that is reduced in MDD (Anand et al., 2005). The UF is an important part of the system for regulating emotional responses to auditory stimuli (Schmahmann et al., 2008), and could also play a role in attaching emotional valence to visual stimuli. Reduced FA in the UF might support a mechanism of decreased salience and emotional network connectivity and activity in limbic regions, including the amygdala and subgenual anterior cingulate cortex, which lie at both ends of the UF.
The strongest evidence for reduced FA in the UF in ED is from studies of MDD. For example, tractography studies (excluded from our analysis as they do not report stereotactic coordinates) have reported reduced FA in the UF in MDD (Zhang et al., 2012, de Kwaasteniet et al., 2013). Murphy et al. (2012) found reduced FA in patients with MDD compared to HC in the left UF. Another FA study with an upper age limit beyond 65 years also found reduced FA in the left UF in MDD (Steele et al., 2005). However Lai and Wu (2013) failed to demonstrate reduced FA in panic disorder, and results from individual studies of BD are more mixed, with Ambrosi et al. (2013) finding reduced FA in the UF of patients with BD, but Versace et al. (2008) reporting increased FA. Further research is necessary to determine the impact of hypo/manic symptoms on FA in BD.
4.4. Anterior corpus callosum
The CC is critical for interhemispheric communication, particularly the integration of high-level cognitive, emotional, perceptual and linguistic functions across numerous networks (Gazzaniga, 2000). The forceps minor of the CC was implicated in the largest cluster in the ED analysis. Some, but not all studies (Yurgelun-Todd et al., 2007) that were not included because they exceeded the upper age limit to 65 have also reported reduced FA in the forceps minor and genu of the CC in individuals with MDD (Cole et al., 2012, Seok et al., 2013) and BD (Benedetti et al., 2011). Numerous TBSS studies included in the Supplement reported reduced FA in the forceps minor/genu of the CC, including for MDD (Murphy et al., 2012), young adults with first-episode, treatment-responsive depression (Guo et al., 2012) and patients with treatment resistant depression (de Diego-Adelino et al., 2014). One TBSS study of OCD also found reduced FA in the forceps minor bilaterally (Benedetti et al., 2013), consistent with Fig. 4 that showed this region to be affected in the left hemisphere in OCD in the VBA meta-analysis.
4.5. Cerebellum
Tracts within the cerebellum are known to connect with the PFC, subcortical limbic nuclei, and brainstem structures that produce monoamines (Schmahmann, 2000). The anterior lobe has a primary sensorimotor region, and the posterior lobe contains association areas important for higher order behavior, via connections with basis pontis and thalamic nuclei (Schmahmann and Caplan, 2006). Disrupted cerebellar and corticocerebellar connectivity has been proposed as a main neurobiological mechanism of emotion dysregulation (Schmahmann, 2000), as lesions confined to the cerebellum can result in a cerebellar cognitive affective syndrome that includes dysregulation of affect (Schmahmann and Caplan, 2006).
4.6. Distinctiveness of each finding across EDs
Notably, the results of the transdiagnostic meta-analyses are primarily left lateralized. Severity of post-stroke depression has also been correlated with proximity of stroke lesion to the left frontal pole, which may align with the shared negative affect across these EDs. However this association has been found both in cortical lesions and lesions restricted to subcortical regions (Starkstein and Robinson, 1987), thus depression following brain lesions cannot be attributed to disruptions to WM alone. Furthermore, stroke lesions impair signal transport by destroying nerve fiber bundles locally, in contrast to decreased signal transport due to a more extensive impairment of neurons. Whilst our focus was largely on shared WM alterations across all three disorders, there is some evidence of differences. Most notably, the PTSD findings were the most distinct, demonstrating no overlap with any other EDs for any of the significant clusters in the separate meta-analyses for each disorder. The OCD group showed overlap with both categories of mood disorders, including for clusters in the right hemisphere. OCD also showed overlap with SAD for two of the clusters, one of which it also shared with MDD (the largest cluster, i.e. the IFOF/ATR/UF/forceps minor cluster). The BD findings were more bilateral relative to the tendency for left laterality observed across the other groups. This laterality finding harkens back to early lesion research suggesting right hemisphere stroke is associated with a hypomanic state characterized by inappropriate cheerfulness, loss of interest, anxiety, slowness and agitation (Robinson et al., 1983). Left prefrontal hypometabolism has also been reported in patients with BD in the depressed phase (Baxter et al., 1989). However, subsequent studies have found little consistent support for lateralization of mood or emotion, and only limited support for the valence hypothesis of emotion in the frontal cortex (Wager et al., 2003). BD also differed from the other disorders in that it was the only one to find increased FA in any region. This finding supports previous findings that BD loads on a separate factor from MDD and AD (Watson, 2005, Kotov et al., 2015); however it may also suggest BD is the addition of a separate manic disorder to MDD and/or AD. Future studies might examine the relationship between increases in FA for contrasts between internalizing and externalizing symptoms.
4.7. Limitations
The present study had several limitations. First, there were insufficient GAD studies using VBA that met our inclusion criteria, and all studies of panic disorder used TBSS thus were only able to be included in the Supplemental analysis. Second, we chose to exclude studies with any subjects outside of the age range 18–65 to limit the potential influence of degenerative conditions and development, thus numerous studies primarily involving adults were excluded (Steele et al., 2005, Seok et al., 2013, Benedetti et al., 2011). The included studies differed in diffusion imaging methods, which could limit the interpretability of the findings. For example, more recent studies tend to use correcting procedures for EPI distortions that more dated studies did not, which could impact findings in proximity of brain regions that are sensitive to B0 inhomogeneities. There are also limitations in the interpretation of FA, as it is only an indirect marker of WM microstructure. Although commonly interpreted to reflect decreased myelination or progressive myelin degradation due to a loss of oligodendrocyte cells, there are other interpretations of reduced FA. For instance, low FA could also be the result of axonal damage, reduced fibers (axonal density), or disorganized fibers (Thomason and Thompson, 2011, Kumar et al., 2015). It could all be the result of inadequate stimulation of anatomic structures or pathways. Furthermore, microstructural alterations in the brain may be an antecedent and/or a consequence of disease. It is unclear at this point how length and severity of illness might affect DTI measurements. The diffusion tensor method has received criticism for underestimating FA in regions where fibers cross. Thus findings in such areas of crossing fasciculi should be interpreted with caution. Newer models such as mode of anisotropy avoid such limitations and allow a more meaningful interpretation of FA results (Kumar et al., 2015). Finally, our discussion of the functionality of the decreased FA in each area in relation to the EDs are speculative and require additional research to verify whether these functions are indeed reduced in these disorders, and whether they precede or are a consequence of them.
5. Conclusions
Consistent with the NIMH RDoC initiative, we attempted to identify WM alterations common across different types of ED compared to HCs. Reduced FA occurred in tracts that connect emotion, salience and cognitive control networks. Future studies would benefit from looking at transdiagnostic symptom, cognitive, and affective domains, given the high co-occurrence of many depressive and/or anxious disorders. Through these multi-method studies the field will develop a more complete understanding of common and specific symptoms and associated phenotypes and develop improved treatment targets.
The following are the supplementary data related to this article.
Supplementary material.
White matter tracts implicated in VBA meta-analysis of emotional disorders. Significant clusters are shown in green, and tracts implicated in yellow.
Conflict of interest
The authors disclose no conflicts of interests, including financial interests, activities, relationships and affiliations.
Acknowledgements
This work was supported by MH101487 (LMJ and SAL) and MH081911 (LMJ and SAL) (design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation of manuscript).
References
- Admon R., Bleich-Cohen M., Weizmant R., Poyurovsky M., Faragian S., Hendler T. Functional and structural neural indices of risk aversion in obsessive-compulsive disorder (OCD) Psychiatry Res. Neuroimaging. 2012;203:207–213. doi: 10.1016/j.pscychresns.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Ambrosi E., Rossi-Espagnet M.C., Kotzalidis G.D. Structural brain alterations in bipolar disorder II: a combined voxel-based morphometry (VBM) and diffusion tensor imaging (DTI) study. J. Affect. Disord. 2013;150(2):610–615. doi: 10.1016/j.jad.2013.02.023. [DOI] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol. Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Arnold J.F., Zwiers M.P., Fitzgerald D.A. Fronto-limbic microstructure and structural connectivity in remission from major depression. Psychiatry Res. Neuroimaging. 2012;204(1):40–48. doi: 10.1016/j.pscychresns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Ayling E., Aghajani M., J-P F., van der Wee N. Diffusion tensor imaging in anxiety disorders. Curr. Psychiatry Rep. 2012;14:197–202. doi: 10.1007/s11920-012-0273-z. [DOI] [PubMed] [Google Scholar]
- Baur V., Hanggi J., Rufer M. White matter alterations in social anxiety disorder. J. Psychiatr. Res. 2011;45(10):1366–1372. doi: 10.1016/j.jpsychires.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Baxter L.R., Schwartz J.M., Phelps M.E. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46(3):243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Yeh P.H., Bellani M. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol. Psychiatry. 2011;69(4):309–317. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Benedetti F., Giacosa C., Radaelli D. Widespread changes of white matter microstructure in obsessive-compulsive disorder: effect of drug status. Eur. Neuropsychopharmacol. 2013;23:581–593. doi: 10.1016/j.euroneuro.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Blood A.J., Iosifescu D.V., Makris N. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One. 2010;5(11) doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T.A., Campbell L.A., Lehman C.L., Grisham J.R., Mancill R.B. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J. Abnorm. Psychol. 2001;110(4):585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Bruno S., C M., R A.M. White matter abnormalities in bipolar disorder: a voxel-based diffusion tensor imaging study. Bipolar Disord. 2008;10(4):460–468. doi: 10.1111/j.1399-5618.2007.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro P.A., Makris N., Howard J.D., Wedig M.M., Hodge S.M., Wilhelm S., Kennedy D.N., Rauch S. A diffusion tensor imaging study of white matter in obsessive-compulsive disorder. Depress. Anxiety. 2007;24:440–446. doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- Cassidy F., Ahearn E.P., Carroll B.J. Substance abuse in bipolar disorder. Bipolar Disord. 2001;3(4):181–188. [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Chaddock C., Barker G.J., Marshall N. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br. J. Psychiatry. 2009;194(6):527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- Chan W.-Y., Yang G.-L., Chia M.-Y. Cortical and subcortical white matter abnormalities in adults with remitted first-episode mania revealed by tract-based spatial statistics. Bipolar Disord. 2010;12(4):383–389. doi: 10.1111/j.1399-5618.2010.00829.x. [DOI] [PubMed] [Google Scholar]
- Chen S., Lisha L., Zhuang K., Xiao L., Zhang J., Li J. Imaging changes in neural circuits in patients with depression using H-magnetic resonance spectroscopy and diffusions tensor imaging. Neural Regen. Res. 2012;7(24):1881–1888. doi: 10.3969/j.issn.1673-5374.2012.24.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.S., Holtzheimer P.E., Franco A.R. Reconciling variable findings of white matter integrity in major depressive disorder. Neuropsychopharmacology. 2014;39(6):1332–1339. doi: 10.1038/npp.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L.A., Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol. 1991;100(3):316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cole J., Chaddock C.A., Farmer A.E. White matter abnormalities and illness severity in major depressive disorder. Br. J. Psychiatry. 2012;201(1):33–39. doi: 10.1192/bjp.bp.111.100594. [DOI] [PubMed] [Google Scholar]
- Cui L., Chen Z., Deng W. Assessment of white matter abnormalities in paranoid schizophrenia and bipolar mania patients. Psychiatry Res. Neuroimaging. 2011;194(3):347–353. doi: 10.1016/j.pscychresns.2011.03.010. [DOI] [PubMed] [Google Scholar]
- de Diego-Adelino J., Pires P., Gomez-Anson B. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol. Med. 2014;44(6):1171–1182. doi: 10.1017/S003329171300158X. [DOI] [PubMed] [Google Scholar]
- de Kwaasteniet B., Ruhe E., Caan M. Relation between structural and functional connectivity in major depressive disorder. Biol. Psychiatry. 2013;74:40–47. doi: 10.1016/j.biopsych.2012.12.024. [DOI] [PubMed] [Google Scholar]
- Fan S., van den Heuvel O.A., Cath D.C. Mild white matter changes in unmedicated obsessive-compulsive disorder patients and their unaffected siblings. Front. Neurosci. 2016;9(495):1–9. doi: 10.3389/fnins.2015.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., King T.Z., Jovanic T. White matter integrity in highly traumatised adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:2740–2746. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M., Rankin M.A., Wright E.C. Anxiety disorders in major depression. Compr. Psychiatry. 2000;41(2):97–102. doi: 10.1016/s0010-440x(00)90140-8. [DOI] [PubMed] [Google Scholar]
- Freeman M.P., Freeman S.A., McElroy S.L. The comorbidity of bipolar and anxiety disorders: prevalence, psychobiology and treatment issues. J. Affect. Disord. 2002;68(1):1–23. doi: 10.1016/s0165-0327(00)00299-8. [DOI] [PubMed] [Google Scholar]
- Garibotto V., Scifo P., Gorini A., Alonso C.R., Brambati S., Bellodi L., Perani D. Disorganization of anatomical connectivity in obsessive compulsive disorder: a multi-parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiol. Dis. 2010;37:468–476. doi: 10.1016/j.nbd.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M.S. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Goisman R.M., Goldenberg I., Vasile R.G., Keller M.B. Comorbidity of anxiety disorders in a multicenter anxiety study. Compr. Psychiatry. 1995;36(4):303–311. doi: 10.1016/s0010-440x(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Guo W., Liu F., Xue Z. Altered white matter integrity in young adults with first-episode treatment-naïve, and treatment-responsive depression. Neurosci. Lett. 2012;522(2):139–144. doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Ha T.H., Her J.Y., Kim J.H., Chang J.S., Cho H.S., Ha K. Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neurosci. Lett. 2011;505(2):150–154. doi: 10.1016/j.neulet.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Henry C., van den Bulke D., Bellivier F., Etain B., Rouillon F., Leboyer M. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J. Clin. Psychiatry. 2003;64(3):331–335. [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jia Z., Huang X., Wu Q. High-field magnetic resonance imaging of Suicidality in patients with major depressive disorder. Am. J. Psychiatr. 2010;167(11):1381–1390. doi: 10.1176/appi.ajp.2010.09101513. [DOI] [PubMed] [Google Scholar]
- Kendler K.S., Prescott C.A., Myers J., Neale M.C. The structure of genetic and environmental risk factors for common psychiatric and substances use disorders in men and women. Arch. Gen. Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kennis M., van Rooij S.J.H., Tromp D.P.M., Fox A.S., Rademaker A.R., Kahn R.S., Kalin N.H., Geuze E. Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology. 2015;40:2434–2442. doi: 10.1038/npp.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic-stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Nelson C.B., McGonagle K.A., Liu J., Swartz M., Blazer D.G. Comorbidity of DSM_III_R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br. J. Psychiatry. 1996;168:17–30. [PubMed] [Google Scholar]
- Kessler R.C., Chiu W.T., Demler O., Walters E.E. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseppa T., E M., Mantyla R. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J. Affect. Disord. 2010;120(1–3):240–244. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Kim M.J., Lyoo I.K., Kim S.J., Sim M., Kim M., Kim N., Choi N., Jeong D.-U., Covell J., Renshaw P.F. Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport. 2005;16(10):1049–1053. doi: 10.1097/00001756-200507130-00004. [DOI] [PubMed] [Google Scholar]
- Korgaonkar M.S., Grieve S.M., Koslow S.H., Gabrieli J.D.E., Gordon E., Williams L.M. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum. Brain Mapp. 2011;32(12):2161–2171. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R., Perlman G., Gamez W., Watson D. The structure and short-term stability of the emotional disorders: a dimensional approach. Psychol. Med. 2015;45(8):1687–1698. doi: 10.1017/S0033291714002815. [DOI] [PubMed] [Google Scholar]
- Kumar J., Iwabuchi S., Oowise S., Balain V., Palaniyappan L., Liddle P.F. Shared white-matter dysconnectivity in schizophrenia and bipolar disorder with psychosis. Psychol. Med. 2015;45:759–770. doi: 10.1017/S0033291714001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.H., Wu Y.T. Front-occipital fasciculus, corpus callosum and superior longitudinal fasciculus tract alterations of first-episode, medication-naïve and late-onset panic disorder patients. J. Affect. Disord. 2013;146(3):378–382. doi: 10.1016/j.jad.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Li Z., Ji W., Li D., Li X., Feng W. Microstructural abnormality in left nucleus accumbens predicts dysfunctional beliefs in treatment-resistant obsessive-compulsive disorder. Med. Sci. Monit. 2014;20:2275–2282. doi: 10.12659/MSM.891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-X., Chen Y.-S., Hsieh J.-C., Su T.-P., Yeh T.-C., Chen L.-F. Differences in white matter abnormalities between bipolar I and II disorders. J. Affect. Disord. 2010;127(1–3):309–315. doi: 10.1016/j.jad.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Ma N., Li L., Shu N. White matter abnormalities in first-episode, treatment-Naïve young adults with major depressive disorder. Am. J. Psychiatr. 2007;164(5):823–826. doi: 10.1176/ajp.2007.164.5.823. [DOI] [PubMed] [Google Scholar]
- Mahon K., Wu J., Malhotra A.K. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34(6):1590–1600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Kennedy D.N., McInerney S. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb. Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- McElroy S.L., Altshuler L.L., Suppes T. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am. J. Psychiatr. 2001;158(3):420–426. doi: 10.1176/appi.ajp.158.3.420. [DOI] [PubMed] [Google Scholar]
- Menzies L., Williams G.B., Chamberlain S.R., Ooi C., Fineberg N., Suckling J., Sahakian B.J., Robbins T.W., Bullmore E.T. White matter abnormalities in patients with obsessive-compulsive disorder and their first-degree relatives. Am. J. Psychiatr. 2008;165(10):1308–1315. doi: 10.1176/appi.ajp.2008.07101677. [DOI] [PubMed] [Google Scholar]
- Murphy M., Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol. Mood Anxiety Disord. 2011;1(3):1–12. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M.L., Carballedo A., Fagan A.J. Neurotrophic tyrosine kinase polymorphism impacts white matter connections in patients with major depressive disorder. Biol. Psychiatry. 2012;72(8):663–670. doi: 10.1016/j.biopsych.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Nakamae T., Narumoto J., Shibata K., Matsumoto R., Kitabayashi Y., Yoshida T., Yamada K., Nishimura T., Fukui K. Alteration of fractional anisotropy and apparent diffusion coefficient in obsessive-compulsive disorder: a diffusion tensor imaging study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1221–1226. doi: 10.1016/j.pnpbp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kawashima R., Ito K. Activation of the right inferior frontal cortex during assessment of facial emotion. J. Neurophysiol. 1999;82(3):1610–1614. doi: 10.1152/jn.1999.82.3.1610. [DOI] [PubMed] [Google Scholar]
- Nortje G., Stein D.J., Radua J., Mataix-Cols D., Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J. Affect. Disord. 2013;150:192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- Osoba A., Hanggi J., Li M. Disease severity is correlated to tract specific changes of fractional anisotropy in MD and CM thalamus – a DTI study in major depressive disorder. J. Affect. Disord. 2013;149(1–3):116–128. doi: 10.1016/j.jad.2012.12.026. [DOI] [PubMed] [Google Scholar]
- Ouyang X., Tao H.J., Liu H.H. White matter integrity deficit in treatment-naïve adult patients with major depressive disorder. East Asian Arch. Psychiatr. 2011;21(1):5–9. [PubMed] [Google Scholar]
- Peng H., Zheng H., Ning Y. Abnormalities of cortical-limbic-cerebellar white matter networks may contribute to treatment-resistant depression: a diffusion tensor imaging study. BMC Psychiatry. 2013;13:72. doi: 10.1186/1471-244X-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan L.K., Orlichenko A., Boyd E. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol. Psychiatry. 2009;66(7):691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzrak R.H., Goldstein R.B., Southwick S.M., Grant B.F. Prevalence and axis comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the National Epidemiologic Survey on alcohol and related conditions. J. Anxiety Disord. 2011;25(3):456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pini S., Cassano G.B., Simonini E., Savino M., Russo A., Montgomery S.A. Prevalence of anxiety disorders comorbidity in bipolar depression, unipolar depression and dysthymia. J. Affect. Disord. 1997;42(2–3):145–153. doi: 10.1016/s0165-0327(96)01405-x. [DOI] [PubMed] [Google Scholar]
- Qiu C., Zhu C., Zhang J. Diffusion tensor imaging studies on chinese patients with social anxiety disorder. Biomed. Res. Int. 2014;2014:860658. doi: 10.1155/2014/860658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., Matrix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br. J. Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Radua J., Via E., Catani M., Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol. Med. 2011;41:1539–1550. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- Radua J., Mataix-Cols D., Phillips M.L. A new meta-analytic method for neuroimaging studies that combine reported peak coordinates and statistical parametric maps. Eur. Psychiatry. 2012;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua R., Grau M., van den Heuvel O.A. Multimodal voxel-based meta-analysis of white matter abnormalities in obsessive-compulsive disorder. Neuropsychopharmacology. 2014;39(7):1547–1557. doi: 10.1038/npp.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.G., Starr L.B., Kubos K.L. PriceTR. A two-year longitudinal study of post-stroke mood disorders: findings during the initial evaluation. Stroke. 1983;14(5):736–741. doi: 10.1161/01.str.14.5.736. [DOI] [PubMed] [Google Scholar]
- Rojkova K., Volle E., Urbanski M., Humbert F., Dell'Acqua F. Thiebaut de Schotten M. Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study. Brain Struct. Funct. 2016;221:1751–1766. doi: 10.1007/s00429-015-1001-3. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. The role of the cerebellum in affect and psychosis. J. Neurolinguistics. 2000;13(2–3):189–214. [Google Scholar]
- Schmahmann J.D., Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129(2):290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Smith E.E., Eichler F.S., Filley C.M. Cerebral white matter: Neuroanatomy, clinical neurology and neurobehavioural correlates. Ann. N. Y. Acad. Sci. 2008;1142:266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok J.H., Choi S., Lim H.K., Lee S.H., Kim I., Ham B.J. Effects of the COMT val158met polymorphism on white matter connectivity in patients with major depressive disorder. Neurosci. Lett. 2013;545:35–39. doi: 10.1016/j.neulet.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Spalletta G., Piras F., Fagioli S., Caltagirone C., Piras F. Brain microstructural changes and cognitive correlates in patients with pure obsessive compulsive disorder. Brain Behav. 2014;4(2):261–277. doi: 10.1002/brb3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein S.E., Robinson R.G. Comparison of cortical and subcortical lesions in the production of post-stroke mood disorders. Brain. 1987;110:1045–1059. doi: 10.1093/brain/110.4.1045. [DOI] [PubMed] [Google Scholar]
- Steele J.D., Bastin M.E., Wardlaw J.M., Ebmeier K.P. Possible structural abnormality of the brainstem in unipolar depressive illness: a transcranial ultrasound and diffusion tensor magnetic resonance imaging study. J. Neurol. Neurosurg. Psychiatry. 2005;76(11):1510–1515. doi: 10.1136/jnnp.2004.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang Z., Ding W., Wan J., Zhuang Z., Zhang Y., Liu Y., Zhou Y., Xu J. Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmann J.E., Lymer G.K.S., McKirdy J. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11(1):11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Szeszko P., Ardekani B.A., Ashtari M., Malhotra A.K., Robinson D.G., Bilder R.M., Lim K.O. White matter abnormalities in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2005;62:782–790. doi: 10.1001/archpsyc.62.7.782. [DOI] [PubMed] [Google Scholar]
- Tekin S., Cummings J.L. Frontal-subcortical neuronal circuits and clinical neuropsychiatry. J. Psychosom. Res. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Tha K.K., Terae S., Nakagawa S. Mpaired integrity of the brain parenchyma in non-geriatric patients with major depressive disorder revealed by diffusion tensor imaging. Psychiatry Res. Neuroimaging. 2013;212(3):208–215. doi: 10.1016/j.pscychresns.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Dell'Acqua F., Forkel S.J. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M., Ffytche D.H., Bizzi A. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage. 2011;54(1):49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Thompson P.M. Diffusion imaging, white matter, and psychopathology. Annu. Rev. Clin. Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Versace A., Almeida J.R., Hassel S. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch. Gen. Psychiatry. 2008;65(9):1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A., Almeida J.R., Quevedo K. Right orbitofrontal corticolimbic and left corticocortical white matter connectivity differentiate bipolar and unipolar depression. Biol. Psychiatry. 2010;68(6):560–567. doi: 10.1016/j.biopsych.2010.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollebergh W.A.M., Iedema J., Bijl R.V., de Graaf R., Smit F., Ormel J. The structure and stability of common mental disorders: the NEMESIS study. Arch. Gen. Psychiatry. 2001;58(6):597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Phan K.L., Liberzon I., Taylor S.F. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Wang T., Huang X., Huang P. Early-stage psychotherapy produces elevated frontal white matter integrity in adult major depressive disorder. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0063081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J. Abnorm. Psychol. 2005;114(4):522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Wessa M., Houenou J., Leboyer M., Chanraud S., Poupon C., Martinot J.-L. Microstructural white matter changes in euthymic bipolar patients: a whole-brain diffusion tensor imaging study. Bipolar Disord. 2009;11(5):504–514. doi: 10.1111/j.1399-5618.2009.00718.x. [DOI] [PubMed] [Google Scholar]
- Wu F., Tang Y., Xu K. Whiter matter abnormalities in medication-naïve subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. Neuroimaging. 2011;191(1):80–83. doi: 10.1016/j.pscychresns.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerevanian B.I., Koek R.J., Ramdev S. Anxiety disorders comorbidity in mood disorder subgroups: data from a mood disorders clinic. J. Affect. Disord. 2001;67(1–3):167–173. doi: 10.1016/s0165-0327(01)00448-7. [DOI] [PubMed] [Google Scholar]
- Yoo S.Y., Jang J.H., Shin Y.-W., Kim D.J., Park H.-J., Moon W.-J., Chung E.C., Lee J.-M., Kim I.Y., Kim S.I., Kwon J.S. White matter abnormalities in drug-naïve patients with obsessive-compulsive disorder: a diffusion tensor study before and after citalopram treatment. Acta Psychiatr. Scand. 2007;166:211–219. doi: 10.1111/j.1600-0447.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D.A., Silveri M.M., Gruber S.A., Rohan M.L., Pimentel P.J. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9(5):504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- Zanetti M.V., Jackowski M.P., Versace A. State-dependent microstructural white matter changes in bipolar I depression. Eur. Arch. Psychiatry Clin. Neurosci. 2009;259(6):316–328. doi: 10.1007/s00406-009-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang Y., Li L., Li Z., Li W., Ma N., Hou C., Zhang Z., Zhang Z., Wang L., Duan L., Lu G. Different white matter abnormalities between the first-episode, treatment-naïve patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J. Affect. Disord. 2011;133:294–299. doi: 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Zhang A., Leow A., Ajilore O. Quantitative tract-specific measures of uncinate and cingulum in major depression using diffusion tensor imaging. Neuropsychopharmacology. 2012;37(4):959–967. doi: 10.1038/npp.2011.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K., Huang X., Li T. Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J. Psychiatry Neurosci. 2008;33(6):525–530. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
White matter tracts implicated in VBA meta-analysis of emotional disorders. Significant clusters are shown in green, and tracts implicated in yellow.