Abstract
Neurogenic dysphagia frequently occurs after stroke and deglutitive aspiration is one of the main reasons for subacute death after stroke. Although promising therapeutic interventions for neurogenic dysphagia are being developed, the functional neuroanatomy of recovered swallowing in this population remains uncertain. Here, we investigated 18 patients post-stroke who recovered from dysphagia using an event related functional magnetic resonance imaging (fMRI) study of swallowing. Patients were characterized by initial dysphagia score (mild to severe), lesion mapping, white matter fractional anisotropy (FA) of the pyramidal tracts, and swallowing performance measurement during fMRI scanning. Eighteen age matched healthy participants served as a control group. Overall, patients showed decreased fMRI-activation in the entire swallowing network apart from an increase of activation in the contralesional primary somatosensory cortex (S1). Moreover, fMRI activation in contralesional S1 correlated with initial dysphagia score. Finally, when lesions of the pyramidal tract were more severe, recovered swallowing appeared to be associated with asymmetric activation of the ipsilesional anterior cerebellum. Taken together, our data support a role for increased contralesional somatosensory resources and ipsilesional anterior cerebellum feed forward loops for recovered swallowing after dysphagia following stroke.
Keywords: Dysphagia, Neurorehabilitation, Recovery, Stroke, Swallowing
Highlights
-
•
Recovered dysphagic stroke patients showed decreased fMRI-activation of swallowing network.
-
•
Contralesional S1 activation was higher in patient vs. control group and positively associated with initial dysphagia score.
-
•
Ipsilesional anterior cerebellum was asymmetrically activated with severe lesions of pyramidal tract.
1. Introduction
Swallowing is a complex sensorimotor behaviour involving a coordinated neural interplay at both cortical and subcortical levels. Damage to this swallowing network can lead to swallowing problems (dysphagia), and furthermore 80% of dysphagia occurrences are a direct result of stroke (Prosiegel, 2002). About 50% of patients post-stroke experience dysphagia in the acute phase of which two thirds may aspirate silently (Daniels et al., 1998). This can lead to pneumonia and malnutrition (Kidd et al., 1995) in addition to lengthier hospital stays with afflicted patients being more likely to be transferred to a nursing home than patients without dysphagia.
Interestingly, Yamamoto et al. (2014) have suggested that a left hemispheric stroke is associated with higher aspiration rates than a right hemispheric stroke, while territorial infarcts in posterior brain areas may result in more frequent penetration and aspiration with swallowing (Kim et al., 2014). Within the cortex, lesions of the insula (Daniels and Foundas, 1997, Daniels et al., 2006, Riecker et al., 2009), the frontal operculum (Meadows, 1973, Pender and Ferguson, 2007), and the primary sensorimotor cortex (Daniels et al., 1996) are most commonly associated with impairment of swallowing. Damage to the posterior limb of the internal capsule is associated with motor deficits also seen in swallowing (Galovic et al., 2013). Moreover, brainstem lesions [predominantly the dorsal part, which coordinates reflexive swallowing function (Lang, 2009)] can often lead to profound dysphagia (Vuilleumier et al., 1995).
The cortical and subcortical networks of swallowing have been widely investigated using functional magnetic resonance imaging (fMRI). Cortically, the bilateral inferior primary motor (M1) and somatosensory cortex (S1), the bilateral insula [anterior insula (Ant Ins) and posterior insula (Post Ins)], the cingulate cortex together with the supplementary motor area (SMA), and the premotor cortex (ventral and dorsal PMC) have been implicated during swallowing tasks (Birn et al., 1999, Hamdy et al., 1999, Kern et al., 2001, Malandraki et al., 2009, Martin et al., 2001, Martin et al., 2004, Mihai et al., 2013, Mosier and Bereznaya, 2001, Mosier et al., 1999b, Suzuki et al., 2003, Toogood et al., 2005). Subcortically the cerebellum and basal ganglia, along with the brainstem, and the thalamus have also been reported (Malandraki et al., 2009, Mihai et al., 2013, Mosier and Bereznaya, 2001, Mosier et al., 1999b, Suzuki et al., 2003).
Recovery patterns in the cerebral representation of swallowing after stroke are clinically highly relevant but poorly understood. This is due in part to the difficulty in examining swallowing in an fMRI-scanner in a supine position, which even in healthy participants is sensitive to a number of artefacts. Indeed, the investigation of dysphagic patients lying in a scanner bore while performing the swallowing task is problematic because of the risk of aspiration, excessive head movement and general patient frailty.
However, in an early study in ten acute patients with moderate dysphagia, Li et al. (2009) were able to show that recovery is associated with cerebral activation in cortical swallowing areas compensating for increased effort or recruited areas in the intact hemisphere. In a later study the same authors reported that dysphagia secondary to stroke is associated with disruptive functional (resting state fMRI) and structural integrity in the large-scale brain networks involved in motor control (Li et al., 2014). Up to now, no studies have systematically investigated differences of the functional representation of swallowing in a group of recovered chronic patients post-stroke with initial dysphagia, characterized lesions (lesion mapping) and combined this with white matter disturbances of the pyramidal tract using DTI.
The main objectives of this work were to examine the recovered neuronal swallowing control in subacute patients post-stroke using structural, functional and diffusion MRI, and compare how these changes differ from a control group. More specifically: (1) We hypothesised that swallowing representation will be increased in several functional areas (contralesional S1 and M1, cerebellar hemispheres, insula, vPMC and SMA, prefrontal cortex) as compared to controls. We further hypothesised an increase of functional representation of the contralesional hemisphere as indicated by a more asymmetric lateralization index. (2) We additionally hypothesised that differences would exist between patients with predominantly cortical and subcortical lesions. (3) Lastly, we hypothesised that there would be an association between the ability to perform our swallowing protocol (compliance) and the amount of white matter lesion (lateralization index of fractional anisotropy).
2. Materials and methods
2.1. Participants
We recruited patients post-stroke from the local neurorehabilitation centre (BDH-Klinik Greifswald, Germany.) Patients with a single ischaemic cerebral infarction who had recovered from neurogenic post-stroke dysphagia were included in the study. Patients post-stroke, who were severely dysphagic during the rehabilitation period (within the last 3 years prior to the study), were invited to a follow-up swallowing function assessment. Severe dysphagia during the subacute phase after stroke was operationalised using the Bogenhausen Dysphagia Score sum of scores (BODS1 and BODS2; Schiele et al., 2015, Starrost et al., 2012). These scores are used by therapists and physicians in the German speaking areas of Europe and show impairments of saliva swallow and food intake in clinical/neurological testing.
Exclusion criteria were lack of capacity to consent due to neuropsychological, linguistic or psychiatric disorders. Procedures were approved by the Ethics Committee of the Medical Faculty of The University of Greifswald (registration number BB 101/08).
Eighteen patients (mean age and standard deviation: 56.6 ± 15.3 years, 25–73 years, 5 female) were included in the study. Each participant provided informed, written consent. Based on the Edinburgh Handedness Inventory [EHI (Oldfield, 1971)] 15 subjects were right handed and 3 were left handed before stroke onset (Table 1).
Table 1.
Patients characteristics.
Patient characteristics including age at fMRI study date, gender, handedness as described by the Edinburgh Handedness Inventory, time after stroke at fMRI study date rounded to the nearest week, date of admission, i.e. date of infarct, hospital stay including rehabilitation, affected hemisphere, volume of lesion in cubic centimetres, location of lesion, BODS sum of scores at admission and discharge, and compliance.
| Patient | Age at fMRI | Gender | EHI LQ | Time after stroke (weeks, rounded) | Hospital stay (weeks) | Aff. hem. | Lesion size (cm3) | Lesion location | BODS sum of scores admission | BODS sum of scores discharge | Compliance (# of extra thyroid cartilage movements) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | 90.91 | 16 | 16 | R | 0.88 | sc: medial pons | 16 | 2 | 0.5 (10) |
| 2 | 40 | M | 75 | 53 | 13 | R | 112.57 | c: complete arteria media | 4 | 2 | 0.6 (8) |
| 3 | 38 | M | 50 | 54 | 7 | R | 39.54 | sc: internal capsule, c: occipital | 11 | 2 | 0.7 (6) |
| 4 | 67 | M | 100 | 157 | 24 | L | 74.22 | c: fronto-parietal | 9 | 2 | 0.2 (16) |
| 5 | 54 | M | 91.7 | 157 | 6 | R | 22.54 | sc: cerebell. hem./vermis | 11 | 2 | 0.85 (3) |
| 6 | 70 | M | 100 | 67 | 15 | L | 62.63 | c: parietal, posterior insula | 3 | 2 | 0.35 (13) |
| 7 | 59 | M | 100 | 75 | 22 | R | 1.82 | sc: medial pons, brainstem | 16 | 3 | 0.55 (9) |
| 8 | 66 | M | 100 | 35 | 13 | L | 5.86 | sc: insula, putamen | 3 | 2 | – |
| 9 | 70 | M | 83.3 | 45 | 15 | R | 3.48 | sc: pyramidal tract | 3 | 3 | 1 (0) |
| 10 | 70 | F | − 63.6 | 32 | 12 | R | 14.71 | sc: temporal | 6 | 2 | 0.85 (3) |
| 11 | 56 | M | 100 | 42 | 14 | L | 134.54 | c/sc: complete art. media | 5 | 2 | 0.35 (13) |
| 12 | 72 | F | 83.3 | 4 | 5 | L | 0.07 | sc: medial brainstem | 8 | 2 | 0.9 (2) |
| 13 | 60 | F | 100 | 18 | 18 | R | 15.93 | sc: internal capsule, putamen, thalamus | 9 | 2 | 0.95 (1) |
| 14 | 40 | M | − 28.57 | 22 | 22 | L | 14.11 | sc: medial cingulate | 11 | 2 | 0.85 (3) |
| 15 | 25 | F | 100 | 87 | 20 | L | 5.82 | c: precentral gyrus | 3 | 2 | 0.85 (3) |
| 16 | 55 | F | − 0.58 | 49 | 8 | R | 3.4 | Thalamus, putamen, nucl. caud. | 3 | 2 | 1 (0) |
| 17 | 71 | M | 100 | 41 | 6 | L | 4.52 | sc: ant. cerebell. hem. | 3 | 2 | 0.95 (1) |
| 18 | 69 | M | 100 | 66 | 19 | R | 15.55 | c: precentral gyrus | 16 | 2 | – |
BODS: Bogenhausener Dysphagia Score quantifies the degree of dysphagia; 1–2: no dysphagia, 2–3: mild dysphagia, 3–5: mild to moderate dysphagia, 4/5–7: moderate to severe dysphagia, 6/7–10: severe dysphagia, and 11–16: profound dysphagia. Compliance refers to the ability to follow protocol and subjects avoided unwanted coughing or throat clearing throughout scanning; please refer to methods for further details. R: right; L: left; M: male; F: female; c: cortical; sc: subcortical; int.: internal; cerebell. hem.: cerebellar hemisphere; art. media: medial cerebral artery; nucl. caud.: nucleus caudatus; ant.: anterior; EHI LQ: Edinburgh Handedness Inventory Lateralisation Quotient. For two patients the compliance data was missing due to pneumatic cushion signal dropout.
During their prior inpatient rehabilitation stay, all patients received standard dysphagia therapy based on their clinical dysphagia assessment as described in Section 2.2 (Bartolome and Schröter-Morasch, 2010, Becker et al., 2011). At the start of the therapy, adaptive procedures were employed, such as specification of safe food type and consistency. Further, patients were trained to improve the motor and/or the sensory control of the swallowing act. Compensatory measures such as change in position (anteflexion of head, head turns to the affected/non-affected side or head tilts), in conjunction with adaptive as well as restitutive therapeutic procedures were performed on an individual basis as indicated. All patients received a 30-minute sessions of therapy at least five times per week for the whole duration of their hospital stay.
The control group consisted of 18 healthy participants (mean age and standard deviation: 61.94 ± 9.78 years, 50–72 years, 14 female), whom had no history of dysphagia. The results of these data were discussed in a previous article (Windel et al., 2015). The purpose of these data was to act as a control for the patient data, and is not discussed further. Additionally, only 18 of the 27 controls were included in this study in order to match the age distribution of the patient group. We screened controls with a questionnaire for signs of chronic pain, medication status, chronic medical conditions, motor, sensory, swallowing, speech and otolaryngologic deficits, epilepsy, and history of stroke or other diseases provoking problems with swallowing. All healthy participants were free of any swallowing symptoms at the time of study. Ten participants documented use of medication to regulate blood pressure, one took analgesic medication regularly, one used anti-diabetic medication, and another used medication to treat anxiety. All control participants were right handed based on the EHI.
2.2. Testing of swallowing function
During their initial inpatient rehabilitation treatment each patient underwent a clinical examination at admission and discharge based on the clinical part of the neurogenic oropharyngeal dysphagia test (NOD, Ickenstein et al., 2009). The following components were investigated: facio-oral motor and somatosensory functions, inspection of the oral cavity and an assessment of somatomotor pharyngeo-laryngeal function. Additionally, the water swallowing test developed by Daniels et al. (1997) was performed, which includes 2 × 5 ml, 2 × 10 ml, and 2 × 20 ml water swallowing tasks. If two of the six predictors were present (dysphonia, dysarthria, impaired random cough, lowered or missing gag reflex, coughing and change in voice quality after water swallow) aspiration risk was considered likely. This test has a sensitivity of 92.7% and a specificity of 66.7%. Further, an assessment concerning the impairment of saliva swallow and food intake was performed based on the Bogenhausen Dysphagia Score [BODS, consisting of BODS1 and BODS2 (Schiele et al., 2015, Starrost et al., 2012) with a maximum sum of scores of 16]. A score between 0 and 2 means no dysphagia and between 14 and 16 means profound dysphagia. In a final analysis, the swallowing impairment scale was also tested [German: “Schluckbeeinträchtigungsskala” (SBS) based on the criteria developed by (Prosiegel, 2002)] together with the functional communication measure [FCM, from the American Speech-Language-Hearing Association (ASHA 2003)]. All these tests were performed to ensure an accurate and robust diagnosis. Based on individual clinical decisions, the clinical dysphagia assessment was complemented by videofluoroscopic dysphagia testing in a proportion of patients using a standard clinical protocol (Bartolome and Schröter-Morasch, 2010).
On the day of fMRI measurement patients were re-tested to ensure swallowing function did not deviate from the discharge measurement using the clinical swallowing examination as part of the NOD, and the water-swallowing test. If patients showed any sign of aspiration on these additional tests, they were excluded from participating in the experiment, as part of the ethical guidance for the study. Furthermore, an MRI cinematographic sequence enabled a view of bolus transportation similar to videofluoroscopy (Mihai et al., 2014), to help monitor silent aspiration in a supine position during scanning.
2.3. Brain fMRI-measurement during swallowing
The experiment comprised a functional and a structural imaging run. For the functional run 20 swallows were recorded with a fixed inter-stimulus interval of 11 s. Room temperature water (2 ml, injection velocity 2 ml/s) was delivered via a MR-safe contrast agent injector (Spectris Solaris; Medrad, Warrendale, PA, USA) through a soft rubber tube (diameter = 1.5 mm) held between the patient's lips in the midline. Water delivery was indicated by a cued colour change (blue: rest, green: water injection) projected onto a translucent screen. Patients were instructed to swallow as soon as they felt that all of the water had reached the mouth and to avoid swallowing in between water delivery. Swallowing timing and task compliance was controlled by thyroid cartilage movement measurements using a pneumatic cushion (Siemens MRI safe respiration bellows) placed around the participant's neck (see also Toogood et al., 2005). The pressure exerted by the thyroid cartilage on the cushion was transformed into an electrical signal measured through an electro-optical biosignal recorder (Varioport-b; Becker Meditec, Karlsruhe, Germany). The total number of swallow events determined with the pneumatic cushion was then divided by the expected number of 20. A compliance score of 1.0 meant the patient swallowed an expected 20 times in one run, and any deviation above 20 swallows resulted in a lower compliance score. Other deflections in the cushion signal were also counted as non-compliance, and included movements such as coughing or throat clearing. Compliance thus refers to the ability to follow the protocol. A higher score means that the participants performed the task well and avoided unwanted coughing or throat clearing throughout scanning.
2.4. MRI data acquisition
A 3 T MRI scanner (Siemens Verio, Erlangen, Germany) using a 32-channel head coil was used to record MRI data. Functional and structural images were recorded. Field homogeneity was optimized by a shimming sequence and a gradient echo sequence (34 phase and magnitude images, 34 slices, 2 × 2 × 2 mm3, field of view [FoV] 192 mm, repetition time (TR) 488 ms, echo time (TE)1 4.92 ms, TE1 7.38 ms, flip angle [α] 60°) was acquired in order to correct geometric distortions in the echo planar images (EPI). Functional EPI images (TR 2 s, TE 22 ms, FoV 192 mm, slice spacing 2.5 mm, matrix 96 × 96, α = 90°, voxel size 2 × 2 × 2 mm3) were sliced in an oblique direction in order to ensure full coverage of brain areas including sensorimotor cortex, cerebellum and brainstem. The scanner discarded three initial scanning volumes automatically. A structural T1-weighted anatomical image was acquired for each subject (magnetization-prepared rapid gradient echo, TR 1690 ms, TE 2.52 ms, α = 9°, FoV 250 mm, voxel size 1 × 1 × 1 mm3, parallel acquisition using a k-space based algorithm [GRAPPA] factor 2, matrix 256 × 256, distance factor 50%, transversal orientation, 176 slices, ascending). Additionally, a cinematographic sequence (cardiovascular imaging sequence, TR 2.7 ms, TE 1.22 ms, a550, FoV 270 mm, voxel size 2.8 × 2.8 × 10 mm, parallel acquisition using a k-space-based algorithm [GRAPPA] factor 2, distance factor 10%, base resolution 96 X96, sagittal orientation, 1 slice, 200 volumes) enabled the visualization of water, tongue, and pharynx movement during ten swallowing trials. Ten trials were considered sufficient for the visual inspection of bolus transportation. Its effective TR was 305 ms or 3.3 frames per second. The frame rate of 3.3 frames per second is comparable to the one used in low dose fluoroscopic diagnostics (3.75 frames per second) (Stueve, 2006) and thus sufficient to follow the bolus. To ensure synchronization of all data acquisition modalities, trigger signals in line with fMRI scans were recorded for the colour change onset. A diffusion-weighted imaging (DWI) data set was also measured (TR 10500 ms, TE 107 ms, α = 90°, FoV 230 mm, voxel size 1.8 × 1.8 × 2.3 mm3, matrix 128 × 128, 55 slices). The sequence used was the standard MDDW (multi directional diffusion weighted) sequence found on Siemens MRI scanners. A diffusion weighting of b = 1000 s/mm2 was applied and 64 diffusion weighting gradient directions were chosen. A reference image with no diffusion weighting (b = 0 s/mm2) was also recorded.
2.5. DWI pre-processing and analysis
Pre-processing of DWI data was performed with FSL 5.08 (Analysis Group, FMRIB, Oxford, UK). After data conversion into the NIFTI format and retrieval of the diffusion gradient vector tables, the individual data sets with lesions to the right hemisphere were left-right flipped. Afterwards correction for artefacts resulting from head movement and eddy currents was implemented (eddy_correct of the FSL Toolbox). Individual T1 images were skull-stripped to improve coregistration. The resulting data sets were then used to calculate non-linear transformations into the aforementioned MNI space. Via a linear coregistration between DWI and T1, a final non-linear transformation of the DWI data into MNI space was created. Diffusion gradient vector tables were adjusted for the rotations found during head movement correction. A reference coordinate system between gradient vectors and the subject in the scanner was formed, a rotation of any of the parts would invalidate the coordinate system, therefore a correction after the coregistrations was necessary.
Pre-processed DWI data sets were fed into FSL dti_fit to calculate diffusion tensors and fractional anisotropy (FA). Additionally FSL bedpostx (Behrens et al., 2007) was used to build up distributions on diffusion parameters at each voxel. Bedpostx uses a Bayesian model to calculate these distributions, and due to the complexity, advanced sampling techniques (Markov Chain Monte Carlo sampling) were then applied. Bedpostx allows modelling of crossing fibres within each voxel of the brain and crucially, it also allows automatical determination of the number of crossing fibres per voxel. The bedpostx data were used to perform probabilistic tractography with FSL probtrackx (Behrens et al., 2007) in each subject. Regions of interest (ROIs) of the tongue-lip region (M1S1 L/R tongue-lip) depicted the seed regions for tractography. Additional ROIs of the posterior limb of the internal capsule (R/L) were used as a tractography waypoint, and the Corpus Callosum as an exclusive ROI. Since a mask involving the mouth and laryngeal representation of M1-S1 and the dorsal part of the internal capsule were used as seeds for the DTI analysis, we were not able to differentiate between the corticobulbar and corticospinal tracts. This resulted in a focused fibre reconstruction between M1 and the internal capsule. The M1 mask was taken from the Anatomy toolbox (Eickhoff et al., 2005), the other masks from the JHU ICBM-DTI-81 White-Matter Labels (Hua et al., 2008). The reconstructed tracts were used to mask the previously created FA maps and to calculate a weighted mean of fractional anisotropy for each tract. Since tracts reconstructed by probtrackx contain a connectivity distribution at every voxel coordinate, a voxel with a high connectivity contributes more to the reconstructed tract than a voxel with very low connectivity. The connectivity value is used to weight the FA value at the respective voxel coordinate, thus a weighted mean FA for the tract was calculated.
2.6. Preprocessing of fMRI data
Pre-processing of fMRI data was done in SPM8 rev. 5236 (Wellcome Department of Imaging Neuroscience, London, UK) running on MATLAB R2011a. A maximum translational motion of 2 mm was considered appropriate to reduce the influence of head motion related artefacts. Pre-processing of fMRI data included realignment and unwarping with the help of a voxel displacement map calculated from the field map data, coregistration of the structural T1 image to the mean EPI image. Head motion and susceptibility distortion by movement interaction was corrected using the Realign and Unwarp method (Andersson et al., 2001). Additionally, the ArtRepair Toolbox (Mazaika et al., 2009) was used to correct any data sets which showed movement greater than 2 mm as reported by Michou et al. (2015). Four patient and five control data sets required repairing. For easy comparison brains were left-right flipped so that the lesions for each subject were localized to the left hemisphere (Lotze et al., 2012). Ten data sets were therefore flipped (01, 02, 03, 05, 07, 09, 10, 13, 16, 18). T1 images were segmented into grey and white matter, bias corrected and spatially normalized using the New Segment function of SPM8. An additional tissue probability map was used as a prior, consisting of white matter and cerebro-spinal fluid to ensure proper segmentation and normalization of lesioned brains (Ripollés et al., 2012). The DARTEL toolbox (Ashburner, 2007) was used to create a normalizing template. DARTEL's “Normalize to Montreal Neurological Institute (MNI)” Space function was used to normalize the functional and structural images of each participant using the individual flow fields and the created template. Functional images were smoothed in the normalization step using a Gaussian full width at half-maximum kernel of 6 × 6 × 6 mm3 to increase the signal-to-noise ratio and reduce inter-subject differences.
We measured lesion volumes by manually drawing the border of the lesion in the high spatial resolution T1-weighted image for each slice and then calculating the resulting volume (cm3) with MRICron (http://www.sph.sc.edu/comd/rorden/mricron).
2.7. Statistical analysis of fMRI data
Two different general linear models were set up. One consisted of the smoothed healthy control data, and the other of the patient data. Low frequency components were filtered with a high-pass filter having a cutoff of 128 s. BOLD responses were modelled using SPM's hemodynamic response function (HRF). Onsets were modelled for each swallow and chosen to correspond to the water injection time based on results from previous work (Mihai et al., 2013). Swallows and other air cushion deflections, which did not correspond to the standard onsets, as reflected in the thyroid cartilage movement data, were modelled with a HRF and included as regressors of no interest in the design matrix. For the patient data, if the thyroid cartilage response showed an ambiguous signal not corresponding to swallowing, then this time-frame was also included as a regressor of no interest in the design matrix.
The autocorrelation in the data was estimated by an autoregressive AR(1) model. An explicit mask provided by SPM8 (brainmask.nii) was used to exclude inference on voxels outside the brain. Model parameters were estimated using the Restricted Maximum Likelihood.
At the first level analysis, contrasts were computed for the onsets of both groups. The control data were then taken to second level analysis and a random effects one-sample t-test was computed. The MarsBar toolbox (Brett et al., 2002) was used to extract mean parameter estimates (betas) for the individual subjects using the following pre-specified regions of interests (ROIs). Left and right primary motor cortex that included the hand region (M1 L/R hand) and another one that included the tongue-lip region (M1 L/R tongue-lip); left and right primary somatosensory cortex for the hand and tongue-lip regions (S1 L/R hand, S1 L/R tongue-lip); secondary somatosensory cortex (S2 L/R), anterior and posterior insula (Insula L/R ant, Insula L/R post), cerebellum using Larsell's parcellation IV–VIIb (Cereb. L/R), left trigeminal nerve and solitary nucleus. M1, S1, and S2 were taken from the Anatomy toolbox (Eickhoff et al., 2005). Cerebellar masks were created using the WFU Pick Atlas (Maldjian et al., 2003). The anterior and posterior insula were provided by Neuromorphometrics, Inc. (http://neuromorphometrics.com) under academic subscription, taken from SPM12. The trigeminal nerve (MNI coordinate: − 10, − 30, − 34) and solitary nucleus (MNI coordinate: 4, − 36, − 44) ROIs consisted of spheres (diameter 6 mm because of the small areas relevant for brainstem processing) created using the coordinates from a previous swallowing experiment (Mihai et al., 2013). Betas provide a numerical estimate of the effect size during the swallowing condition. Mean betas were chosen because they offer more moderate activation values as opposed to the extreme values given by the peak t-statistic (Fox et al., 2014), which tend to deviate greatly between subjects. Furthermore, the time course for a single voxel can be noisy, and could represent an outlier in the ROI (Nieto-Castanon et al., 2003).
Main effect of voxel-based analysis (swallowing all patients, Fig. 2) was corrected for multiple comparisons for the whole brain volume (Family-Wise-Error-correction, FWE-correction). Comparisons between groups were corrected for the areas hypothesised to show a significant effect, i.e. vPMC, dPMC, SMA, M1 (hand, lip-tongue), S1 (hand, lip-tongue), S2, posterior and anterior insula, cerebellum (Larsell's IV, V, VI, VIIb), thalamus and brainstem. For the comparison between patients and controls, we expected a significant increase of contralesional activation for the M1 and S1 somatotopic swallowing representation area. Therefore we corrected for a sphere of 10 mm around the activation maxima reported previously for young healthy participants [(Windel et al., 2015); S1 right MNI: (45, − 22, 36), M1 right: (46, − 6, 40)]. The same procedure was used for associations with dysphagia scores (BODS) for patients, since we expected increased (compensatory) recruitment of M1/S1 of the healthy hemisphere with increasing dysphagia. The swallowing tests prior to fMRI measurements resulted in uniform results and an additional correlation with these scores was not warranted. Therefore, we applied BODS at admission to the further analysis, due to its well-defined scale, and also to test if greater swallowing impairment produces a distinct pattern of reorganization in the recovery phase of the patients. Additionally, a Pearson's correlation between lesion size and BODS score was calculated to test the influence of lesion size on impairment. Comparisons for voxel based analyses were restricted on volumes of interest [M1, S1, S2, anterior and posterior insula, vPMC, SMA, thalamus, brain stem (nucleus solitarius, trigeminal nucleus), and cerebellar hemisphere].
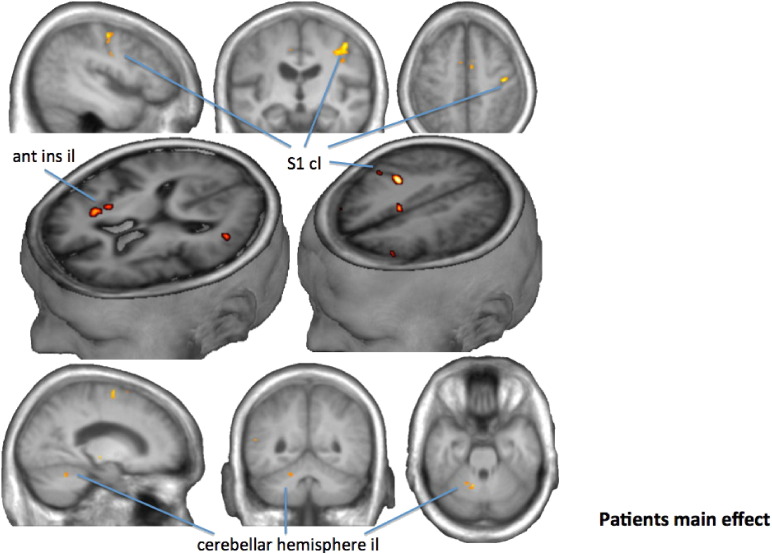
Fig. 2.
Voxel wise group analysis of the comparison swallow onset against baseline in patients (FWE-corrected, p < 0.05). Since lesions were all flipped to the left hemisphere brain areas here shown on the left hemisphere were described as ipsilesional (il), those on the right as contralesional (cl). We found ipsilesional activation in the cerebellar hemisphere and contralesional in the primary somatosensory cortex (S1 and in the anterior insula (ant ins)).
Lateralization indices [LI; defined as (betaleft − betaright)/(betaleft + betaright)] of fMRI-activation magnitude (betas) of cortical regions (M1, S1, S2, anterior insula, cerebellum) were compared between the patients and controls using t-tests for independent variables. fMRI-activation magnitude in bilateral vPMC and SMA were compared between patients and controls using t-tests for independent variables. Clinical motor and somatosensory scores at admission were also correlated with the lateralization index of betas.
Lastly, the lateralization index of fractional anisotropy (LI FA) was compared between patients and controls using a t-test for independent variables. Further, LI FA was correlated with compliance (as defined above).
3. Results
3.1. Characterization of patients and behavioural data
Table 1 provides an overview of the clinical and demographical characteristics of our patients. Initially six patients were profoundly dysphagic (BODS 11–16), five moderate to severely dysphagic (5–9) and seven were mild to moderately dysphagic (3–4). By discharge swallowing had normalized in all but two patients, both of whom were left with very mild impairment (BODS 3; one brainstem and one pyramidal tract lesion). Thyroid cartilage movement data indicated that patients needed more time to swallow than healthy controls [t(35) = 2.31, p > 0.03]. For the controls, the average time between the visual cue and peak thyroid cartilage deflection was 3.4 ± 0.5 s compared to patients: 4.1 ± 1.2 s. The water delivery time of 1 s was included in this calculation. A Pearson's correlation between lesion size and severity of dysphagia (BODS at admission) was not significant (r = − 0.22, p < 0.38). None of the patients showed signs of aspiration during the cinematographic scanning of the 10 swallows prior to the functional imaging acquisition.
All non-compliant patients showed additional thyroid cartilage movements. Compliance data from two patients were missing, due to signal loss from the pneumatic cushion. An analysis of the movement artefacts showed that within these limits the patients swallowed according to the 20 required swallows. The low temporal resolution of the movement data, however, provided less information than needed to calculate compliance robustly. We included these patients to increase power and analysed the data accordingly.
3.2. Neuronal representation in patients vs. controls
Lesion mapping, fMRI, tractography and compliance were investigated for each patient and Fig. 1 shows an example for a single patient. Fig. 2 shows the activation of swallowing vs. baseline for all patients masked using the ROIs described in the Methods section. Activations were found bilaterally in the dPMC, vPMC, SMA, M1 and S1 of the hand and tongue-lip regions, S2, anterior and posterior insula, cerebellum in Larsell's IV, V, VI, VIIb, and in the premotor, motor and somatosensory thalamus (Table 2). Paired t-test between hemispheres of activation magnitude per ROIs showed no relevant differences indicating an overall highly symmetric representation for cortical and cerebellar representations, other than that seen from the unilateral lesion.
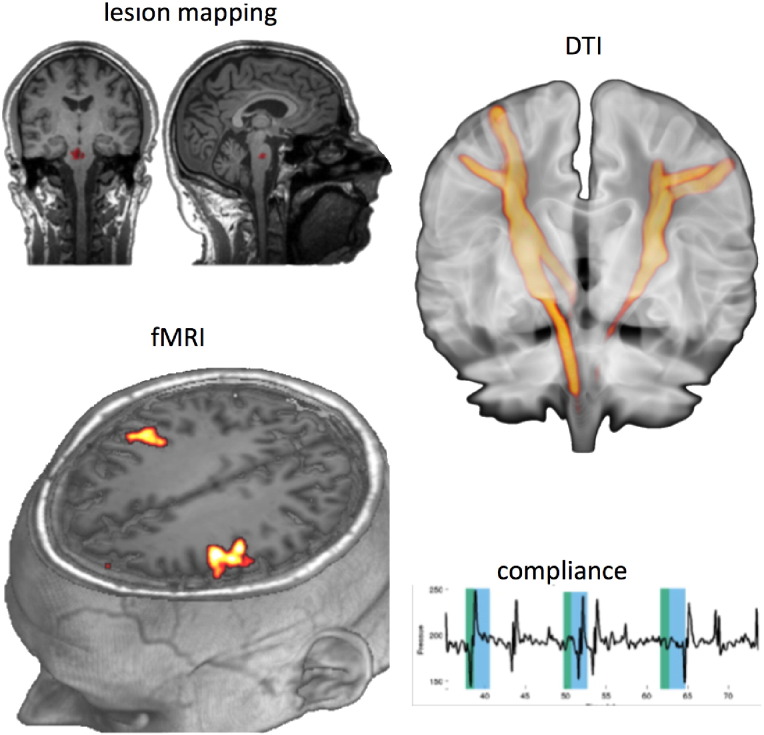
Fig. 1.
An example of the material collected for each patient. Left: patient with pontine lesion (patient 1; top left, lesion volume of 0.88 cm3 indicated in red on the T1-weighted anatomical MRI scan) showed moderately good compliance of the swallowing task (bottom right; thyroid cartilage movements over time indicated a factor of 0.5). The fMRI showed bilateral activation in M1S1 (left bottom, FEW-corrected). White matter diffusion imaging between the tongue/larynx/lip area of the primary sensorimotor cortex and pons showed strong symmetry of the pyramidal tracts descending from the face area (right top).
Table 2.
MNI coordinates for contrast Swallowing vs. Baseline in patients (FWE-corrected, p < 0.05).
| Area | t-Value | k | MNI coordinate |
||
|---|---|---|---|---|---|
| x | y | z | |||
| SMA il | 9.89 | 2021 | − 12 | − 10 | 55 |
| SMA cl | 7.95 | 1956 | 2 | − 4 | 61 |
| dPMC il | 8.15 | 2928 | − 16 | − 12 | 61 |
| dPMC cl | 8.11 | 2640 | 27 | − 3 | 51 |
| vPMC il | 8.04 | 4202 | − 48 | − 6 | 16 |
| vPMC cl | 7.8 | 3464 | 39 | 6 | 15 |
| M1 hand il | 7.3 | 364 | − 33 | − 21 | 52 |
| M1 hand cl | 7.13 | 328 | 38 | − 22 | 46 |
| M1 tongue-lip il | 6.35 | 480 | − 32 | − 13 | 45 |
| M1 tongue-lip cl | 8.6 | 537 | 34 | − 21 | 42 |
| S1 hand il | 5.75 | 141 | − 34 | − 33 | 48 |
| S2 hand cl | 10.33 | 278 | 40 | − 22 | 46 |
| S1 tongue-lip il | 7.64 | 1280 | − 34 | − 25 | 40 |
| S1 tongue-lip cl | 11.09 | 1502 | 36 | − 24 | 39 |
| S2 il | 8.04 | 1695 | − 48 | − 6 | 16 |
| S2 cl | 6.94 | 1159 | 54 | 2 | 10 |
| Ant. insula il | 5.92 | 125 | − 44 | − 1 | 4 |
| Ant. insula cl | 7.77 | 431 | 30 | 15 | 7 |
| Post. insula il | 4.34 | 18 | − 33 | − 13 | 15 |
| Post. insula cl | 5.24 | 117 | 36 | − 13 | 6 |
| Cer IV, V, VI, VIIb il | 9.08 | 2267 | − 9 | − 61 | − 20 |
| Cer IV, V, VI, VIIb cl | 5.4 | 53 | 9 | − 66 | − 21 |
| Thalamus Pre, Mot, Som il | 5.87 | 169 | − 20 | − 13 | 1 |
| Thalamus Pre, Mot, Som cl | 6.39 | 256 | 24 | − 15 | 9 |
| Trigeminal nerve il | – | – | – | – | – |
| Trigeminal nerve cl | – | – | – | – | – |
| Solitary nucleus cl | – | – | – | – | – |
MNI coordinates of highest activation for each specific area including t-value Thalamus, and cluster size in voxels (k). il: ipsilesional; cl: contralesional; SMA: supplementary motor area; dPMC: dorsolateral premotor cortex; vPMC: ventrolateral premotor cortex; M1: primary motor cortex; S1: primary somatosensory cortex; S2: secondary somatosensory cortex; Ant.: anterior; Post: posterior; Cer IV, V, VI, VIIb: cerebellar hemisphere including specified Larsell's areas; Pre: premotor; Mot: motor; Som: somatosensory.
Generally, healthy controls showed higher activation levels than patients during deglutition in almost all areas involved in swallowing (Supplementary Fig. 1). Patients thus showed an overall decreased level of activation compared to controls, but the symmetry of swallowing representation appeared to be maintained.
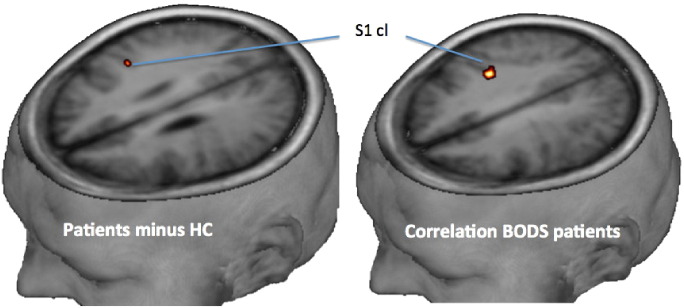
When testing patients minus controls, patients showed increased primary somatosensory representation in the unaffected hemisphere [MNI (43, − 18, 30), t = 3.57, p = 0.05]. Furthermore, contralesional S1 activation was positively associated with the BODS at admission [MNI (36, − 24, 33), t = 5.60, p = 0.005]. The initially more impaired stroke victims showed higher recruitment of recovered swallowing function in contralesional S1 (Fig. 3) suggesting a greater recruitment of this region was driving the compensated function.
Fig. 3.
Primary somatosensory cortex (S1) representation (FWE-corrected, p < 0.05) of the contralesional hemisphere when contrasting patients minus controls (left) and when performing a linear regression of BODS at admission and fMRI during recovered swallowing (right).
3.3. Cortical vs. subcortical lesion
A sub-group difference between cortical and subcortical lesions in the patient cohort could not be calculated, as the amount of subjects in the cortical group (6) was too low for a statistically valid group analysis. Instead, we calculated the difference between sub-cortically lesioned patients and the controls. An increase in contralesional S1 activation was enhanced when patients with predominantly subcortical lesions (12 patients) were compared to controls [MNI (45, − 18, 30), t = 3.96, p = 0.028].
3.4. Correlation analysis: compliance and white matter lesions
LI FA was positively associated with LI of the cerebellum (r = 0.71; p = 0.002): greater FA asymmetry was observed when there was increasing lateralization of BOLD-magnitude to the ipsilesional anterior cerebellum.
3.5. Lesion size, FA of pyramidal tracts and associations with clinical data
The lateralization index of fractional anisotropy (LI FA) between patients and controls differed (t(32) = 3.21; p = 0.005). Patients showed an asymmetric LI FA of the pyramidal tract between the tongue area and the posterior limb of the internal capsule with less FA ipsilesionally (− 0.885) whereas controls were highly symmetric (− 0.004). As expected, the larger the lesion the more asymmetric was the lateralization index of the FA-values of the pyramidal tract (r = − 0.76; p = 0.001). LI FA was positively associated with compliance (r = 0.65; p = 0.009) implying that less compliant patients showed a lower level of symmetry.
4. Discussion
The aim of this study was to better understand the neuronal reorganization associated with swallowing recovery from subacute to chronic dysphagic stroke. To our knowledge, this study is first to elucidate these changes at the whole brain level. We used high field fMRI and an event related design to study the neural network of swallowing in 18 formerly dysphagic patients post-therapy. A group of age matched controls without neurologic disease or dysphagia was used for comparison. Patients differed in dysphagic impairment in the acute stage from profound to mild impairment, and after functional recovery, despite almost completely normalizing swallowing function, they took a longer time to perform the swallowing act than controls, and in most cases had lower compliance scores.
The results of our study show an overall decreased level of activation in the patient group compared to normal function in controls, but the symmetry of swallowing representation appeared to be maintained. For patients, the laterality index of fractional anisotropy was negatively associated with lesion size and highly asymmetric: being most relevant in white matter lesions of the pyramidal tract between the M1/S1 tongue area and the posterior limb of the internal capsule. Those patients who complied more accurately in following the swallowing protocol had less damage in the pyramidal tract. Furthermore, lesion size had no influence on the severity of dysphagia. This result is unsurprising, since a small lesion in the medulla can have a sizeable impact on motor coordination, as well as indirectly influencing somatosensory input to higher cortical areas. Lesion location (rather than size) seems thus to play a more important role in deglutition disorders. Unilateral cerebral infarction in either hemisphere can, however, induce dysphagia.
By contrast, we found increased fMRI-activation in the contralesional S1 when comparing patients and controls. This finding was more pronounced when comparing patients with subcortical lesions to the controls. Those patients who were initially more severely impaired (higher BODS) also showed higher activation magnitude in the contralesional S1.
4.1. Patterns of fMRI-activation in recovered dysphagic patients post-stroke
Overall, swallowing related brain activation was lower for patients when compared to control subjects. This result differs from the one published by Li et al. (2009). However, they studied acute patients that may perform differently compared to recovered patients. Furthermore, they used saliva, which is known to elicit a higher BOLD response than swallowing water (Humbert et al., 2009). The activations were also more bilaterally represented, as seen from both voxel based comparisons as well as lateralization indices of activation magnitudes in ROIs. Bi-hemispheric activation patterns have been observed in previous fMRI studies of early post-stroke reorganization, hinting at increased compensatory activity in the ipsilesional and contralesional motor and supplementary motor cortices (Cramer et al., 1997, Loubinoux et al., 2003, Nair et al., 2007, Seitz et al., 1998, Weiller et al., 1993). Additionally, a resting state fMRI analysis in acute dysphagic patients after stroke provided evidence for a bilateral redistribution of swallowing networks after unilateral damage (Li et al., 2014). One might postulate that the contralesional activation seen in the S1 region is a reflection of this bihemispheric engagement of swallowing regions, perhaps with a greater demand on the contralesional side (Hamdy et al., 1998).
4.2. Lesion location and representation of recovered swallowing
For the representation of recovered hand function, it has been demonstrated that the representation maps are influenced by the associated lesion location. Subcortical lesions show characteristic differences in the representation of the affected hand than cortical lesions (Luft et al., 2004). We therefore expected comparable effects for recovered swallowing representation. For instance, a unilateral large cortical lesion might be compensated by activation of the same contralesional sensorimotor areas. However, a lesion in the brain stem with the representation of automatized loops of swallowing might be accompanied by a more general increase of cortical activation. This in turn may recruit additional cortical motor neurons with intact pathways to the nuclei of cranial nerves (V, VII, IX and X, XII) or other parts of the dorsal brainstem (Vuilleumier et al., 1995), which controls the more automatic swallowing function (Lang, 2009).
Comparing subcortically lesioned patients to controls, revealed a functionally relevant increase in activation in contralesional S1. Thus, our data imply that patients recovered from dysphagia after stroke may require increased somatosensory resources to successfully perform the task. This finding was supported by the observed correlation between the BODS with BOLD activity. The initially more affected patients recruited the unaffected S1 more strongly after functional recovery. Subcortical damage may thus be more critical in the acute stage of dysphagia and hence may need to compensate through sensory reorganization. One might then postulate that physical inputs such as tactile stimulation during swallowing may be an important factor in recovery, as has been shown in studies involving oro-pharyngeal stimulation (Teismann et al., 2009). Presumably, S1 involvement in re-learning the ability to swallow is important, especially when the starting point is severe dysphagia caused by subcortical lesions.
4.3. Correlates of BOLD-lateralization with damage
Lateralization indices of the cerebellum were associated with LI FA, implying that higher asymmetry of the pyramidal tract (negative LI FA) resulted in higher cerebellar involvement (positive LI). However, the more the cerebellum was involved, the lower the compliance levels seen, as observed in the association of compliance and LI of the ipsilesional cerebellum. The cerebellum may play a role in feed-forward mechanisms and timing control, sequencing, and internal coordination for oral-lingual and pharyngeal musculature (Rangarathnam et al., 2014). The implication of this phenomenon in respect of dysphagia recovery is not yet fully understood. However, in a recent study on the effect of transcranial magnetic stimulation (TMS) of the human cerebellum, directly evoked motor responses in the pharynx were observed which were believed to have originated from the cerebellum itself (Jayasekeran et al., 2011). Furthermore, when cerebellar stimulation was employed as a preconditioning stimulus before cortical stimulation, significant facilitation of the pharyngeal motor responses to cortical stimulation was found. The cerebellum may thus act to intensify or modulate the cortical firing for swallowing. In our case, the higher cerebellar involvement may have compensated for the loss of function at the cortical level, and may have facilitated motor responses, as well as modulated intact cortical areas during deglutition.
Taking our findings together, recovery after stroke-induced dysphagia can occur across different lesion locations and sizes outlined in this study. Subcortical lesions, such as in the brainstem and internal capsule, although small, produced the greatest impairment but did not impede the recovery process. Furthermore, white matter tracts, although not symmetrically distributed, were recruited in aiding reorganization of the swallowing network. However, it is possible that some of the brain changes seen at fMRI may be a function of routine swallowing therapy that all studied patients were given, a potentially important factor in the recovery process after stroke.
4.4. Limitations
One limitation of our study is the location of the lesion for each patient. Ideally lesions should be similar in size and location, or at best be localized to the same single hemisphere when analysing group data. As a consequence of not fulfilling this criteria, we were required to flip some of the subjects' brains in order to obtain a more homogeneous picture. Studies on healthy participants hint at hemispheric differences in normal swallowing function (see introduction); hence flipping some of the brains reduces the inferences we could make about lateralization. Although we tried to create at least two different clinical groups, they were small and unequal in size, and thus resulted in loss of power, especially in the cortical group. A larger group may be necessary to allow better categorization. Furthermore, it would have been interesting to measure patients in the acute stage of dysphagia and thereby assess the evolution of recovery-related functional cerebral re-organization in post-stroke dysphagia directly. However, with the method used here only patients with recovered dysphagia were investigated. Diagnosis of recovery from dysphagia at the time of study entry was based on medical records and confirmed by clinical assessment only; accordingly, we might have underdiagnosed certain aspects of dysphagia that may have been detected through diagnostic tools such as videofluoroscopy or fibreoptic endoscopy. Each patient received an individualised therapy, which might be a confounding factor for the study. Future studies might consider using a more standardized single therapy across the whole group to control for differential effects due to different treatment strategies.
Compliance is an important confounder and was hard to control for, as patients post-stroke can find it difficult to follow the protocol. A training run before the actual measurement might be effective in increasing compliance. We could not verify compliance directly, only indirectly through air cushion deflections. For better compliance recording an extra slice encompassing the tongue and larynx may provide a more direct visualization of task performance, as has been done by Babaei et al. (2012). Furthermore, we suggest that future studies use video recording to check if thyroid cartilage movements are swallow related or due to coughing or throat clearing.
The observations outlined in this manuscript do not necessarily reflect a mechanism of recovery, per se. Instead functional imaging studies such as ours can only reflect a snapshot of the recovery process. This may also be subject specific as the functional reorganization after stroke may have either completed or remain ongoing. Functional relevance of observed activation differences between patients and controls remains thus uncertain. Thus, our preliminary results should thus be interpreted accordingly, but provide a useful platform further studies in this field.
4.5. Conclusion
In summary, our data support the notion for an increase of contralesional somatosensory resources and increased ipsilesional cerebellar anterior hemisphere feed forward loops for recovered swallowing after dysphagia following stroke. Our findings are in line with the idea that recovery of swallowing function is possible with both cortical and subcortical lesions in either hemisphere and with intact cortical-subcortical asymmetrical connectivity.
The following is the supplementary data related to this article.
Contrast healthy controls (HC) minus recovered dysphagic patients (FWE-corrected, p < 0.05).
Funding
This study was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation, LO 795/12-2).
References
- Andersson J.L., Hutton C., Ashburner J., Turner R., Friston K. Modeling geometric deformations in EPI time series. NeuroImage. 2001;13(5):903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Association, A.S.-L.-H. Rockville; MD ASHA: 2003. National Outcomes Measurement System (NOMS): Adult Speech-Language Pathology User's Guide. [Google Scholar]
- Babaei A., Ward B.D., Ahmad S., Patel A., Nencka A., Li S.-J.…Shaker R. Reproducibility of swallow-induced cortical BOLD positive and negative fMRI activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303(5):G600–G609. doi: 10.1152/ajpgi.00167.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolome G., Schröter-Morasch H. fourth ed. Urban & Fischer; München: 2010. Schluckstörungen - Diagnostik und Rehabilitation. [Google Scholar]
- Becker R., Nieczaj R., Egge K., Moll A., Meinhard M., Schulz R.J. Functional dysphagia therapy and PEG treatment in a clinical geriatric setting. Dysphagia. 2011;26:108–116. doi: 10.1007/s00455-009-9270-8. [DOI] [PubMed] [Google Scholar]
- Behrens T.E.J., Berg H.J., Jbabdi S., Rushworth M.F.S., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Bandettini P.a., Cox R.W., Shaker R. Event-related fMRI of tasks involving brief motion. Hum. Brain Mapp. 1999;7:106–114. doi: 10.1002/(SICI)1097-0193(1999)7:2<106::AID-HBM4>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. Abstract Presented at the 8th International Conference on Functional Mapping of the Human Brain. 2002. Region of interest analysis using an SPM toolbox. [Google Scholar]
- Cramer S.C., Nelles G., Benson R.R., Kaplan J.D., Parker R.A., Kwong K.K., Kennedy D.N., Finklestein S.P., Rosen B.R. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997 doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Foundas a.L. The role of the insular cortex in dysphagia. Dysphagia. 1997;12:146–156. doi: 10.1007/PL00009529. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Foundas A.L., Iglesia G.C., Sullivan M.A. Lesion site in unilateral stroke patients with dysphagia. J. Stroke Cerebrovasc. Dis. 1996;6:30–34. doi: 10.1016/s1052-3057(96)80023-1. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., McAdam C.P., Brailey K., Foundas A.L. Clinical assessment of swallowing and prediction of dysphagia severity. Am. J. Speech Lang. Pathol. 1997;6:17–23. [Google Scholar]
- Daniels S.K., Brailey K., Priestly D.H., Herrington L.R., Weisberg L.a., Foundas A.L. Aspiration in patients with acute stroke. Arch. Phys. Med. Rehabil. 1998;79:14–19. doi: 10.1016/s0003-9993(98)90200-3. [DOI] [PubMed] [Google Scholar]
- Daniels S.K., Corey D.M., Fraychinaud A., DePolo A., Foundas A.L. Swallowing lateralization: the effects of modified dual-task interference. Dysphagia. 2006;21:21–27. doi: 10.1007/s00455-005-9007-2. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K., Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fox K.C.R., Nijeboer S., Dixon M.L., Floman J.L., Ellamil M., Rumak S.P., Sedlmeier P., Christoff K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 2014;43C:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Galovic M., Leisi N., Müller M., Weber J., Abela E., Kägi G., Weder B. Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke. 2013;44:2760–2767. doi: 10.1161/STROKEAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- Hamdy S., Aziz Q., Rothwell J.C., Power M., Singh K.D., Nicholson D.A., Tallis R.C., Thompson D.G. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- Hamdy S., Rothwell J.C., Brooks D.J., Bailey D., Aziz Q., Thompson D.G. Identification of the cerebral loci processing human swallowing with H2(15)O PET activation. J. Neurophysiol. 1999;81:1917–1926. doi: 10.1152/jn.1999.81.4.1917. [DOI] [PubMed] [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S., Calabresi P.A., Pekar J.J., van Zijl P.C.M., Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert I.a., Fitzgerald M.E., McLaren D.G., Johnson S., Porcaro E., Kosmatka K., Hind J., Robbins J. Neurophysiology of swallowing: effects of age and bolus type. Neuroimage. 2009;44:982–991. doi: 10.1016/j.neuroimage.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ickenstein G.W., Hofmayer A., Lindner-Pfleghar B., Pluschinski P., Riecker A., Schelling A., Prosiegel M. Standardisierung des Untersuchungsablaufs bei neurogener oropharyngealer Dysphagie (NOD) Neuropsychol. Rehabil. 2009;15:290–300. [Google Scholar]
- Jayasekeran V., Rothwell J., Hamdy S. Non-invasive magnetic stimulation of the human cerebellum facilitates cortico-bulbar projections in the swallowing motor system. Neurogastroenterol. Motil. 2011;23 doi: 10.1111/j.1365-2982.2011.01747.x. [DOI] [PubMed] [Google Scholar]
- Kern M.K., Jaradeh S., Arndorfer R.C., Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G354–G360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- Kidd D., Lawson J., Nesbitt R., MacMahon J. The natural history and clinical consequences of aspiration in acute stroke. QJM. 1995;88:409–413. [PubMed] [Google Scholar]
- Kim S.Y., Kim T.U., Hyun J.K., Lee S.J. Differences in videofluoroscopic swallowing study (VFSS) findings according to the vascular territory involved in stroke. Dysphagia. 2014 doi: 10.1007/s00455-014-9525-x. [DOI] [PubMed] [Google Scholar]
- Lang I.M. Brain stem control of the phases of swallowing. Dysphagia. 2009;24:333–348. doi: 10.1007/s00455-009-9211-6. [DOI] [PubMed] [Google Scholar]
- Li S., Luo C., Yu B., Yan B., Gong Q., He C., He L., Huang X., Yao D., Lui S., Tang H., Chen Q., Zeng Y., Zhou D. Functional magnetic resonance imaging study on dysphagia after unilateral hemispheric stroke: a preliminary study. J. Neurol. Neurosurg. Psychiatry. 2009;80:1320–1329. doi: 10.1136/jnnp.2009.176214. [DOI] [PubMed] [Google Scholar]
- Li S., Ma Z., Tu S., Zhou M., Chen S., Guo Z., Gong Q., He L., Huang X., Yao D., Lui S., Yu B., Wang X., Zhou D., He C. Altered resting-state functional and white matter tract connectivity in stroke patients with dysphagia. Neurorehabil. Neural Repair. 2014;28:260–272. doi: 10.1177/1545968313508227. [DOI] [PubMed] [Google Scholar]
- Lotze M., Beutling W., Loibl M., Domin M., Platz T., Schminke U., Byblow W. dPMC activation of the contralesional hemisphere is associated with the decrease of DTI-traces in chronic subcortical stroke patients. Neurorehabil. Neural Repair. 2012;26(6):594–603. doi: 10.1177/1545968311427706. [DOI] [PubMed] [Google Scholar]
- Loubinoux I., Carel C., Pariente J., Dechaumont S., Albucher J.F., Marque P., Manelfe C., Chollet F. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Luft A.R., McCombe-Waller S., Whitall J., Forrester L.W., Macko R., Sorkin J.D., Schulz J.B., Goldberg A.P., Hanley D.F. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malandraki G.a., Sutton B.P., Perlman A.L., Karampinos D.C., Conway C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum. Brain Mapp. 2009;30:3209–3226. doi: 10.1002/hbm.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.a., Laurienti P.J., Kraft R.a., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Martin R.E., Goodyear B.G., Gati J.S., Menon R.S. Cerebral cortical representation of automatic and volitional swallowing in humans. J. Neurophysiol. 2001;85:938–950. doi: 10.1152/jn.2001.85.2.938. [DOI] [PubMed] [Google Scholar]
- Martin R.E., MacIntosh B.J., Smith R.C., Barr A.M., Stevens T.K., Gati J.S., Menon R.S. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J. Neurophysiol. 2004;92:2428–2443. doi: 10.1152/jn.01144.2003. [DOI] [PubMed] [Google Scholar]
- Mazaika P.K., Whitfield-Gabrieli S., Reiss A., Glover G. Poster at the Human Brain Mapping Conference. 2009. Artifact repair for fMRI data from high motion clinical subjects; p. 2009. [Google Scholar]
- Meadows J.C. Dysphagia in unilateral cerebral lesions. J. Neurol. Neurosurg. Psychiatry. 1973;36:853–860. doi: 10.1136/jnnp.36.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michou E., Williams S., Vidyasagar R., Downey D., Mistry S., Edden R.a.E., Hamdy S. fMRI and MRS measures of neuroplasticity in the pharyngeal motor cortex. Neuroimage. 2015;117:1–10. doi: 10.1016/j.neuroimage.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihai P.G., von Bohlen Und Halbach O., Lotze M. Differentiation of cerebral representation of occlusion and swallowing with fMRI. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;304:847–854. doi: 10.1152/ajpgi.00456.2012. [DOI] [PubMed] [Google Scholar]
- Mihai P.G., Otto M., Platz T., Eickhoff S.B., Lotze M. Sequential evolution of cortical activity and effective connectivity of swallowing using fMRI. Hum. Brain Mapp. 2014;35:5962–5973. doi: 10.1002/hbm.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier K., Bereznaya I. Parallel cortical networks for volitional control of swallowing in humans. Exp. Brain Res. 2001;140:280–289. doi: 10.1007/s002210100813. [DOI] [PubMed] [Google Scholar]
- Mosier K., Patel R., Liu W.C., Kalnin a., Maldjian J., Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope. 1999;109:1417–1423. doi: 10.1097/00005537-199909000-00011. [DOI] [PubMed] [Google Scholar]
- Nair D.G., Hutchinson S., Fregni F., Alexander M., Pascual-Leone A., Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage. 2007;34:253–263. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Castanon A., Ghosh S.S., Tourville J.a., Guenther F.H. Region of interest based analysis of functional imaging data. Neuroimage. 2003;19:1303–1316. doi: 10.1016/s1053-8119(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pender M.P., Ferguson S.M. Dysarthria and dysphagia due to the opercular syndrome in multiple sclerosis. Mult. Scler. 2007;13:817–819. doi: 10.1177/1352458506073481. [DOI] [PubMed] [Google Scholar]
- Prosiegel M. Neurologie von Schluckstörungen. In: Prosiegel M., editor. Praxisleitfaden Dysphagie. Verlag Hygieneplan; Bad Homburg: 2002. pp. 9–46. [Google Scholar]
- Rangarathnam B., Kamarunas E., McCullough G.H. Role of cerebellum in deglutition and deglutition disorders. Cerebellum. 2014:767–776. doi: 10.1007/s12311-014-0584-1. [DOI] [PubMed] [Google Scholar]
- Riecker A., Gastl R., Kühnlein P., Kassubek J., Prosiegel M. Dysphagia due to unilateral infarction in the vascular territory of the anterior insula. Dysphagia. 2009;24:114–118. doi: 10.1007/s00455-008-9164-1. [DOI] [PubMed] [Google Scholar]
- Ripollés P., Marco-Pallarés J., de Diego-Balaguer R., Miró J., Falip M., Juncadella M., Rubio F., Rodriguez-Fornells a. Analysis of automated methods for spatial normalization of lesioned brains. Neuroimage. 2012;60:1296–1306. doi: 10.1016/j.neuroimage.2012.01.094. [DOI] [PubMed] [Google Scholar]
- Schiele J.T., Penner H., Schneider H., Quinzler R., Reich G., Wezler N., Micol W., Oster P., Haefeli W.E. Swallowing tablets and capsules increases the risk of penetration and aspiration in patients with stroke-induced dysphagia. Dysphagia. 2015 doi: 10.1007/s00455-015-9639-9. [DOI] [PubMed] [Google Scholar]
- Seitz R.J., Höflich P., Binkofski F., Tellmann L., Herzog H., Freund H.J. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch. Neurol. 1998;55:1081–1088. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- Starrost U., Bartolome G., Schröter-Morasch H., Ziegler W., Fussenegger C., Marano C., Schilling B.K. Der Bogenhausener Dysphagiescore–BODS: Inhaltsvalidität und Reliabilität. DysphagieForum. 2012;2:2–11. [Google Scholar]
- Stueve D. Management of pediatric radiation dose using Philips fluoroscopy systems DoseWise: perfect image, perfect sense. Pediatr. Radiol. 2006;36(Suppl. 2):216–220. doi: 10.1007/s00247-006-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Asada Y., Ito J., Hayashi K., Inoue H., Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–77. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- Teismann I.K., Steinsträter O., Warnecke T., Suntrup S., Ringelstein E.B., Pantev C., Dziewas R. Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. 2009;10:71. doi: 10.1186/1471-2202-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toogood J.a., Barr A.M., Stevens T.K., Gati J.S., Menon R.S., Martin R.E. Discrete functional contributions of cerebral cortical foci in voluntary swallowing: a functional magnetic resonance imaging (fMRI) “Go, No-Go” study. Exp. Brain Res. 2005;161:81–90. doi: 10.1007/s00221-004-2048-1. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Bogousslavsky J., Regli F. Infarction of the lower brainstem. Brain. 1995;118:1013–1025. doi: 10.1093/brain/118.4.1013. [DOI] [PubMed] [Google Scholar]
- Weiller C., Ramsay S.C., Wise R.J., Friston K.J., Frackowiak R.S. Individual patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann. Neurol. 1993;33:181–189. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- Windel A.-S., Mihai P.G., Lotze M. Neural representation of swallowing is retained with age. A functional neuroimaging study validated by classical and Bayesian inference. Behav. Brain Res. 2015 doi: 10.1016/j.bbr.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Koh H., Shimada H., Takeuchi J., Yamakawa Y., Kawamura M., Miki T. Cerebral infarction in the left hemisphere compared with the right hemisphere increases the risk of aspiration pneumonia. Osaka City Med. J. 2014;60(2):81–86. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contrast healthy controls (HC) minus recovered dysphagic patients (FWE-corrected, p < 0.05).