Abstract
Auditory verbal hallucinations (AVH) of schizophrenia are associated with a disrupted connectivity between frontal and temporoparietal language areas. We hypothesized that this dysconnectivity is underpinned by white matter abnormalities in the left arcuate fasciculus, the main fiber bundle connecting speech production and perception areas. We therefore investigated the relationship between AVH severity and the integrity of the arcuate fasciculus measured by diffusion tensor imaging (DTI) tractography in patients with schizophrenia.
Thirty-eight patients with treatment-resistant schizophrenia were included: 26 presented with daily severe treatment-resistant AVH, 12 reported prominent negative symptoms and no AVH. Fractional anisotropy (FA) was measured along the length of the left and right anterior arcuate fasciculi and severity of AVH was assessed using P3 PANSS item.
FA values were significantly higher in the left arcuate fasciculus in patients with AVH than in no AVH patients (F(1,35) = 3.86; p = 0.05). No difference was observed in the right arcuate fasciculus. There was a significant positive correlation between FA value in the left arcuate fasciculus and the severity of AVH (r = 0.36; p = 0.02). No correlation was observed between FA values and PANSS total score suggesting a specific relationship between AVH severity and the left arcuate fasciculus integrity.
These results support the hypothesis of a relationship between left frontotemporal connectivity and AVH in patients with schizophrenia and suggest that whilst a disruption of frontotemporal connectivity might be present to ensure the emergence of AVH, more severe anatomical alterations may prevent the occurrence of AVH in patients with schizophrenia.
Keywords: Schizophrenia, Auditory verbal hallucinations, Diffusion tensor imaging, Arcuate fasciculus, White matter, Tractography, DTI
Highlights
-
•
Fractional anisotropy (FA) in the left arcuate fasciculus (AF) correlated with severity of hallucinations.
-
•
Hallucinating patients had greater FA in the left AF than non-hallucinating patients.
-
•
We did not find any association between hallucinations and FA in the right AF.
1. Introduction
Auditory verbal hallucinations (AVH) are key symptoms of schizophrenia. The brain networks underlying such experiences are still unclear. Several neuroimaging studies have associated AVH with impaired functional connectivity within large-scale networks (Hoffman and Hampson, 2012). More specifically, it has been reported that patients with AVH displayed an abnormal resting functional connectivity between frontal and temporal areas including brain networks involved in the perception and production of speech (Lawrie et al., 2002; for a review see Alderson-Day et al., 2015). Post mortem and genetic studies suggest that such abnormalities in functional connectivity between frontal and temporal regions may be underpinned at the structural level by white matter (WM) microstructural abnormalities within bundles connecting these two regions (for review see Takahashi et al., 2011). However, the direct link between AVH symptoms and WM anatomy remains unclear. Thanks to recent advances in brain imaging, it is possible to noninvasively explore and quantify WM microstructure in the living human using magnetic resonance diffusion tensor imaging (DTI). Fractional anisotropy (FA) is the most frequently used measure of anisotropic diffusion and is assumed to reflect WM fiber organization, or axonal and myelin integrity (Beaulieu, 2002). DTI studies investigating WM integrity in schizophrenia reported fiber tracts abnormalities in the left frontal lobe and the left temporal lobe in various populations: individuals at risk to develop schizophrenia (Samartzis et al., 2014), medicated and unmedicated patients with schizophrenia (Ellison-Wright and Bullmore, 2009, Mandl et al., 2013), patients at the early stage of disorder and patients with first-episode schizophrenia (Kyriakopoulos and Frangou, 2009, Luck et al., 2011).
For example, as compared with healthy controls, some studies reported a decreased FA within several fiber tracts including the uncinate fasciculus, the inferior longitudinal fasciculi, the cingulum bundle and the arcuate fasciculus in patients with schizophrenia (Minami et al., 2003, Phillips et al., 2009; for a review see Kubicki et al., 2007). By contrast, other studies reported an increased FA in bundles such as the arcuate fasciculus (Knöchel et al., 2012; for a review see Kubicki et al., 2007). To date, the discrepancy in findings is not explained. The clinical heterogeneity of schizophrenia patients included in published studies could be a confounding factor. The stratification of schizophrenia patients according to their prominent symptoms (such as AVH or negative symptoms) could help to clarify contradictory results.
Several studies investigated the relationship between FA and AVH and most of them focused on fiber tracts belonging to the networks involved in language such as the arcuate fasciculus and the superior longitudinal fasciculus (SLF). These bundles connect frontal areas with the posterior part of the temporoparietal junction. In a recent metaanalysis including 5 DTI studies that compared patients with schizophrenia suffering from AVH (AVH +; n = 106) to matched healthy controls (n = 150), Geoffroy et al. (2014) reported a significant mean reduced FA in the left, but not in the right, arcuate fasciculus in AVH + patients.
Despite the fact that some studies compared WM integrity of the arcuate fasciculus between AVH + patients with schizophrenia and patients with schizophrenia without AVH (no-AVH) in order to distinguish AVH-specific from schizophrenia-specific effects, the relationship between severity of AVH and integrity of the left and/or right arcuate fasciculus remains unclear. For instance, McCarthy-Jones et al. (2015) and de Weijer et al. (2011) reported that FA was significantly reduced in the left arcuate fasciculus in AVH + patients as compared with no-AVH patients. A reduced FA was also observed in right arcuate fasciculus of AVH + as compared with no-AVH patients (Catani et al., 2011). Conversely, other studies showed an increased FA in the superior temporal gyrus (Lee et al., 2009) and in the arcuate fasciculus of AVH + patients as compared to no-AVH patients (Hubl et al., 2004). Moreover, Seok et al. (2007) observed an increased FA in the left SLF of AVH + patients as compared to no-AVH patients. Remarkably, few studies investigated the relationship between AVH severity and WM integrity of the arcuate fasciculus in patients with schizophrenia. Rotarska-Jagiela et al. (2009) observed a positive correlation between FA in the arcuate fasciculus and AVH whereas Ćurčić-Blake et al. (2015) reported the inverse relationship. Two studies reported a positive correlation between AVH severity and FA in the SLF either in the left part (Seok et al., 2007) or bilaterally (Shergill et al., 2007). Finally, a recent study in unmedicated subjects at risk to develop psychosis reported larger abnormalities in WM integrity and functional connectivity within the left perisylvian language network in subjects who had not developed AVH as compared with subjects who had developed AVH (Benetti et al., 2015).
Several considerations can explain these discrepancies between studies ranging from clinical characteristics of included patients, age and medication to DTI methodology and anatomical definition of the bundle. In the present study, we aimed to investigate the relationship between AVH and the integrity of the arcuate fasciculus in patients with treatment-resistant schizophrenia according to the presence or absence of AVH. The resistance was defined as the presence of symptoms after two well-conducted antipsychotic treatments, in sufficient dose and duration. Patients included in this study were clinically characterized either by the presence of daily resistant AVH or absence of daily resistant AVH. Hence, we undertook a DTI-tractography study comparing FA values in both left and right arcuate fasciculi between these two samples of patients. We also investigated the relationship between the integrity of the arcuate fasciculus and AVH severity. We hypothesized that severity of AVH was associated with abnormal FA values especially in the left arcuate fasciculus.
2. Material and methods
2.1. Subjects
Thirty-eight patients with DSM IV-TR criteria for schizophrenia were enrolled in the study (mean PANSS score 76.3 ± standard deviation 11.8). Patients were free from any other DSM IV disorders as assessed by a structural interview using the Mini-International Neuropsychiatric Interview (MINI, Sheehan et al., 1998). The study was approved by a local ethic committee (CPP Sud EST 6, France) and all patients gave their written informed consent.
The sample was separated into two groups based on the presence of daily AVH (AVH + and no-AVH patients). The severity of AVH was assessed by the Positive and Negative Syndrome Scale (PANSS) P3 item (i.e., hallucinations). Since all the recruited patients experienced mainly AVH, the P3 item was used to measure exclusively AVH severity in our study. Twenty-six patients presented with daily severe treatment-resistant AVH (PANSS P3 item > 3), 12 patients had no current or past history of AVH (PANSS P3 item ≤ 3) and presented with disabling treatment-resistant negative symptoms.
Since brain lateralization and thickness of WM fibers may vary with handedness (Parker et al., 2005, Knecht et al., 2000), only right-handed subjects were included in this study. All included patients were treated with antipsychotic medication for at least three months without changes in dose (713 equivalent chlorpromazine mg/day ± 584). Patients (11 women and 27 men) were aged between 22 and 57 years old (mean: 38.3 ± standard deviation: 9.4) with an educational level of 11.5 ± 2.6 years and an illness duration of 11.2 ± 8.2 years. Characteristics of groups are given in Table 1.
Table 1.
Socio-demographic and clinical characteristics of patients with schizophrenia.
| AVH | no-AVH | p | |
|---|---|---|---|
| n | 26 | 12 | |
| Female/Male | 10/26 | 1/12 | 0.12 |
| Age (years) | 36.4 ± 8.3 | 42.4 ± 10.7 | 0.06 |
| Educational level | 11.4 ± 2.7 | 11.5 ± 2.5 | 0.93 |
| Illness duration (years) | 9.9 ± 6.8 | 13.7 ± 10.1 | 0.19 |
| Medication (eq cpz mg/day) | 745 ± 561 | 650 ± 648 | 0.65 |
| PANSS | 73.5 ± 12.3 | 82.4 ± 8.3 | 0.02 |
| P3 item (hallucinations) | 5.8 ± 0.9 | 1.7 ± 0.9 | < 0.001 |
2.2. Image acquisition
DTI and T1-weighted MRI images were acquired on a 1.5-T Siemens Magnetom Sonata Maestro Class system equipped with a standard headcoil, at the “CERMEP-Imagerie du vivant” research imaging center of Lyon, France. Head motion was restricted using foam padding. Structural images were obtained with a standard T1-weighted pulse sequence: TR = 1.97 ms, TE = 3.93 ms, flip angle of 15°, FOV = 256 × 256 mm, voxel size: 1.0 × 1.0 × 1.0 mm. Whole brain DTI images were then acquired using a spin-echo EPI sequence (TR = 3800 ms, TE = 96 ms) with 128 × 128 phase-encoding over a FOV of 320 × 320 mm2 and 51 axial slices of 2.5 mm thickness. DTI images were acquired in 24 directions with b values of 0 and 1000 s/mm2. Six acquisitions were made and averaged at b = 0 and 3 acquisitions in other directions. The scan duration for the DTI sequence was about 10 min.
2.3. Data analyses
2.3.1. Image processing
DTI data of each subject were processed using MedINRIA software. Each run was linearly registered to the first diffusion series using the b = 0 image, while assuming that no motion occurred within a set (visual check). No eddy current correction was performed. Color FA map was obtained and tractography (DTI track) for the arcuate fasciculus was performed by seeding the voxels with a FA > 0.2 and a constraining angle lower than 60°.
2.3.2. Region of interest definition
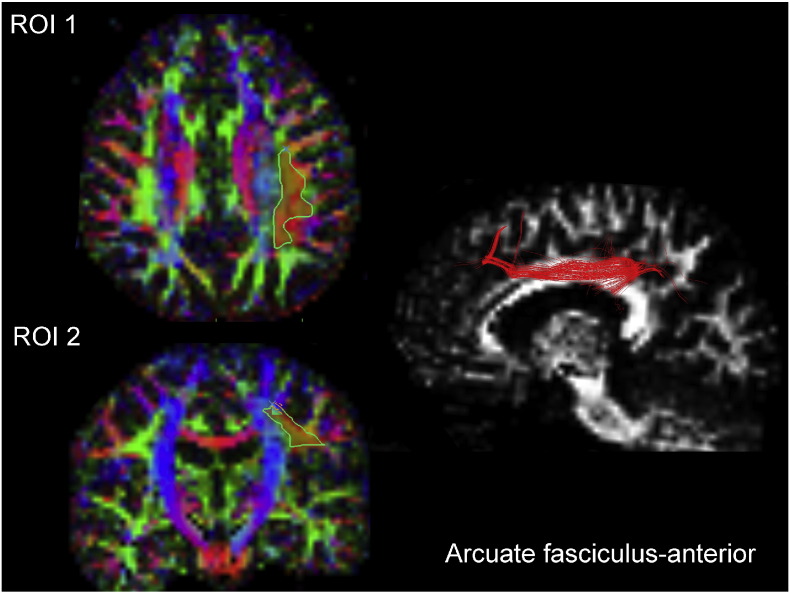
Here, with a 2-ROI approach based on FA color maps, only green fibers were selected on an axial and coronal plan for each subject. These fibers corresponded to fibers with anterior-posterior orientation (arcuate fasciculus-anterior). In each hemisphere, two regions of interest (ROI) were used to extract the arcuate fasciculus fibers. All ROIs were independently drawn on the FA weighted colored maps by two investigators blinded to the hallucination status. The ROIs were placed referring to Oishi et al.'s MRI atlas of human white matter (Oishi et al., 2011) as follows: the first ROI was placed on an axial slice corresponding to Talairach 5/6, MNI z = 27.5 to 32.5 and the second ROI was placed on a coronal slice corresponding to Talairach E2/E3, MNI y = − 12.5 to − 17.5 as described on the MRI atlas of human white matter, second edition (Oishi et al., 2011) pages 95–99 and 155–159 respectively (Fig. 1). The obtained fiber tract throughout these 2 ROIs was then analyzed using bundle manager tool in MedINRIA. The value used in the analysis was the mean of the FA values obtained by the 2 independent experimenters (MP & CF), with an inter-rater correlation of 0.93. The fiber was tracked separately on each of the hemisphere using the same methodology.
Fig. 1.
Left panel (ROI 1/ROI 2) shows FA-weighted colored maps with ROI positions (in red and delineated in green). Right panel shows representative 3D illustrations of the arcuate fasciculus-anterior in a patient with schizophrenia (in red).
2.4. Statistical analyses
Clinical characteristics of patients were compared using 2-tailed Student t-tests and using Fischer's exact test for gender. As a trend toward a significant difference in age was observed between groups (p = 0.06) and since age is known to influence FA values (Voineskos et al., 2010, Bijanki et al., 2015), age was added as covariate in the analysis. For each hemisphere, FA values of the arcuate fasciculus were compared between AVH + and no-AVH patients using an ANCOVA with age as covariate. Intra group comparisons (right versus left FA) were analyzed using Student t-test and Effect size (Hedges' g) were calculated.
Relationships between FA values (left and right) and severity of symptoms (AVH measured by P3 item PANSS score and symptoms of schizophrenia measured by PANSS total scores) were analyzed using Pearson correlation tests. Significance was set at p < 0.05.
3. Results
3.1. Comparison between AVH + and no-AVH patients
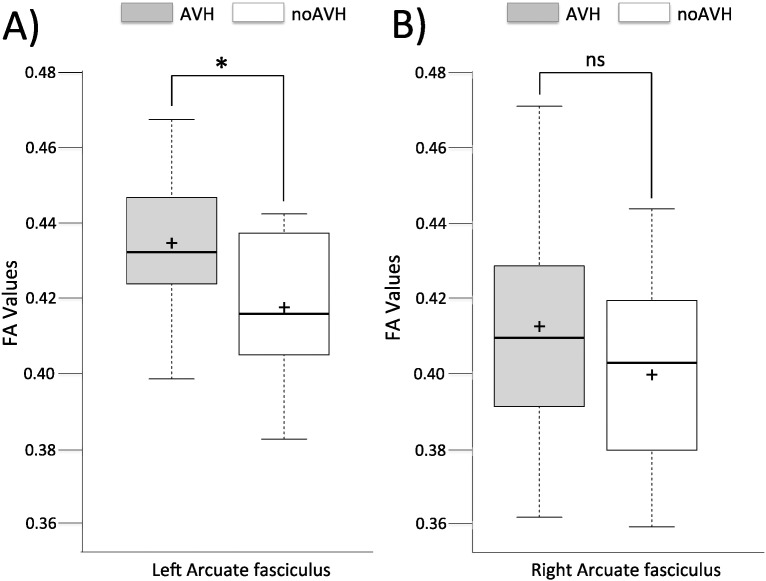
The ANCOVA with age as a covariate revealed a significant difference (F(1,35) = 3.86; p = 0.05) in FA values of the left arcuate fasciculus between AVH + (0.434 ± 0.019) and no-AVH patients (0.417 ± 0.019; Fig. 2, part A). No difference was observed for FA values in the right arcuate fasciculus (F(1,35) = 1.09; p = 0.30) between AVH + (0.412 ± 0.027) and no-AVH patients (0.399 ± 0.027; Fig. 2, part B).
Fig. 2.
FA value in the left (part A) and right (part B) arcuate fasciculus in patients with schizophrenia with and without daily auditory hallucinations.
Center lines show the medians; box limits indicate the 25th and 75th percentiles as determined by R software; whiskers extend 1.5 times the interquartile range from the 25th and 75th percentiles, outliers are represented by dots; crosses represent sample means. n = 26, 12, 26, 12 sample points.
3.2. Comparison between right and left arcuate fasciculus
In AVH + patients, the FA value was significantly greater in the left arcuate fasciculus as compared to the right arcuate fasciculus (Hedges g = 0.93; 95% CI = 0.36–1.51; p = 0.001) whereas only a trend toward a significant difference between left and right arcuate fasciculi was observed in no-AVH patients (Hedges' g = 0.73; 95% CI = − 0.09–1.56; p = 0.08).
3.3. Relationship analysis between FA and AVH severity
A significant positive correlation was observed between the FA value in the left arcuate fasciculus and the severity of AVH measured by P3 PANSS item (r = 0.36; p = 0.02). No significant correlation was observed between the FA value in the left arcuate fasciculus and the severity of general symptoms of schizophrenia measured by PANSS total score (r = − 0.04; p = 0.80).
No correlations were found between the FA value in the right arcuate fasciculus and the severity of AVH measured by P3 PANSS item (r = 0.165; p = 0.32) as well as between the FA value in the right arcuate fasciculus and the severity of symptoms measured by PANSS total score (r = 0.023; p = 0.89).
4. Discussion
In this study, we used DTI-tractography to investigate WM abnormalities in both left and right arcuate fasciculi in AVH + patients as compared to no-AVH patients with schizophrenia. We reported that FA was greater in the left arcuate fasciculus of AVH + patients as compared with no-AVH patients whereas no difference was observed for the right arcuate fasciculus between the 2 groups. Moreover, the FA value was significantly greater in the left arcuate fasciculus as compared to the right arcuate fasciculus in AVH + patients, whereas only a trend toward a significant difference between left and right arcuate fasciculi was observed in no-AVH patients.
We also found a significant positive correlation between FA values in the left arcuate fasciculus and the severity of AVH measured with ‘P3’ item of the PANSS. This correlation seems to be specifically lateralized since no correlation was found between FA in the right arcuate fasciculus and severity of AVH. The left-side specificity of the correlation between AVH and WM integrity of the arcuate fasciculus may be related to the fact that speech-relevant areas are predominantly located in the left hemisphere in > 90% of right-handed subjects (Catani et al., 2007, Vernooij et al., 2007). The observed increase in FA within the left arcuate fasciculus seems to be specifically associated with AVH severity since no significant relationship was observed between FA (right and left) and total PANSS score. We ensured that our results were not influenced by individual characteristics that are known to influence FA values in patients with schizophrenia by introducing age (Mori et al., 2007, Voineskos et al., 2010, Bijanki et al., 2015) as a covariate in our analysis. Moreover, we ensured that AVH + and no-AVH groups were not different for illness duration, gender and medication (Minami et al., 2003, Okugawa et al., 2004, Takase et al., 2004).
Our results are in line with previous findings investigating WM abnormalities in patients with schizophrenia and reporting increased FA value in the left arcuate fasciculus in AVH + patients with schizophrenia as compared with no-AVH patients (Hubl et al., 2004, Seok et al., 2007). However, these results are in contradiction with other studies reporting a reduced FA value in the left arcuate fasciculus in AVH + patients as compared to no-AVH patients and healthy controls (de Weijer et al., 2011, Catani et al., 2011, Geoffroy et al., 2014, Ćurčić-Blake et al., 2015, McCarthy-Jones et al., 2015). These discrepancies between findings might be explained by difference in DTI methodology. Indeed, most previous studies have used a VBM approach that assesses WM alterations on large regions without an a priori hypothesis by calculating a mean FA value on a ROI on a FA map with WM templates. Thus VBM approach does not take advantage of DTI's ability to depict WM systems through fiber tracking. Here, we used DTI-tractography that assembles the local diffusion tensor data to infer the paths of fiber tracts (Mori et al., 1999), based on a priori hypothesis, where scalar metrics, such as FA, along these tracts allow for the precise localization of WM abnormalities in the arcuate fasciculus.
The heterogeneity in the definition of the tract between studies could also be taken into account (e.g., parts of the arcuate fasciculus, the perisylvian language network, parts of the SLF). Indeed, tractography studies have revealed that anatomy of the arcuate fasciculus is complex (Catani et al., 2005) and delimitation between the SLF and the arcuate fasciculus is not yet clearly established (Dick and Tremblay, 2012). Here, we used a tract-specific measurements method that allowed us to quantify the microstructural integrity of the arcuate fasciculus. However, tractography approach has limitations such as sensitivity to ROI placement and partial volume effect (Lifshits et al., 2009). In this way, with the same objective and using a tractography method, the portion of the arcuate fasciculus considered differs from one study to the other according to the parameters used to specify anatomical boundaries. Here, with a 2-ROIs approach based on FA color maps, only green fibers were selected on an axial and coronal plan for each subject. These fibers corresponded to fibers with anterior-posterior orientation (arcuate fasciculus anterior, van Beek et al., 2014). In contrast, de Weijer et al. and McCarthy-Jones et al. developed measurement designs giving access to U-shape fibers of the arcuate fasciculus (de Weijer et al., 2011, McCarthy-Jones et al., 2015).
The heterogeneity in clinical characteristics of included patients in the different studies could also in part explain the observed differences. The characteristics of AVH and the characteristic of the no-AVH groups can have influenced FA values. The main discrepancies concern the clinical characteristics of no-AVH populations who are either patients with no lifetime AVH (this study and Seok et al., 2007) or patients with no AVH in the last month (Ćurčić-Blake et al., 2015). The AVH + populations included patients with remitted AVH (McCarthy-Jones et al., 2015), or treatment-resistant AVH (Seok et al., 2007). In the current study, we included patients with treatment-resistant daily chronic AVH and patients with treatment-resistant negative symptoms and no current or past history of AVH. Interestingly, the clinical characteristics of our populations and our results are similar to those of Seok and collaborators suggesting that the increase of FA in left arcuate fasciculus in AVH + patients compared with no-AVH patients is specific to treatment-resistant AVH (Seok et al., 2007).
The findings of a reduced FA value in the left arcuate fasciculus of patients with prominent treatment resistant negative symptoms as compared with AVH + patients are consistent with studies reporting lower FA in patients with prominent negative symptoms as compared with healthy controls (Hovington et al., 2015). Furthermore, in healthy subjects, studies show that most subjects have leftward lateralization of the arcuate fasciculus (Catani et al., 2007). In our results, this leftward lateralization of the arcuate fasciculus is found in AVH + patients but not in no-AVH patients. One can hypothesize that whilst disruption of connectivity might be present to ensure the emergence of AVH as compared with healthy controls (Geoffroy et al., 2014), more severe WM alterations may prevent the occurrence of AVH in patients with predominantly negative symptoms as suggested by Benetti et al. (2015). This hypothesis is consistent with McCarthy-Jones and collaborators findings showing that patients with larger FA decreases in the arcuate were less likely to have current AVH (McCarthy-Jones et al., 2015). Our findings could also be interpreted as meaning that AVH + patients may have normal FA and no-AVH patients abnormal low FA. The lack of a healthy subject group doesn't allow us to solve this point. However, the positive correlation found between FA of the left arcuate fasciculus and AVH severity suggests that the FA in AVH + patients is abnormal.
Our results are in line with functional imaging studies reporting a hypercoupling between frontal and temporal regions in non-psychotic individuals with hallucinations (Diederen et al., 2013) and in AVH + patients with schizophrenia (Lavigne et al., 2015). The WM alterations observed in patients with predominantly negative symptoms could be linked to a high susceptibility of WM tracts to inflammatory mediators in this population (Alba-Ferrara and de Erausquin, 2013). Indeed, inflammatory mediators, such as C-reactive protein (CRP), were associated with reduced FA (Prasad et al., 2015) and higher CRP levels in schizophrenia in patients with more severe negative symptoms (Garcia-Rizo et al., 2012, Joseph et al., 2015). Regarding to patient with prominent AVH, to our knowledge, no data linking cytokine levels and AVH severity in patients with schizophrenia are available to date.
Differences in the types of fibers altered could also explain the differences in FA value between patients with prominent negative symptoms and patients with treatment-resistant daily chronic AVH. In white mater regions, higher FA is typically associated with favorable neurobiological factors, such as increased tissue density and fiber organization and conversely. Thus, a reduced FA is commonly associated with clinical populations and interpreted as reflecting axonal disorganization or alterations of myelin. However, evidence for increased FA has been reported in some clinical populations, such as anorexia nervosa (Travis et al., 2015) and individuals born preterm (Groeschel et al., 2014). Interestingly, in these two populations increased FA was found in the left superior fasciculus and supposed to reflect increased fiber coherence from a reduction in the number or density of crossing fibers (Groeschel et al., 2014). Thus, a reduction in crossing fibers within the arcuate fasciculus could increase FA in AVH + patients and offset the FA decrease due to alterations specific to the arcuate fasciculus fibers. Investigating the integrity of other bundles might also be of interest to better understand the relationship between AVH and WM abnormalities. For instance, a significantly greater FA in the fiber connecting homotopic auditory areas via the corpus callosum was observed in AVH + as compared to no-AVH patients, a trendwise correlation between FA values and severity of AVH symptoms was also reported (Mulert et al., 2012). Moreover, a recent study performing cluster analysis on 18 fiber tracts showed two patterns of WM abnormalities in patients with schizophrenia as compared to healthy subjects. These patterns of WM alteration allowed discriminating two subgroups of patients: patients with widespread WM abnormalities showing more severe negative symptoms than patients with circumscribed regional WM abnormalities, mostly in the left superior longitudinal fasciculus (Sun et al., 2015). Combined with our findings, these results suggest the existence of two neurobiologically distinct subgroups of patients with schizophrenia according to their prominent symptomatology. Moreover, it suggests that WM abnormalities can provide a biomarker for subtyping patients.
Finally, abnormalities in the left arcuate fasciculus integrity, the most important fiber bundle between Broca's area and Wernicke's area could in part explain the emergence of AVH in patients with frequent AVH. More severe WM abnormalities in patients with prominent negative symptoms may prevent the occurrence of AVH. The correlation between FA and AVH symptom severity suggest that integrity of the left arcuate fasciculus could be a biological marker of AVH. Our results outline the importance of stratifying patients with schizophrenia according to their prominent symptoms in further imaging studies.
Role of the funding source
This study was supported by a grant from the “Conseil Scientifique de la Recherche” from the Hôpital Le Vinatier, Bron, France. JB & DL hold a travel grant from Centre de Recherche Fernand Seguin de l'Hôpital Louis H Fontaine, Montréal, QC, Canada.
JB is supported by the 2013 NARSAD Young Investigator award from the Brain & Behavior Research foundation (grant number # 20988). MM holds a Canadian Institutes of Health Research fellowship award (CIHR#140867).
The funding source had no further role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Contributors
JB & MM wrote the first draft of manuscript. MM assisted with the data collection. JB & DL assisted with data analyses; MP & CF have analyzed imaging data. FH assessed clinical characteristics of patients. MFSC supervised analyses. All authors contributed to and have approved the final manuscript.
Conflict of interest
None of the authors have any conflicts of interest related to this work.
References
- Alba-Ferrara L.M., de Erausquin G.A. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front. Integr. Neurosci. 2013;7:9. doi: 10.3389/fnint.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B., McCarthy-Jones S., Fernyhough C. Hearing voices in the resting brain: a review of intrinsic functional connectivity research on auditory verbal hallucinations. Neurosci. Biobehav. Rev. 2015;55:78–87. doi: 10.1016/j.neubiorev.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benetti S., Pettersson-Yeo W., Allen P., Catani M., Williams S., Barsaglini A., Kambeitz-Ilankovic L.M., McGuire P., Mechelli A. Auditory verbal hallucinations and brain dysconnectivity in the perisylvian language network: a multimodal investigation. Schizophr. Bull. 2015;41(1):192–200. doi: 10.1093/schbul/sbt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijanki K.R., Hodis B., Magnotta V.A., Zeien E., Andreasen N.C. Effects of age on white matter integrity and negative symptoms in schizophrenia. Schizophr. Res. 2015;161(1):29–35. doi: 10.1016/j.schres.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Catani M., Allin M.P., Husain M., Pugliese L., Mesulam M.M., Murray R.M. Symmetries in human brain language pathways correlate with verbal recall. Proc. Natl. Acad. Sci. U. S. A. 2007;104(43):17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Craig M.C., Forkel S.J., Kanaan R., Picchioni M., Toulopoulou T., Shergill S., Williams S., Murphy D.G., McGuire P. Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol. Psychiatry. 2011;70(12):1143–1150. doi: 10.1016/j.biopsych.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Ćurčić-Blake B., Nanetti L., van der Meer L., Cerliani L., Renken R., Pijnenborg G.H.M., Aleman A. Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain Struct. Funct. 2015;220(1):407–418. doi: 10.1007/s00429-013-0663-y. [DOI] [PubMed] [Google Scholar]
- de Weijer A.D., Mandl R.C.W., Diederen K.M.J., Neggers S.F.W., Kahn R.S., Hulshoff Pol H.E., Sommer I.E.C. Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr. Res. 2011;130:68–77. doi: 10.1016/j.schres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Dick A.S., Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135(Pt 12):3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Diederen K.M., Neggers S.F., de Weijer A.D., van Lutterveld R., Daalman K., Eickhoff S.B., Clos M., Kahn R.S., Sommer I.E. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol. Med. 2013;43(8):1685–1696. doi: 10.1017/S0033291712002541. (Epub 2012 Nov 16) [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr. Res. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Garcia-Rizo C., Fernandez-Egea E., Oliveira C., Justicia A., Bernardo M., Kirkpatrick B. Inflammatory markers in antipsychotic-naïve patients with nonaffective psychosis and deficit vs. nondeficit features. Psychiatry Res. 2012;198(2):212–215. doi: 10.1016/j.psychres.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy P.A., Houenou J., Duhamel A., Amad A., De Weijer A.D., Curčić-Blake B., Linden D.E., Thomas P., Jardri R. The Arcuate Fasciculus in auditory-verbal hallucinations: a meta-analysis of diffusion-tensor-imaging studies. Schizophr. Res. 2014;159(1):234–237. doi: 10.1016/j.schres.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Groeschel S., Tournier J.D., Northam G.B., Baldeweg T., Wyatt J., Vollmer B. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. NeuroImage. 2014;87:209–219. doi: 10.1016/j.neuroimage.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., Hampson M. Functional connectivity studies of patients with auditory verbal hallucinations. Front. Hum. Neurosci. 2012;6:6. doi: 10.3389/fnhum.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovington C.L., Bodnar M., Chakravarty M.M., Joober R., Malla A.K., Lepage M. Investigation of white matter abnormalities in first episode psychosis patients with persistent negative symptoms. Psychiatry Res. 2015;233(3):402–408. doi: 10.1016/j.pscychresns.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Hubl D., Koenig T., Strik W., Federspiel A., Kreis R., Boesch C., Maier S.E., Schroth G., Lovblad K., Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch. Gen. Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Joseph J., Depp C., Martin A.S., Daly R.E., Glorioso D.K., Palmer B.W. Associations of high sensitivity C-reactive protein levels in schizophrenia and comparison groups. Schizophr. Res. 2015;168(1–2):456–460. doi: 10.1016/j.schres.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S., Drager B., Deppe M., Bobe L., Lohmann H., Floel A., Ringelstein E.B., Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Knöchel C., O'Dwyer L., Alves G., Reinke B., Magerkurth J., Rotarska-Jagiela A., Prvulovic D., Hampel H., Linden D.E., Oertel-Knöchel V. Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenia patients and unaffected relatives. Schizophr. Res. 2012;140(1–3):129–135. doi: 10.1016/j.schres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Kubicki M., McCarley R., Westin C.F., Park H.J., Maier S., Kikinis R., Jolesz F.A., Shenton M.E. A review of diffusion tensor imaging studies in schizophrenia. J. Psychiatr. Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos M., Frangou S. Recent diffusion tensor imaging findings in early stages of schizophrenia. Curr. Opin. Psychiatry. 2009;22:168–176. doi: 10.1097/YCO.0b013e328325aa23. [DOI] [PubMed] [Google Scholar]
- Lavigne K.M., Rapin L.A., Metzak P.D., Whitman J.C., Jung K., Dohen M., Lœvenbruck H., Woodward T.S. Left-dominant temporal-frontal hypercoupling in schizophrenia patients with hallucinations during speech perception. Schizophr. Bull. 2015;41(1):259–267. doi: 10.1093/schbul/sbu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie S.M., Buechel C., Whalley H.C., Frith C.D., Friston K.J., Johnstone E.C. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol. Psychiatry. 2002;51(12):1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lee K., Yoshida T., Kubicki M., Bouix S., Westin C.F., Kindlmann G., Niznikiewicz M., Cohen A., McCarley R.W., Shenton M.E. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophr. Res. 2009;108:33–40. doi: 10.1016/j.schres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshits S., Tamir A., Assaf Y. Combinatorial fiber-tracking of the human brain. NeuroImage. 2009;48(3):532–540. doi: 10.1016/j.neuroimage.2009.05.086. [DOI] [PubMed] [Google Scholar]
- Luck D., Buchy L., Czechowska Y., Bodnar M., Pike G.B., Campbell J.S.W., Achim A., Malla A., Joober R., Lepage M. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. J. Psychiatr. Res. 2011;45:369–377. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Mandl R.C., Rais M., van Baal G.C., van Haren N.E., Cahn W., Kahn R.S., Hulshoff Pol H.E. Altered white matter connectivity in never-medicated patients with schizophrenia. Hum. Brain Mapp. 2013;34(9):2353–2365. doi: 10.1002/hbm.22075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy-Jones S., Oestreich L.K., Australian Schizophrenia Research Bank, Whitford T.J. Reduced integrity of the left arcuate fasciculus is specifically associated with auditory verbal hallucinations in schizophrenia. Schizophr. Res. 2015;162(1–3):1–6. doi: 10.1016/j.schres.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Minami T., Nobuhara K., Okugawa G., Takase K., Yoshida T., Sawada S. Diffusion tensor magnetic resonance imaging of disruption of regional white matter in schizophrenia. Neuropsychobiology. 2003;47:141–145. doi: 10.1159/000070583. [DOI] [PubMed] [Google Scholar]
- Mori S., Crain B.J., Chacko V.P., van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mori T., Ohnishi T., Hashimoto R., Nemoto K., Moriguchi Y., Noguchi H. Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Res. 2007;154:133–145. doi: 10.1016/j.pscychresns.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mulert C., Kirsch V., Whitford T.J., Alvarado J., Pelavin P., McCarley R.W., Kubicki M., Salisbury D.F., Shenton M.E. Hearing voices: a role of interhemispheric auditory connectivity? World J. Biol. Psychiatry. 2012;13(2):153–158. doi: 10.3109/15622975.2011.570789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A.V., van Zijl P.C.M., Mori S. second ed. Elsevier; Academic Press: 2011. MRI Atlas of Human White Matter. (266 pp.) [Google Scholar]
- Okugawa G., Nobuhara K., Minami T., Tamagaki C., Takase K., Sugimoto T. Subtle disruption of the middle cerebellar peduncles in patients with schizophrenia. Neuropsychobiology. 2004;50:119–123. doi: 10.1159/000079101. [DOI] [PubMed] [Google Scholar]
- Parker G.J., Luzzi S., Alexander D.C., Wheeler-Kingshott C.A., Ciccarelli O., Lambon Ralph M.A. Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage. 2005;24:656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Phillips O.R., Nuechterlein K.H., Clark K.A., Hamilton L.S., Asarnow R.F., Hageman N.S., Toga A.W., Narr K.L. Fiber tractography reveals disruption of temporal lobe white matter tracts in schizophrenia. Schizophr. Res. 2009;107(1):30–38. doi: 10.1016/j.schres.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K.M., Upton C.H., Nimgaonkar V.L., Keshavan M.S. Differential susceptibility of white matter tracts to inflammatory mediators in schizophrenia: an integrated DTI study. Schizophr. Res. 2015;161(1):119–125. doi: 10.1016/j.schres.2014.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., Oertel-Knoechel V., DeMartino F., van de Ven V., Formisano E., Roebroeck A., Rami A., Schoenmeyer R., Haenschel C., Hendler T., Maurer K., Vogeley K., Linden D.E. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2009;174:9–16. doi: 10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Samartzis L., Dima D., Fusar-Poli P., Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J. Neuroimaging. 2014;24(2):101–110. doi: 10.1111/j.1552-6569.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- Seok J.H., Park H.J., Chun J.W., Lee S.K., Cho H.S., Kwon J.S., Kim J.J. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res. 2007;156:93–104. doi: 10.1016/j.pscychresns.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- Shergill S.S., Kanaan R.A., Chitnis X.A., O'Daly O., Jones D.K., Frangou S., Williams S.C., Howard R.J., Barker G.J., Murray R.M., McGuire P. A diffusion tensor imaging study of fasciculi in schizophrenia. Am. J. Psychiatry. 2007;164:467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Sun H., Lui S., Yao L., Deng W., Xiao Y., Zhang W., Huang X., Hu J., Bi F., Li T., Sweeney J.A., Gong Q. Two patterns of white matter abnormalities in medication-naive patients with first-episode schizophrenia revealed by diffusion tensor imaging and cluster analysis. JAMA Psychiatry. 2015;72(7):678–686. doi: 10.1001/jamapsychiatry.2015.0505. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Sakurai T., Davis K.L., Buxbaum J.D. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog. Neurobiol. 2011;93(1):13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase K., Tamagaki C., Okugawa G., Nobuhara K., Minami T., Sugimoto T., Sawada S., Kinoshita T. Reduced white matter volume of the caudate nucleus in patients with schizophrenia. Neuropsychobiology. 2004;50:296–300. doi: 10.1159/000080956. [DOI] [PubMed] [Google Scholar]
- Travis K.E., Golden N.H., Feldman H.M., Solomon M., Nguyen J., Mezer A. Abnormal white matter properties in adolescent girls with anorexia nervosa. Neuroimage Clin. 2015;9:648–659. doi: 10.1016/j.nicl.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beek L., Ghesquière P., Lagae L., De Smedt B. Left fronto-parietal white matter correlates with individual differences in children's ability to solve additions and multiplications: a tractography study. NeuroImage. 2014;90:117–127. doi: 10.1016/j.neuroimage.2013.12.030. (Apr 15) [DOI] [PubMed] [Google Scholar]
- Vernooij M.W., Smits M., Wielopolski P.A., Houston G.C., Krestin G.P., van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. NeuroImage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. (15) [DOI] [PubMed] [Google Scholar]
- Voineskos A.N., Lobaugh N.J., Bouix S., Rajji T.K., Miranda D., Kennedy J.L., Mulsant B.H., Pollock B.G., Shenton M.E. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133(Pt 5):1494–1504. doi: 10.1093/brain/awq040. (Epub 2010 Mar 17) [DOI] [PMC free article] [PubMed] [Google Scholar]