ABSTRACT
Streptococcus gallolyticus is a commensal bacterium responsible for infectious endocarditis in the elderly, which has frequently been associated with colonic carcinoma. Whether this species is a cause or a consequence of colorectal cancer remains unknown. We recently demonstrated that S. gallolyticus Pil3 pilus is required for adhesion to colonic mucus and for colonization of mouse distal colon. We show here that Pil3 pilus binds equally well to human colonic mucins derived from HT29-MTX cells and to human stomach mucins from healthy donors. In addition, we have found that Pil3 also binds to human fibrinogen, which expands the repertoire of Pil3 host ligands.
KEYWORDS: colon, colonization, fibrinogen, MUC2, MUC5AC, mucins, Pil3 pilus, S. bovis, S. gallolyticus
Addendum
Streptococccus gallolyticus subsp. gallolyticus, formerly known as S. bovis biotype I,1,2 is one of the few opportunistic pathogens which has been unambiguously linked to colorectal cancer (CRC).3-10 This bacterium is responsible for a growing number of infective endocarditis (IE) and septicemia cases in elderly and immunocompromised individuals.11-16 Firstly isolated from Koala feces and named for its capacity to degrade host tannins,17 S. gallolyticus is a common inhabitant of the rumen flora of herbivores. This species has also been found in the gastrointestinal (GI) tract of a wide spectrum of animals,17-21 including marsupials, mammals and birds. In humans, S. gallolyticus is detected at a low carriage rate (2.5 to 15%) from stool samples.3-5 In 1977, Klein et al reported that patients displaying colonic neoplasia had an increased level (up to 5-fold) of S. gallolyticus in fecal samples as compared to similar samples from healthy individuals.3 In 2010, Abdulamir et al. provided the first molecular evidence for a specific enrichment of S. gallolyticus in CRC-mucosal tissues as compared to healthy tissue.5 Nevertheless, it remains unclear whether S. gallolyticus is taking advantage of the tumor environment to outcompete other microbiota species or if it can promote colonic transformation through other unknown mechanisms.22,23
Bacterial colonization of host tissues is often considered to be a crucial step for the establishment of a successful infection. Adhesion to host cells is mediated by surface-exposed adhesins, which can be found in Gram-positive bacteria at the tip of long covalent polymers of covalently bound proteins called pili.24 S. gallolyticus reference strain UCN34,25 recovered from the blood of a 70 year-old patient with IE and subsequently diagnosed for CRC, possesses 3 pilus loci: pil1, pil2 and pil3. Among these, pil1 and pil3 are the most conserved loci in clinical isolates.26 We previously showed that Pil1 pilus allows S. gallolyticus to bind to collagen type I, promoting IE in a rat model of experimental endocarditis.27 These surface-exposed structures are highly immunogenic and have been proposed as vaccine candidates in pathogenic streptococci.28 Interestingly, Pil1 is expressed heterogeneously at the single bacterium level by a novel regulatory mechanism in the promoter region, combining phase variation in the leader peptide and transcriptional attenuation.29 Some evidence suggests that heterogeneity in Pil1 expression confers a fitness advantage to the bacterial population as it alleviates selective pressure from the host immune system.29
How does S. gallolyticus interact with the human colon was unexplored until our recent work.26 We characterized the functional role of Pil3 pilus in binding to colonic mucins isolated from HT29-MTX cells. Pil3 is composed of 2 subunits, the putative adhesin Pil3A and the backbone pilin Pil3B, forming a surface-exposed appendage. As for Pil1, Pil3 was found to be expressed heterogeneously at the single cell level in UCN34 as well as in other clinical isolates.26,29
Pil3A was shown to mediate S. gallolyticus binding to HT29-MTX cells derived mucus. Interestingly, no significant binding was observed when using bovine maxillary mucins (data not shown), which suggests specific traits in host mucins serving as Pil3 ligand. We previously developed a mouse model of gut colonization by S. gallolyticus requiring pre-treatment of C57BL/6 mice with a cocktail of antibiotics to reduce endogenous microbiota and repeated oral inoculation with the strain UCN34.26 This protocol resulted in significant colonization of mice intestinal tissues (small intestine, cecum, colon). The UCN34Δpil3 mutant was significantly impaired (2-log reduction) in distal colon colonization as compared to a Pil3-overexpressing variant (Pil3+). Examination by confocal microscopy of mouse intestinal tissues showed that S. gallolyticus localized within the colonic mucus layer. These in vivo experiments revealed the importance of Pil3 pilus for the colonization of mouse distal colon.26
Role of Pil3 pilus in the interaction with human mucins and extracellular matrix (ECM) components
We previously showed that the adhesin Pil3A binds to MUC5AC mucin isolated from HT29-MTX cells mucus. HT29-MTX is a human colonic cell line of particular relevance, because in contrast to other colonic cell lines such as HT29, Caco-2 or T84, it secretes a thick mucus layer upon differentiation for 15 to 20 days.30,31 Among all glycoproteins, mucins, which are major components of the mucus gel covering and protecting the intestinal epithelial layer, are frequently altered in CRC. Indeed, specific mucins (e.g. MUC5AC) can be overexpressed and differentially glycosylated in CRC, and HT29-MTX is the cell line that better mimics this tumor characteristic. In healthy conditions, MUC5AC mucin is only expressed in the stomach and not in the colon, whereas MUC2 is the predominant mucin in the colon. Interestingly, aberrant and mislocalized expression of MUC5AC mucin in colonic adenomas and carcinomas has been reported,32-34 as well as modification of mucins glycosylation patterns during colonic carcinogenesis.35-39
To investigate S. gallolyticus ligand preferences, we tested the binding capacities of UCN34 WT, Pil3+ and Δpil3 strains to human mucins isolated from colon (MUC2) and stomach (MUC5AC) of healthy donors. As shown in Figure 1A, Pil3 pilus was equally able to interact with MUC2 and MUC5AC. Binding of Pil3+ strain to purified mucins was significantly increased when compared to WT and Δpil3 strains. We hypothesized that binding of Pil3 pilus to MUC2 mucin may be important for S. gallolyticus commensal colonization, whereas binding to MUC5AC mucin could provide a colonization advantage over other microbiota species in the context of colonic tumor environment. This might contribute to the higher prevalence of S. gallolyticus in patient colorectal tumor tissues.5
Figure 1.
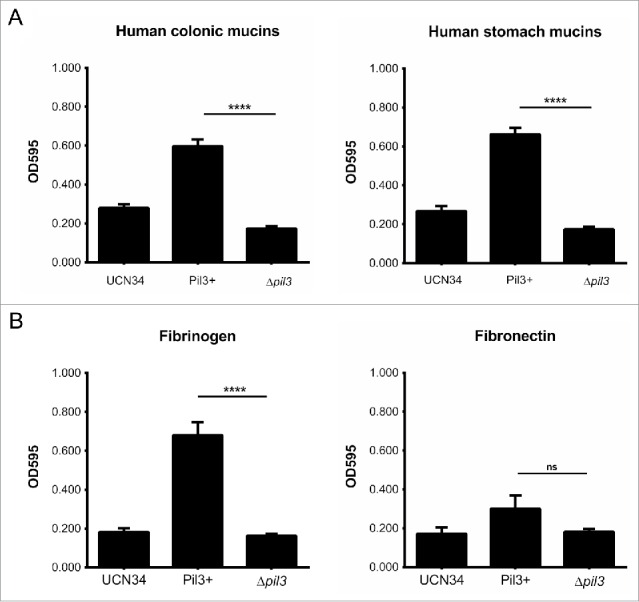
Pil3 pilus interaction with mucins and ECM components. Adhesion of UCN34, Pil3+ and Δpil3 strains to (A) human purified mucins from healthy colon, stomach, (B) fibrinogen and fibronectin. Values indicate the mean of 3 independent experiments assayed in triplicate. Statistical analysis was performed using a 2-way ANOVA test (****p < 0.0001; ns, not significant).
Next, we tested the capacity of UCN34, Pil3+ and Δpil3 strains to interact with other ECM components, such as human fibrinogen and fibronectin. As shown in Figure 1B, Pil3+ strain adhered more efficiently to fibrinogen compared to UCN34 and Δpil3. No significant difference was found between the strains in fibronectin binding. Hence, Pil3 pilus contributes to the binding of S. gallolyticus to fibrinogen, but not to fibronectin. Binding to fibrinogen contributes to the development of endovascular infections through multiple events: increased biofilm formation, adherence to endothelial cells and most importantly binding to the surface of human platelets. Since cardiac valve endothelial lesion activates platelet recruitment, this allows the bacteria attached to the platelets via fibrinogen cross-bridges, to colonize these injured sites then contributing to IE development.40-44 Thus, Pil3 pilus of S. gallolyticus might also be important in a later stage of infection, such as survival in the blood or in adhesion to platelets through fibrinogen-mediated cross-bridging, thereby contributing in concert with Pil1 pilus to the development of IE.
Pil3A binds to mucins and to fibrinogen through its amino-terminal part
We previously showed that heterologous expression of Pil3A in Lactococcus lactis strain NZ9000 conferred to the recombinant bacteria an enhanced capacity for binding to colonic mucins, as compared to the control L. lactis strain harboring the empty vector. Pil3A is a large LPXTG protein of 1664 amino acids containing a putative mucus- binding domain (position 1154 to 1238). In order to identify the binding domains of Pil3A, the amino-terminal region (from AA 43 to 847) and the C-terminal region (from AA 848 to 1627) were cloned in the shuttle vector pAT28-covSP+SPA containing a promoter, a signal peptide, a spacer and an LPXTG motif allowing expression, secretion and cell wall anchoring of each domain separately (Pil3A-N or Pil3-C). Recombinant plasmids were introduced by electroporation into L. lactis strain NZ9000. We first checked the expression of the Pil3A-N and Pil3A-C at the surface of L. lactis by flow cytometry using specific antibodies raised against the N- or the C-terminal part of Pil3A. As shown in Figure 2A, each fragment of Pil3A is expressed properly at the surface of L. lactis and recognized by the cognate antibody. We then tested the capacity of these recombinant bacteria to bind to HT29-MTX mucus and to fibrinogen. As shown in Figure 2B, only the N-terminal domain of Pil3A confers enhanced binding to mucus and to fibrinogen as compared to the control L. lactis strain harboring the empty vector. These results question the significance of the mucin-binding domain identified in silico located in the C-terminal part of Pil3A.
Figure 2.
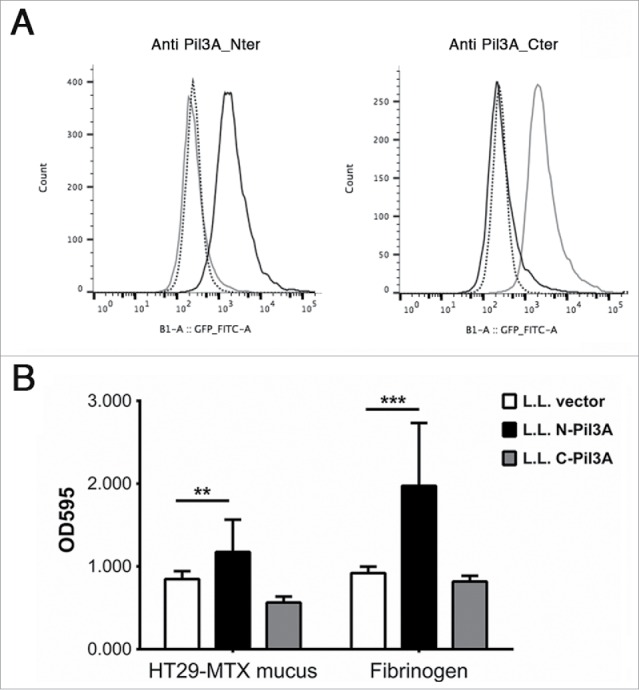
Adhesion of recombinant L. lactis expressing Pil3A to mucus and to fibrinogen. (A) Surface display of Pil3A-N (black line) or Pil3A-C (gray line) regions in recombinant L. lactis was determined by flow-cytometry using specific polyclonal antibodies directed against the amino- (Pil3A_Nter) or carboxyl- (Pil3A_Cter) part of Pil3A. The negative control (L. lactis carrying the empty vector) is shown in dotted line. In this representative experiment, 10,000 bacteria were analyzed for each strain. (B) Recombinant L. lactis strains expressing only the first 800 aa of Pil3A (N-Pil3A) displays higher binding to human HT29-MTX mucus and to fibrinogen as compared to the respective controls harboring the empty vector. Values indicate the mean of 3 independent experiments assayed in triplicate. Statistical analysis was performed using a 2-way ANOVA test (** p < 0.01; ***p < 0.001).
How does S. gallolyticus cause disease?
S. gallolyticus is an opportunistic pathogen responsible for increased number of septicemia and/or IE in the elderly and immunocompromised individuals. Strong evidences indicate a link between the presence of S. gallolyticus and the occurrence of CRC in humans. Whether this bacterium is the cause or the consequence of CRC is not known. Although highly prevalent in the rumen of herbivores, S. gallolyticus is considered as a weak colonizer of the human GI characterized by a low fecal carriage ranging around 5–10%.3 Recent experimental data indicate that metabolic alterations associated to CRC may favor S. gallolyticus outgrowth in this environment.46 In addition, physiological alterations occurring during CRC, such as the expression of mislocalized MUC5AC mucin32-34 and displaced epithelium with exposed collagen IV,47 could favor adhesion of S. gallolyticus through Pil3 and Pil1 pili respectively, and thereby explain the increased numbers of S. gallolyticus bacteria at these particular sites. CRC conditions may also increase the ability of S. gallolyticus to translocate through the epithelial barrier and reach the lamina propria. Mimicking this route of infection, Boleij et al have shown experimentally that S. gallolyticus is able to cross the intestinal barrier using a paracellular route.48 Moreover, the same study showed that S. gallolyticus remains quite invisible or silent to the host immune system (no IL8 and IL1-β-dependent pro-inflammatory responses), which in turn increases the chances of the bacteria to reach the bloodstream – Then, heterogeneity in Pil1 and Pil3 expression probably attenuates exposure and pressure from the immune system. Once in the bloodstream, S. gallolyticus expressing Pil3 probably binds to fibrinogen highly enriched in human plasma, which in turn will contribute to host-bacteria interactions in unknown manner. Pil1-expressing S. gallolyticus cells are then able to colonize collagen I rich surfaces, such as damaged heart valves and cause IE.27 This physiopathological scenario is summarized in Figure 3.
Figure 3.
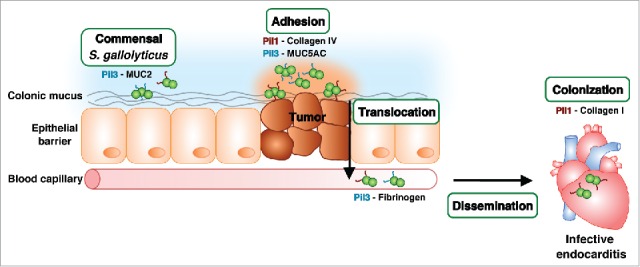
Roles of S. gallolyticus Pil1 and Pil3 pili during an invasive infection. S. gallolyticus enters the human intestine via an oral route, is outcompeted by resident microbiota and exits through fecal excretion. Nevertheless, colonic neoplasia conditions provide a potential colonization site due to physiological modifications with altered nutrient availability and exposure of collagen IV fibers. The subsequent translocation across the epithelial barrier by a paracellular mechanism contributes to bacterial dissemination in the bloodstream, which allows the bacteria to eventually reach the heart and colonize damaged heart valves with exposed collagen I.
The contribution of S. gallolyticus to CRC certainly needs further experimental exploration using relevant animal models. Identification and characterization of S. gallolyticus specific factors contributing to pathogenesis will definitely help in the development of new strategies for CRC diagnosis, treatment and prevention.
Abbreviations
- AA
Amino acids
- CRC
Colorectal cancer
- ECM
Extracellular matrix
- FG
Fibrinogen
- GI
Gastrointestinal
- IE
Infective endocarditis
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Antonin Weckel and Agnes Fouet for the kind gift of plasmid pAT_AW1.
References
- [1].Schlegel L, Grimont F, Ageron E, Grimont PA, Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol 2003; 53:631-45; PMID:12807180; http://dx.doi.org/ 10.1099/ijs.0.02361-0 [DOI] [PubMed] [Google Scholar]
- [2].Poyart C, Quesne G, Trieu-Cuot P. Taxonomic dissection of the Streptococcus bovis group by analysis of manganese-dependent superoxide dismutase gene (sodA) sequences: reclassification of “Streptococcus infantarius subsp. coli” as Streptococcus lutetiensis sp. nov. and of Streptococcus bovis biotype 11.2 as Streptococcus pasteurianus sp. nov. Int J Syst Evol Microbiol 2002; 52:1247-55; PMID:12148636 [DOI] [PubMed] [Google Scholar]
- [3].Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med 1977; 297:800-2; PMID:408687; http://dx.doi.org/ 10.1056/NEJM197710132971503 [DOI] [PubMed] [Google Scholar]
- [4].Boleij A, van Gelder MM, Swinkels DW, Tjalsma H. Clinical Importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and meta-analysis. Clin Infect Dis 2011; 53:870-8; PMID:21960713; http://dx.doi.org/ 10.1093/cid/cir609 [DOI] [PubMed] [Google Scholar]
- [5].Abdulamir AS, Hafidh RR, Bakar FA. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer 2010; 9:249; PMID:20846456; http://dx.doi.org/ 10.1186/1476-4598-9-249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Klein RS, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Streptococcus bovis septicemia and carcinoma of the colon. Ann Intern Med 1979; 91:560-2; PMID:484953; http://dx.doi.org/ 10.7326/0003-4819-91-4-560 [DOI] [PubMed] [Google Scholar]
- [7].Boleij A, Schaeps RMM, Tjalsma H. Association between Streptococcus bovis and colon cancer. J Clin Microbiol 2009; 47:516; PMID:19189926; http://dx.doi.org/ 10.1128/JCM.01755-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Corredoira J, Alonso MP, Coira A, Casariego E, Arias C, Alonso D, Pita J, Rodriguez A, López MJ, Varela J. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis 2008; 27:285-91; PMID:18183440; http://dx.doi.org/ 10.1007/s10096-007-0441-y [DOI] [PubMed] [Google Scholar]
- [9].Ellmerich S, Schöller M, Duranton B, Gossé F, Galluser M, Klein JP, Raul F. Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis 2000; 21:753-6; PMID:10753212; http://dx.doi.org/ 10.1093/carcin/21.4.753 [DOI] [PubMed] [Google Scholar]
- [10].Gupta A, Madani R, Mukhtar H. Streptococcus bovis endocarditis, a silent sign for colonic tumour. Colorectal Dis 2010; 12:164-71; PMID:19226366; http://dx.doi.org/ 10.1111/j.1463-1318.2009.01814.x [DOI] [PubMed] [Google Scholar]
- [11].Facklam RR. Recognition of group D streptococcal species of human origin by biochemical and physiological tests. Appl 1972; 23:1131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parker MT, Ball LC. Streptococci and aerococci associated with systemic infection in man. J Med Microbiol 1976; 9:275-302; PMID:785000; http://dx.doi.org/ 10.1099/00222615-9-3-275 [DOI] [PubMed] [Google Scholar]
- [13].Watanakunakorn C. Streptococcus bovis endocarditis. Am J Med 1974; 56:256-60; PMID:4812081; http://dx.doi.org/ 10.1016/0002-9343(74)90605-6 [DOI] [PubMed] [Google Scholar]
- [14].Ruoff KL, Miller SI, Garner CV, Ferraro MJ, Calderwood SB. Bacteremia with Streptococcus bovis and Streptococcus salivarius: clinical correlates of more accurate identification of isolates. J Clin Microbiol 1989; 27:305-8; PMID:2915024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Duval X, Papastamopoulos V, Longuet P, Benoit C, Perronne C, Leport C, Vildé JL. Definite streptococcus bovis endocarditis: characteristics in 20 patients. Clin Microbiol Infect 2001; 7:3-10; PMID:11284936; http://dx.doi.org/ 10.1046/j.1469-0691.2001.00190.x [DOI] [PubMed] [Google Scholar]
- [16].Herrero IA, Rouse MS, Piper KE, Alyaseen SA, Steckelberg JM, Patel R. Reevaluation of Streptococcus bovis endocarditis cases from 1975 to 1985 by 16S ribosomal DNA sequence analysis. J Clin Microbiol 2002; 40:3848-50; PMID:12354897; http://dx.doi.org/ 10.1128/JCM.40.10.3848-3850.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Osawa R. Formation of a clear zone on tannin-treated brain heart infusion agar by a Streptococcus sp. isolated from feces of koalas. Appl Environ Microbiol 1990; 56:829-31; PMID:2180375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sasaki E, Shimada T, Osawa R, Nishitani Y, Spring S, Lang E. Isolation of tannin-degrading bacteria isolated from feces of the Japanese large wood mouse, Apodemus speciosus, feeding on tannin-rich acorns. Syst Appl Microbiol 2005; 28:358-65; PMID:15997709; http://dx.doi.org/ 10.1016/j.syapm.2005.01.005 [DOI] [PubMed] [Google Scholar]
- [19].Devriese LA, Vandamme P, Pot B, Vanrobaeys M, Kersters K, Haesebrouck F. Differentiation between Streptococcus gallolyticus strains of human clinical and veterinary origins and Streptococcus bovis strains from the intestinal tracts of ruminants. J Clin Microbiol 1998; 36:3520-3; PMID:9817865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Devriese LA, Hommez J, Laevens H, Pot B, Vandamme P, Haesebrouck F. Identification of aesculin-hydrolyzing streptococci, lactococci, aerococci and enterococci from subclinical intramammary infections in dairy cows. Vet Microbiol 1999; 70:87-94; PMID:10591500; http://dx.doi.org/ 10.1016/S0378-1135(99)00124-8 [DOI] [PubMed] [Google Scholar]
- [21].De Herdt P, Haesebrouck F, Devriese LA, Ducatelle R. Prevalence of Streptococcus bovis in racing pigeons. Vet Q 1994; 16:71-4; PMID:7985358; http://dx.doi.org/ 10.1080/01652176.1994.9694421 [DOI] [PubMed] [Google Scholar]
- [22].Hausen H zur. Streptococcus bovis: causal or incidental involvement in cancer of the colon? Int J Cancer 2006; 119:xi-xii; PMID:16947772; http://dx.doi.org/ 10.1002/ijc.22314 [DOI] [PubMed] [Google Scholar]
- [23].Hensler ME. Streptococcus gallolyticus, infective endocarditis, and colon carcinoma: new light on an intriguing coincidence. J Infect Dis 2011; 203:1040-2; PMID:21450993; http://dx.doi.org/ 10.1093/infdis/jiq170 [DOI] [PubMed] [Google Scholar]
- [24].Danne C, Dramsi S. Pili of gram-positive bacteria: roles in host colonization. Res Microbiol 2012; 163:645-58; PMID:23116627; http://dx.doi.org/ 10.1016/j.resmic.2012.10.012 [DOI] [PubMed] [Google Scholar]
- [25].Rusniok C, Couvé E, Da Cunha V, El Gana R, Zidane N, Bouchier C, Poyart C, Leclercq R, Trieu-Cuot P, Glaser P. Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J Bacteriol 2010; 192:2266-76; PMID:20139183; http://dx.doi.org/ 10.1128/JB.01659-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Martins M, Aymeric L, du Merle L, Danne C, Robbe-Masselot C, Trieu-Cuot P, Sansonetti P, Dramsi S. Streptococcus gallolyticus Pil3 Pilus is required for adhesion to colonic mucus and for colonization of mouse distal colon. J Infect Dis 2015; 212:1646-55; PMID:26014801; http://dx.doi.org/ 10.1093/infdis/jiv307 [DOI] [PubMed] [Google Scholar]
- [27].Danne C, Entenza JMM, Mallet A, Briandet R, Débarbouillé M, Nato F, Glaser P, Jouvion G, Moreillon P, Trieu-Cuot P, et al.. Molecular characterization of a Streptococcus gallolyticus genomic island encoding a pilus involved in endocarditis. J Infect Dis 2011; 204:1960-70; PMID:22043018; http://dx.doi.org/ 10.1093/infdis/jir666 [DOI] [PubMed] [Google Scholar]
- [28].Pansegrau W, Bagnoli F. Pilus Assembly in Gram-Positive Bacteria. Curr Top Microbiol Immunol 2016; DOI 10.1007/82_2015_5016; PMID:26847355 [DOI] [PubMed] [Google Scholar]
- [29].Danne C, Dubrac S, Trieu-Cuot P, Dramsi S. Single cell stochastic regulation of pilus phase variation by an attenuation-like mechanism. PLoS Pathog 2014; 10(1):e1003860; PMID:24453966; http://dx.doi.org/ 10.1371/journal.ppat.1003860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lesuffleur T, Violette S, Vasile-Pandrea I, Dussaulx E, Barbat A, Muleris M, Zweibaum A. Resistance to high concentrations of methotrexate and 5-fluorouracil of differentiated HT-29 colon-cancer cells is restricted to cells of enterocytic phenotype. Int J Cancer 1998; 76:383-92; PMID:9579576; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19980504)76:3%3c383::AID-IJC16%3e3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- [31].Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Research 1990; 50:6334-6343; PMID:2205381 [PubMed] [Google Scholar]
- [32].Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer 1999; 80:210-8; PMID:9935202; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19990118)80:2%3c210::AID-IJC9%3e3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- [33].Sylvester PA, Myerscough N, Warren BF, Carlstedt I, Corfield AP, Durdey P, Thomas MG. Differential expression of the chromosome 11 mucin genes in colorectal cancer. J Pathol 2001; 195:327-35; PMID:11673830; http://dx.doi.org/ 10.1002/path.951 [DOI] [PubMed] [Google Scholar]
- [34].Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev 2004; 23:77-99; PMID:15000151; http://dx.doi.org/ 10.1023/A:1025815113599 [DOI] [PubMed] [Google Scholar]
- [35].Devine PL, McKenzie IF. Mucins: structure, function, and associations with malignancy. Bioessays 1992; 14:619-25; PMID:1365918; http://dx.doi.org/ 10.1002/bies.950140909 [DOI] [PubMed] [Google Scholar]
- [36].Itzkowitz SH, Bloom EJ, Lau TS, Kim YS. Mucin associated Tn and sialosyl-Tn antigen expression in colorectal polyps. Gut 1992; 33:518-23; PMID:1582597; http://dx.doi.org/ 10.1136/gut.33.4.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mann B, Klussmann E, Vandamme-Feldhaus V, Iwersen M, Hanski ML, Riecken EO, Buhr HJ, Schauer R, Kim YS, Hanski C. Low O-acetylation of sialyl-Le(x) contributes to its overexpression in colon carcinoma metastases. Int J Cancer 1997; 72:258-64; PMID:9219830; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19970717)72:2%3c258::AID-IJC10%3e3.0.CO;2-C [DOI] [PubMed] [Google Scholar]
- [38].Jenab M, Chen J, Thompson LU. Sialomucin production in aberrant crypt foci relates to degree of dysplasia and rate of cell proliferation. Cancer Lett 2001; 165:19-25; PMID:11248414; http://dx.doi.org/ 10.1016/S0304-3835(00)00706-0 [DOI] [PubMed] [Google Scholar]
- [39].Mesquita P, Peixoto AJJ, Seruca R, Hanski C, Almeida R, Silva F, Reis C, David L. Role of site-specific promoter hypomethylation in aberrant MUC2 mucin expression in mucinous gastric carcinomas. Cancer Lett 2003; 189:129-36; PMID:12490305; http://dx.doi.org/ 10.1016/S0304-3835(02)00549-9 [DOI] [PubMed] [Google Scholar]
- [40].Ford I, Douglas CW. The role of platelets in infective endocarditis. Platelets 1997; 8:285-94; PMID:16793661; http://dx.doi.org/ 10.1080/09537109777159 [DOI] [PubMed] [Google Scholar]
- [41].Moreillon P, Que Y-AA. Infective endocarditis. Lancet 2004; 363:139-49; PMID:14726169; http://dx.doi.org/ 10.1016/S0140-6736(03)15266-X [DOI] [PubMed] [Google Scholar]
- [42].Seo HS, Xiong YQ, Sullam PM. Role of serine-rich surface glycoprotein Srr1 of Streptococcus agalactiae in the pathogenesis of infective endocarditis. PLoS One 2013; 8(5):e64204; PMID:23717569; http://dx.doi.org/ 10.1371/journal.pone.0064204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Seo HS, Xiong YQ, Mitchell J, Seepersaud R, Bayer AS, Sullam PM. Bacteriophage lysin mediates the binding of streptococcus mitis to human platelets through interaction with fibrinogen. PLoS Pathog 2010; 6(8):e1001047; PMID:20714354; http://dx.doi.org/ 10.1371/journal.ppat.1001047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bedran TB, Azelmat J, Spolidorio DP, Grenier D. Fibrinogen-induced streptococcus mutans biofilm formation and adherence to endothelial cells. Biomed Res Int 2013; 2013:431465; PMID:24222906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog 2011; 10:9; PMID:21483655; http://dx.doi.org/ 10.4103/1477-3163.78279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Boleij A, Dutilh BE, Kortman GA, Roelofs R, Laarakkers CM, Engelke UF, Tjalsma H. Bacterial responses to a simulated colon tumor microenvironment. Mol Cell Proteomics 2012; 11:851-62; PMID:22713208; http://dx.doi.org/ 10.1074/mcp.M112.019315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Galbavý S, Lukác L, Porubský J, Cerná M, Labuda M, Kmet'ová J, Papincák J, Durdík S, Jakubovský J. Collagen type IV in epithelial tumours of colon. Acta Histochem 2002; 104:331-4; PMID:12553696; http://dx.doi.org/ 10.1078/0065-1281-00680 [DOI] [PubMed] [Google Scholar]
- [48].Boleij A, Muytjens C, Bukhari S, Cayet N, Glaser P, Hermans P, Swinkels D, Bolhuis A, Tjalsma H. Novel clues on the specific association of Streptococcus gallolyticus subsp gallolyticus with colorectal cancer. The Journal of infectious diseases 2011; 203:1101-9; PMID:21451000; http://dx.doi.org/ 10.1093/infdis/jiq169 [DOI] [PubMed] [Google Scholar]


