ABSTRACT
It has been widely demonstrated that tolerance against gut microbiota is compartmentalized to mucosal sites where microbes mostly reside. How the commensal bacteria are excluded from the entrance into the blood stream via intestinal capillaries that are located beneath the gut epithelium was not clear. We recently described the existence of a new anatomical structure, the ‘gut vascular barrier’ (GVB), both in murine and human intestines that plays a fundamental role in avoiding indiscriminate trafficking of bacteria from the gut into the blood circulation. The vascular barrier integrity could be altered by Salmonella typhimurium, a pathogen capable of systemic dissemination, through the modulation of the Wnt/β-catenin signaling pathway. Here we have analyzed the differences in gut endothelial gene expression profiles during Salmonella infection and have identified some interesting characteristics of endothelial to mesenchymal transition. These findings add new insights in the gut-liver axis.
KEYWORDS: blood-brain barrier, celiac disease, gut-vascular barrier, intestinal endothelium, RNA-sequencing, Salmonella typhimurium
The intestine is continuously exposed to a huge amount of foreign antigens, mostly food proteins and commensal microorganisms. These harmless antigens induce oral tolerance, which is however different when considering gut bacteria or food antigens. Tolerance to food proteins affects both local and systemic immune responses1 indeed fed soluble antigens can gain access to the lymphatics to reach the mesenteric lymph nodes (mLN)2 and the blood stream to reach the liver3 where oral tolerance is established. By contrast, gut microbes are confined at mucosal sites by the mLNs that function like a firewall avoiding the spreading of intestinal microflora through the thoracic duct into the blood circulation.4-6 Therefore at steady state the systemic immune system is left ignorant to the microbiota. Only during intestinal pathology bacteria can reach the liver that acts as a firewall preventing the bacteria from spreading to other systemic sites.7
How the microbiota recirculates through the lymphatics has been widely studied, while how intestinal bacteria are excluded from the blood circulation was completely unexplored, even though it is known that intestinal capillaries are located just underneath the epithelial layer whereas the lymphatics are much deeper in the lamina propria.
In our recent work, we hypothesized the existence of a barrier at the endothelial level, the ‘gut vascular barrier’ (GVB),8 that similarly to the blood brain barrier (BBB) is able to control the type of antigens that can translocate into the blood stream and that is not permissive to bacterial penetration.
Drawing a parallelism between the well-known BBB and the newly identified GVB many similarities can be found. Both are able to avoid the indiscriminate movement of molecules, cells and bacteria from blood to the brain parenchyma in the BBB and from the intestinal lumen into the blood circulation in the case of the GVB. Besides these analogies, the BBB and GVB display many different characteristics due to the fact that these two endothelia should fulfill distinct functions. For instance, the BBB should avoid the uncontrolled movement of any substances from the blood into the brain parenchyma to protect the CNS from the constantly changing milieu of the blood stream.9 For this reason, the BBB is characterized by a continuous endothelial layer without fenestrations and with low pinocytic activity and by junctional complexes formed by tight (TJ) and adherens junction (AJ) proteins which limit paracellular and transcellular trafficking through the endothelial cell layer.10 To allow the delivery of nutrients in the parenchyma and the exclusion of toxin, the brain endothelium is equipped with specific transporters expressed in a polarized manner.11,12
By contrast, the intestinal endothelium is permeable to nutrients, due to the absorptive function of the gut. Indeed we have shown by intravital imaging that small molecules such as 4 KDalton (KDa) dextran could extravasate the intestinal capillaries, however, under steady-state, molecules bigger than 70 KDa could not pass the endothelium. This functional observation suggests that also in the gut, endothelial permeability is regulated and therefore that the endothelial layer has barrier properties.
To characterize the gut endothelial barrier we firstly analyzed the expression of TJ and AJ proteins. We found that, similar to the cerebral endothelium, intestinal endothelial cells (ECs) express the main components of TJs, namely occludin, JAM-A, claudin-12, as well as the cytoplasmic proteins ZO-1 and cingulin, together with VE-cadherin and junctional β-catenin, the main components of the AJs. To further characterize the endothelium of the GVB we purified ECs from small intestine of C57/BL6 mice using fluorescence-activated cell sorting (FACS) and we analyzed the transcriptional profile of CD45−CD31+CD105+LYVE1− blood endothelial cells. Performing a whole transcriptome profiling (Illumina RNA-seq) we have identified a set of genes with high expression in the purified population. As shown in Table 1, as expected, among all the genes expressed by the gut ECs there are many genes belonging to tight and adherens junction molecules, some of them also involved in signaling cascades, and to different classes of transporters (such as ATP-binding cassette (ABC) and sugar transporters). While the expression of some of these proteins, such as claudin (cldn)-12, occludin (ocln), cingulin (cgn), β-catenin (ctnnb1) has been validated by immunofluorescence, the expression of other genes needs to be further analyzed. The transcriptome analysis provides also new insights on how gut endothelium participates to the metabolism and transport of nutrients and other molecules of intestinal origin. Furthermore, the discovery of transporters expressed on the GVB could be important for therapeutic purposes.
Table 1.
Transporters and junctional proteins expressed in gut endothelial cells. Gene list of transporters and adherens and tight junction proteins with an expression value greater than 140 (normalized read count) in CD45−CD31+CD105+LYVE1− gut blood endothelial cell.
| ABC transporters | Sugar Tansporters | Tight Junctions | Adherens Junctions | |||
|---|---|---|---|---|---|---|
| ABCA1 | ATP5F1 | AQP3 | AMICA1 | F11R | ABI2 | GM609 |
| ABCA2 | ATP5L | M6PR | AOC1 | FZD5 | ACTN1 | ITGA6 |
| ABCA9 | ATP5O | SLC23A2 | ARHGAP17 | IGSF5 | APC | JUP |
| ABCB1A | ATP6AP1 | SLC2A1 | ARHGEF2 | INADL | CADM1 | KEAP1 |
| ABCB7 | ATP6V0A1 | SLC2A3 | ASH1L | LIN7C | CD200 | LMO7 |
| ABCB8 | ATP6V0A2 | SLC2A6 | CCND1 | MPP5 | CD226 | LYN |
| ABCC1 | ATP6V0B | SLC35A1 | CDK4 | MPP7 | CDH1 | MLLT4 |
| ABCC3 | ATP6V0C | SLC35A2 | CGN | MTDH | CTNNA1 | MYH9 |
| ABCD1 | ATP6V0D1 | SLC35A3 | CLDN12 | OCLN | CTNNB1 | NDRG1 |
| ABCD3 | ATP6V0E | SLC35A4 | CLDN15 | PARD3 | CTNND1 | PARD3 |
| ABCG1 | ATP6V0E2 | SLC35A5 | CLDN2 | PARD3B | DAG1 | PKP3 |
| ABCG2 | ATP6V1A | SLC45A4 | CLDN23 | PARD6A | DLG1 | PVR |
| ABCG8 | ATP6V1C1 | SLC5A1 | CLDN3 | RAP2B | DSC2 | RDX |
| ANXA5 | ATP6V1D | CLDN4 | RAP2C | DSP | SMAD7 | |
| ATP13A2 | ATP6V1E1 | CLDN7 | STRN | FLOT1 | SPTAN1 | |
| ATP1A1 | ATP6V1F | CRB3 | TGFBR1 | FLOT2 | ZYX | |
| ATP1A3 | ATP6V1G1 | CXADR | TJAP1 | |||
| ATP1B1 | ATP6V1H | CYTH1 | TJP2 | |||
| ATP1B3 | ATP7A | DDX58 | UBN1 | |||
| ATP2A2 | CFTR | DLG1 | VAPA | |||
| ATP2B1 | CPOX | ECT2 | VASP | |||
| ATP2C1 | IPO8 | EPCAM | WNK4 | |||
| ATP5A1 | PCYOX1 | ESAM | ||||
| ATP5B | TAP1 | |||||
| ATP5C1 | TAP2 | |||||
| ATP5D | TCIRG1 | |||||
| ATP5E | ||||||
It has been widely demonstrated that in the BBB the ECs are strictly associated with pericytes, neurons, microglia and astrocyte endfeet that altogether constitute the neurovascular unit (NVU), essential for central nervous system homeostasis.11,13,14 Similarly, we found that in both mouse and human small intestines, blood capillaries are associated to pericytes stained with desmin and enteric glial cells identified with GFAP marker, together forming the “gut-vascular unit” (GVU) (Fig. 1). The influence of these cells on the integrity of the GVB still remains to be elucidated. There is evidence that highlights the importance of enteric glial cells in intestinal homeostasis. For instance, mice lacking GFAP+ cells die for fulminant jejuno-ileitis characterized by massive destruction of the epithelial layer together with microvascular disturbances that result in bacterial spreading into the blood circulation.15,16 Whether gut glial cells may influence endothelial barrier function, resembling the astrocytes in the brain, is unknown. This possibility is supported by the finding that transplantation of enteric glia into the damaged spinal cord accelerates the repair of vasculature at the site of injury and the induction of barrier properties.17 Together with enteric glia also pericytes could be involved in barrier development. Indeed it has been demonstrated that pericytes are necessary for the stabilization of BBB vessels since mice that lack the platelet-derived growth factor-b (PDGF-b) signaling, required for pericyte recruitment in the brain, show ECs hyperplasia, increased vessel diameter and expression of “leaky” vascular barrier markers.18,19
Figure 1.
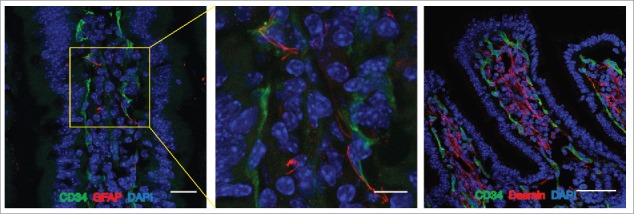
The Gut Vascular Unit. Confocal images of C57BL/6 mice intestine stained with CD34 (green) to identify blood endothelium and GFAP (red), marker of enteric glial cells (left panels; scale bars: 20 and 10 μm) or desmin (red) that marks perycites (scale bar 50 μm). In each section, nuclei were stained with DAPI (blue). Both cell types are in close proximity to CD34-positive endothelial cells, that together for the gut-vascular unit (GVU).
In the intestine the role of the vascular barrier is to exclude the entrance of bacteria or other unwanted molecules that have translocated from the gut lumen into the systemic circulation. In our previous work, we have demonstrated that the GVB is a functional barrier because, similarly to the BBB, Plasmalemma Vescicle Associated Protein-1 (PV1, encoded by the plvap gene), marker of “leaky” endothelial barriers, is expressed at low levels at steady-state.18,19 PV1 is a transmembrane glycoprotein that has been localized to caveolae and trans-endothelial channels of systemic fenestrated capillaries where it regulates vascular permeability.20–22 Furthermore, it has been recently shown that PV1 is not only expressed on blood vessels but also on lymphatic endothelial cells where it forms a physical filter that modulates the passage of soluble antigens and the transmigration of lymphocytes.23
Interestingly, we found that the expression of PV1 is up-regulated on blood capillaries upon infection with Salmonella typhimurium, an enteric pathogen known to disseminate systemically.24 The increase in PV1 expression on ECs correlates also with Salmonella dissemination to systemic sites, with higher serum levels of alanine transaminases (ALT), used as indicators of liver damage, and with an increased extravasation of large molecules out of the intestinal capillaries indicating endothelial leakiness.
A central role for the differentiation and maintenance of the BBB is played by the canonical Wnt/β-catenin signaling pathway.25–27 During development, the Wnt signaling pathway is activated and induces BBB maturation through the up-regulation of claudin-3 and the concomitant decrease of PV1 expression.28
Since PV1 modulation is regulated by Wnt/β-catenin pathway and we found that Salmonella is able to induce PV1 expression, we hypothesized that β-catenin activation may be also involved in the maintenance of the GVB and that Salmonella may be capable of interfering with this pathway. From in vitro studies, we found that the expression of Axin2, a known target gene of active β-catenin,29 is reduced upon infection with S. typhimurium indicating that it could regulate Wnt/β-catenin pathway. Moreover we found that Salmonella pathogenicity islands (Spi) 2 type three-secretion systems (TTSS) is involved in modulating β-catenin activation.
To definitively prove the role of the Wnt/β-catenin signaling pathway in the maintenance of the GVB integrity we used Cdh5(PAC)-CreERT2 mice30 crossed with β-cateninlox(ex3)/lox(ex3) mice.31 Crossing these mouse strains we obtained an inducible β-catenin gain-of-function mouse model in which, upon tamoxifen treatment, in VE-cadherin expressing endothelial cells exon 3 is excised from β-catenin gene and therefore it becomes constitutively active. Using this model we found that when β-catenin is constitutively activated in ECs, mice are more resistant to Salmonella infection since they show lower systemic dissemination. Consistently, in β-catenin gain-of-function mice upon Salmonella challenge neither PV1 expression is induced nor GVB permeability is increased.
With our in vitro and in vivo studies we have demonstrated that the Wnt/β-catenin signaling pathway plays an important role in GVB integrity maintenance. However it cannot be excluded that other molecular mediators could be involved in this process. To have a better insight into the signaling pathways deregulated during GVB injury, we orally challenged mice with S. typhimurium and after 6 hours we isolated and purified CD45−CD31+CD105+LYVE1− blood endothelial cell by FACS sorting and we analyzed their transcriptional profile using Illumina sequencing. The differential expression analysis comparing ECs from untreated and infected mice revealed a significant enrichment of differential expressed genes (DEGs) belonging to the Hedgehog (Hh) targets. To understand whether the Sonic Hedgehog (SHH) pathway is modulated after infection we performed a functional analysis using our DEGs list on Mouse Genome Informatics (MGI) Gene Ontology (GO). The results confirmed the involvement of SHH pathway (GO:0007224; corrected p-value: 9.8×10−03) since we found 17 modulated genes (13 up- and 4 down-regulated) (Fig. 2). The SHH pathway has been described as relevant for BBB establishment and in other adult tissues it is involved in vascular proliferation and tissue repair.32 The inactivation of SHH signaling in ECs leads to a decrease in the expression of TJ proteins and to a higher leakage of plasma proteins indicating an increase in vascular permeability.33 Interestingly, it has been shown that together with a barrier-promoting effect, activation of the SHH pathway favors the immune quiescence of BBB by reducing the expression of pro-inflammatory cytokines and leukocyte transmigration.33 In the gut, SHH signaling is necessary for the formation of villi during development and has homeostatic functions in the adult34,35 indeed Hedgehog ligands, produced mainly by the epithelium, act on mesenchymal cells (smooth muscle cells, myofibroblast-like cells and pericytes located in the lamina propria) and they function as signals to assess epithelial barrier integrity. Our new data suggest an involvement of the SHH pathway also in the maintenance of the GVB but further investigation is required.
Figure 2.
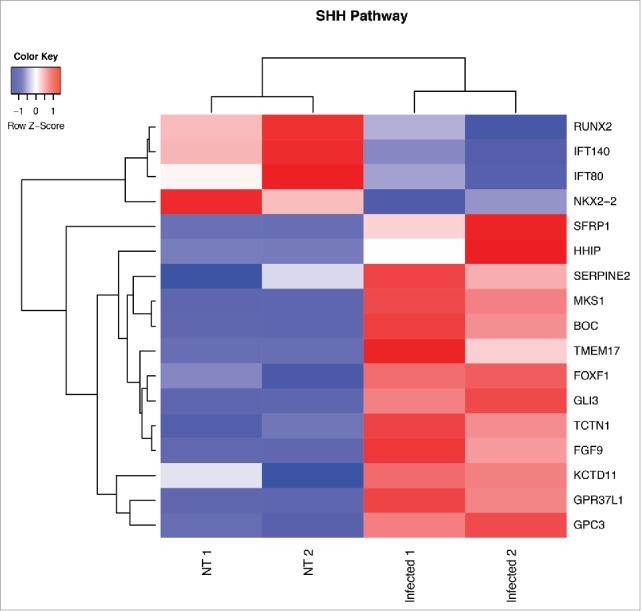
Heat map from hierarchical clustering representing DEGs belonging to SHH pathway. CD45−CD31+CD105+LYVE1− blood endothelial cell were purified by FACS sorting from small intestine of mice orally infected with S. typhimurium for 6 hours. Differential expression analysis was performed to investigate DEGs between infected and untreated mice. The analysis was carried out using DESeq2 R package. After read counts normalization across the samples, the expression of each gene was tested between the two conditions and to avoid false positive expression due to technical sequencing errors only high-quality transcript counts have been analyzed (filter transcript with 0 read count). DEGs with corrected p-value (FDR) less than 0.05 were considered as statistically different between the two conditions. The heat map shows DEGs that are annotated as SHH pathway genes according to Gene Ontology from MGI (GO:0007224). Red upregulated DEGs and blue down-regulated DEGs. NT=untreated.
To explore whether other biological processes correlated to Salmonella infection, we performed a Gene Set Enrichment Analysis (GSEA) using the Hallmark gene sets, a comprehensive list of genes covering the principal molecular functions. From this analysis we found that, unexpectedly, genes involved in the inflammatory immune response were highly downregulated in ECs after infection. Instead, genes belonging to the coagulation cascade, bile acid metabolism, angiogenesis and epithelial/endothelial to mesenchymal transition were positively correlating with the infection (Fig. 3). Among the significantly enriched gene sets related to Salmonella infection the pathway with the greatest normalized enrichment score (NES) is the endothelial to mesenchymal transition (Fig. 3). By analyzing the gene list used for the core enrichment of this pathway, we found that more than 50% (58 out of 103 genes) of those genes were also upregulated DEGs during differential analysis (Fig. 4).
Figure 3.
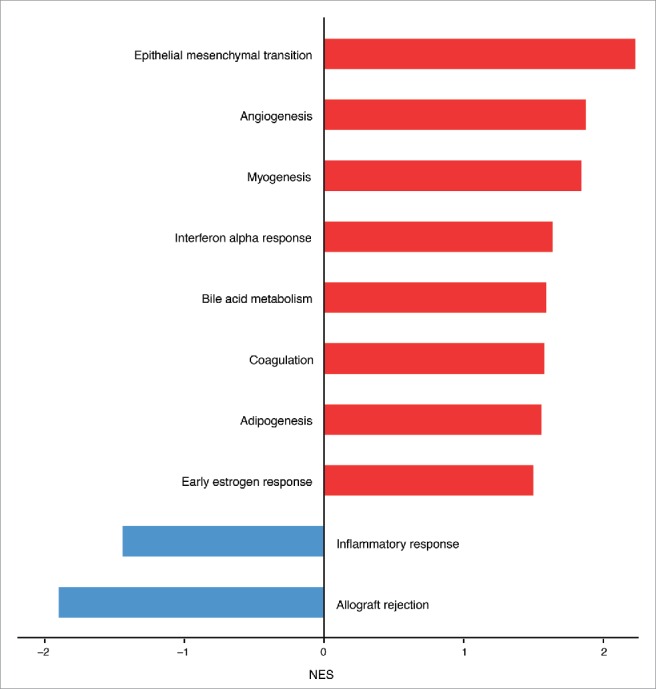
Hallmark molecular functions enriched in endothelial cells upon Salmonella infection. The bars plot shows the statistically significant GSEA Hallmark gene sets (FDR<0.05) that were found enriched in correlation (red bars) or inverse correlation (blue bars) with infected phenotype. The genes were ranked using the Signal2Noise metric (available in GSEA) by taking as reference the treated samples. Gene sets with a FDR lower than 0.05 have been considered statistically significant. The bars were ordered by normalized enrichment score (NES) that indicates the strength of the enrichment.
Figure 4.
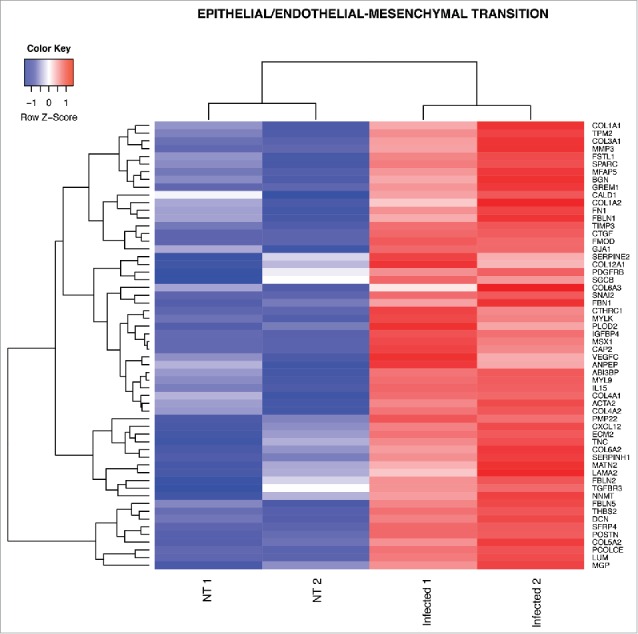
Heat map from hierarchical clustering representing DEGs in EndoMT pathway. The heat map shows DEGs that were used for core enrichment computation for EndoMT gene set in GSEA. All DEGs in the core enrichment were up-regulated (red tiles) upon infection.
Endothelial to mesenchymal transition (EndoMT) is a process in which the endothelium loses endothelial cell markers, such as CD31 and VE-cadherin, and acquires mesenchymal characteristics, such as α-smooth muscle actin and vimentin.36 EndoMT is an essential mechanism of development of different organs but it has also been described in different diseases such as cardiac fibrosis, metastatic cancer, retina diabetes and sepsis.36–38 Interestingly, meningeal pathogens, such as group B Streptococcus, are able to induce EndoMT in the BBB activating Snail1 which represses tight junction protein gene expression resulting in an increased bacterial penetration into the brain parenchyma.39 Similarly, we found that Snail2, another important transcription factor driving EndoMT,36 is up-regulated by Salmonella infection in purified gut endothelial cells together with genes characteristic of mesenchymal cells, such as α-smooth muscle actin (Acta2) and several genes encoding for extracellular matrix proteins characteristic of fibrosis including fibronectin (Fn1), tenascin-C (TnC) and type-I collagen (Col1a1, Col1a2) (Fig. 4). Thus, these data suggest that Salmonella could use the EndoMT process to dismantle the gut endothelial barrier and favor its systemic dissemination.
Finally, in our previous work we have evaluated whether GVB disruption could be involved in human pathology and in particular in celiac disease. We analyzed the GVB in intestinal biopsies isolated from patients following a gluten-free diet and displaying increased transaminases levels and we found that patients with high transaminases have a higher expression of PV1 compared with those with normal circulating liver enzymes. These data suggest that GVB modifications could be responsible for liver damage, which may be due to increased translocation of bacteria/bacterial products from the intestine. Additionally, we can exclude that liver damage can cause GVB disruption because if we treat mice with concanavalin A to induce strong liver inflammation we do not detect any significant upregulation of intestinal PV1.
Together with celiac disease, GVB functional impairment could be involved in other human diseases. For instance, it was shown that in inflammatory bowel disease (IBD) patients there is an increased intestinal vascular permeability that leads to tissue edema and damage.40 Interestingly, the alteration of the vascular permeability in IBD patients is not restricted to the intestinal vessels but affects also the vasculature of other organs such as the brain41 indicating that GVB impairment may also have systemic effects.
Conclusions
In our recent work we have demonstrated the existence of a GVB in the gut with morphological and functional characteristics similar to the BBB.
We found that, similarly to the BBB, Wnt/β-catenin signaling pathway is responsible for GVB maintenance inhibiting vascular permeability and bacterial invasion. However it is still unknown how S. typhimurium disrupts the GVB either via endothelial junction deregulation or through the formation of caveolae where PV1 is localized. Moreover, it remains to be established if Salmonella acts directly on endothelial cells or whether mediators released upon infection by the epithelium, pericytes or enteric glial cells that strictly interact with intestinal endothelium, could drive the effect on ECs. Together with Wnt/β-catenin signaling, other molecular pathways, that we have started to discover through the whole transcriptome analysis of isolated gut ECs, can be also involved in GVB establishment. We found that the activation of other signaling pathways such as SHH signaling as well as EndoMT might be exploited by Salmonella to dismantle the GVB and disseminate systemically.
Our published data on GVB impairment in celiac disease patients with elevated serum transaminases also indicate that gut endothelial barrier modifications may be responsible for liver damage in pathological conditions. Therefore, the identification of a GVB opens a new area of research on the gut/liver axis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by the European Research Council, the Italian Association for Cancer Research (AIRC) and the Italian Ministry of Health (Ricerca finalizzata). IS is the recipient of a FIRC fellowship.
References
- [1].Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 2003; 3:331-341; PMID:12669023; http://dx.doi.org/ 10.1038/nri1057 [DOI] [PubMed] [Google Scholar]
- [2].Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Förster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med 2006; 203:519-527; PMID:16533884; http://dx.doi.org/ 10.1084/jem.20052016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 2008; 29:464-475; PMID:18789731; http://dx.doi.org/ 10.1016/j.immuni.2008.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 2010; 10:159-169; PMID:20182457; http://dx.doi.org/ 10.1038/nri2710 [DOI] [PubMed] [Google Scholar]
- [5].Macpherson AJ, Smith K. Mesenteric lymph nodes at the center of immune anatomy. J Exp Med 2006; 203:497-500; PMID:16533891; http://dx.doi.org/ 10.1084/jem.20060227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science (80-. ) 2004; 303:1662-1665; http://dx.doi.org/ 10.1126/science.1091334 [DOI] [PubMed] [Google Scholar]
- [7].Balmer ML, Slack E, de Gottardi A, Lawson MA, Hapfelmeier S, Miele L, Grieco A, Van Vlierberghe H, Fahrner R, Patuto N, et al.. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 2014; 6:237ra66; PMID:24848256; http://dx.doi.org/ 10.1126/scitranslmed.3008618 [DOI] [PubMed] [Google Scholar]
- [8].Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, et al.. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015; 350:830-4; PMID:26564856; http://dx.doi.org/ 10.1126/science.aad0135 [DOI] [PubMed] [Google Scholar]
- [9].Paolinelli R, Corada M, Orsenigo F, Dejana E. The molecular basis of the blood brain barrier differentiation and maintenance. Is it still a mystery? Pharmacol Res 2011; 63:165-171; PMID:21167284; http://dx.doi.org/ 10.1016/j.phrs.2010.11.012 [DOI] [PubMed] [Google Scholar]
- [10].Liebner S, Czupalla CJ, Wolburg H. Current concepts of blood-brain barrier development. Int J Dev Biol 2011; 55:467-476; PMID:21769778; http://dx.doi.org/ 10.1387/ijdb.103224sl [DOI] [PubMed] [Google Scholar]
- [11].Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41-53; PMID:16371949; http://dx.doi.org/ 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- [12].Siegenthaler JA, Sohet F, Daneman R. ‘Sealing off the CNS’: cellular and molecular regulation of blood-brain barriergenesis. Curr Opin Neurobiol 2013; 23(6):1057–1064; http://dx.doi.org/ 10.1016/j.conb.2013.06.006; PMID:23867075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37:13-25; PMID:19664713; http://dx.doi.org/ 10.1016/j.nbd.2009.07.030 [DOI] [PubMed] [Google Scholar]
- [14].Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev 2010; 64:328-363; PMID:20685221; http://dx.doi.org/ 10.1016/j.brainresrev.2010.05.003 [DOI] [PubMed] [Google Scholar]
- [15].Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 1998; 93:189-201; PMID:9568712; http://dx.doi.org/ 10.1016/S0092-8674(00)81571-8 [DOI] [PubMed] [Google Scholar]
- [16].Bush TG. Enteric glial cells. An upstream target for induction of necrotizing enterocolitis and Crohn's disease? Bioessays 2002; 24:130-140; PMID:11835277; http://dx.doi.org/ 10.1002/bies.10039 [DOI] [PubMed] [Google Scholar]
- [17].Jiang S, Khan MI, Lu Y, Werstiuk ES, Rathbone MP. Acceleration of blood-brain barrier formation after transplantation of enteric glia into spinal cords of rats. Exp Brain Res 2005; 162:56-62; PMID:15599730; http://dx.doi.org/ 10.1007/s00221-004-2119-3 [DOI] [PubMed] [Google Scholar]
- [18].Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al.. Pericytes regulate the blood-brain barrier. Nature 2010; 468:557-561; PMID:20944627; http://dx.doi.org/ 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- [19].Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468:562-566; PMID:20944625; http://dx.doi.org/ 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Herrnberger L, Seitz R, Kuespert S, Bösl MR, Fuchshofer R, Tamm ER. Lack of endothelial diaphragms in fenestrae and caveolae of mutant Plvap-deficient mice. Histochem Cell Biol 2012; 138:709-724; PMID:22782339; http://dx.doi.org/ 10.1007/s00418-012-0987-3 [DOI] [PubMed] [Google Scholar]
- [21].Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A 1999; 96:13203-13207; PMID:10557298; http://dx.doi.org/ 10.1073/pnas.96.23.13203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R, et al.. The diaphragms of fenestrated endothelia: Gatekeepers of vascular permeability and blood composition. Dev Cell 2012; 23:1203-1218; PMID:23237953; http://dx.doi.org/ 10.1016/j.devcel.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rantakari P, Auvinen K, Jäppinen N, Kapraali M, Valtonen J, Karikoski M, Gerke H, Iftakhar-E-Khuda I, Keuschnigg J, Umemoto E, et al.. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat Immunol 2015; 16:386-96; PMID:25665101; http://dx.doi.org/ 10.1038/ni.3101 [DOI] [PubMed] [Google Scholar]
- [24].Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu Rev Med 2001; 52:259-274; PMID:11160778; http://dx.doi.org/ 10.1146/annurev.med.52.1.259 [DOI] [PubMed] [Google Scholar]
- [25].Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon A. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science (80-. ) 2008; 322:1247-1250; http://dx.doi.org/ 10.1126/science.1164594 [DOI] [PubMed] [Google Scholar]
- [26].Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 2009; 106:641-646; PMID:19129494; http://dx.doi.org/ 10.1073/pnas.0805165106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood-brain barrier. Cell and Tissue Research 2014; 355(3):687-699; http://dx.doi.org/ 10.1007/s00441-014-1811-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al.. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 2008; 183:409-417; PMID:18955553; http://dx.doi.org/ 10.1083/jcb.200806024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jho E, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 2002; 22:1172-1183; PMID:11809808; http://dx.doi.org/ 10.1128/MCB.22.4.1172-1183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Lüthi U, et al.. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010; 465:483-486; PMID:20445537; http://dx.doi.org/ 10.1038/nature09002 [DOI] [PubMed] [Google Scholar]
- [31].Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J 1999; 18:5931-5942; PMID:10545105; http://dx.doi.org/ 10.1093/emboj/18.21.5931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med 2013; 19:1584-96; PMID:24309662; http://dx.doi.org/ 10.1038/nm.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science (80-. ) 2011; 334:1727-1731; PMID:22144466; http://dx.doi.org/ 10.1126/science.1206936 [DOI] [PubMed] [Google Scholar]
- [34].Jeon MK, Klaus C, Kaemmerer E, Gassler N. Intestinal barrier: Molecular pathways and modifiers. World J Gastrointest Pathophysiol 2013; 4:94-9; PMID:24244877; http://dx.doi.org/ 10.4291/wjgp.v4.i4.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Büller NVJA, Rosekrans SL, Westerlund J, van den Brink GR. Hedgehog signaling and maintenance of homeostasis in the intestinal epithelium. Physiology (Bethesda) 2012; 27:148-55; PMID:22689790; http://dx.doi.org/ 10.1152/physiol.00003.2012 [DOI] [PubMed] [Google Scholar]
- [36].Troletti CD, de Goede P, Kamermans A, de Vries HE. Molecular alterations of the blood-brain barrier under inflammatory conditions: The role of endothelial to mesenchymal transition. Biochim Biophys Acta 2016; 1862:452-60; PMID:26493443; http://dx.doi.org/ 10.1016/j.bbadis.2015.10.010 [DOI] [PubMed] [Google Scholar]
- [37].Huang X, Pan L, Pu H, Wang Y, Zhang X, Li C, Yang Z. Loss of caveolin-1 promotes endothelial-mesenchymal transition during sepsis: A membrane proteomic study. Int J Mol Med 2013; 32:585-592; PMID:23836408 [DOI] [PubMed] [Google Scholar]
- [38].Krizbai IA, Gasparics Á, Nagyőszi P, Fazakas C, Molnár J, Wilhelm I, Bencs R, Rosivall L, Sebe A. Endothelial-mesenchymal transition of brain endothelial cells: possible role during metastatic extravasation. PLoS One 2015; 10:e0119655; PMID:25742314; http://dx.doi.org/ 10.1371/journal.pone.0119655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim BJ, Hancock BM, Bermudez A, Del Cid N, Reyes E, van Sorge NM, Lauth X, Smurthwaite CA, Hilton BJ, Stotland A, et al.. Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J Clin Invest 2015; 125:2473-83; PMID:25961453; http://dx.doi.org/ 10.1172/JCI74159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter P, Jennings S, et al.. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res 2001; 61:130-143; PMID:11162203; http://dx.doi.org/ 10.1006/mvre.2000.2288 [DOI] [PubMed] [Google Scholar]


