ABSTRACT
Recently, our laboratory demonstrated that bacteriocins produced by commensal enterococci provide an advantage in niche maintenance in the highly competitive environment of the gastrointestinal (GI) tract. Bacterial production of bacteriocins is a conserved defense strategy to help establish an ecological niche. Bacteriocin-encoding genes in enterococci are often carried on mobile genetic elements, including conjugative plasmids, enabling the transfer of such traits to other community members in a shared niche. Use of a novel mouse model for enterococcal colonization of the GI tract allowed us to investigate enterococcal dynamics and the role of enterococcal bacteriocins in the mouse GI tract. We examined the role of bacteriocin-21, carried on the pPD1 plasmid, in enterococcal colonization of the gut. We discovered that Enterococcus faecalis (EF) harboring pPD1 effectively colonizes the GI tract by using Bac-21 to eliminate its competition. In our study, we also present evidence for active conjugation in the GI tract, a strategy EF uses to enhance the number of bacteriocin producers in a given niche and eliminate bacteriocin-susceptible populations. Using an engineered strain of EF that is capable of producing Bac-21 but impaired in its conjugation ability, we were able to reduce pre-existing colonization by vancomycin-resistant enterococci in the mouse gut. Thus, our results suggest a novel therapeutic strategy to de-colonize antibiotic-resistant enterococci from the GI tract of patients and thereby prevent the emergence of resistant enterococcal infections that are otherwise difficult, or impossible, to treat.
KEYWORDS: Animal models of GI infection or GI-diseases with microbial components, Antibiotics in treatment of GI diseases, bacteriocin, conjugation, Enterococcus faecalis, Harnessing microbial strategies for treatment of human disease, intestinal colonization, New/novel treatments for GI infections, niche competition
Enterococci are Gram-positive bacteria that are members of the gut microbiome of a wide range of mammals, including humans.1 While enterococci do not cause enteritis or invade systemically in most cases, they can cause significant disease in immune compromised individuals.2,3 Treating enterococcal infections is challenging due to their intrinsic and acquired resistance to a wide range of antibiotics.3 Additionally, alterations in the intestinal microbiota during antibiotic therapy increases the risk of enterococcal infections, enabling expansion of antibiotic-resistant enterococci in the GI tract of the host and subsequent translocation to extraintestinal sites.4,5 In this study, we tested a strategy designed to reduce colonization of antibiotic-resistant enterococci in the GI tract by using bacteriocin-producing commensal enterococci.6
Bacteriocins often exhibit a relatively narrow spectrum of antimicrobial activity, with activity against bacterial species that are closely related to the producing organism.7-11 Bacteriocin production is one of the tools bacteria can use to enhance the stability of their bacterial communities.10,11 These antimicrobial peptides interfere with other bacterial competitors, prevent invasion of competing species, and thereby establish a stable niche for the producing strain.6,7,10 In our recent study, we explored the effect of enterococcal bacteriocin, Bacteriocin-21 (Bac-21) produced by the laboratory strain of Enterococcus faecalis (OG1RF) in the GI tract.6 Bac-21 is encoded on the sex-pheromone-responding conjugative plasmid pPD1.12,13 The bacterial strain in which pPD1 was first identified was originally called Streptococcus faecalis 39-5 (later re-classified as Enterococcus faecalis), a haemolysin-bacteriocin-producing strain isolated from subgingival scrapings of a patient with periodontitis.14 The prevalence of the pPD1 plasmid among natural populations of enterococci has not been reported so far. However, reports on sex pheromone plasmids of enterococci more generally provide evidence for their prevalence in food samples (in the cheese making industry) or in clinical isolates.15
Genes induced in response to the enterococcal sex pheromone cPD1 (traC-traB-traA-ipd-traE) (Fig. 1) and those involved in bacteriocin (bac-21 operon) production are the only pPD1 determinants that have been sequenced prior to our study.12,13 We determined the complete genetic sequence of pPD1,6 revealing a plasmid of 57,732 bp that encodes 59 predicted open reading frames (ORF). As expected, ORFs homologous to those responsible for conjugation and replication in other well studied pheromone-responsive plasmids (pAD1 and pCF10)6,12,13,16-18 were identified. The genes involved in regulation of the pheromone response are clustered in a 7 kb region present on each plasmid.12,13,16-18 Other ORFs not associated with conjugation, replication, or bacteriocin production were largely of unknown function.6
Figure 1.
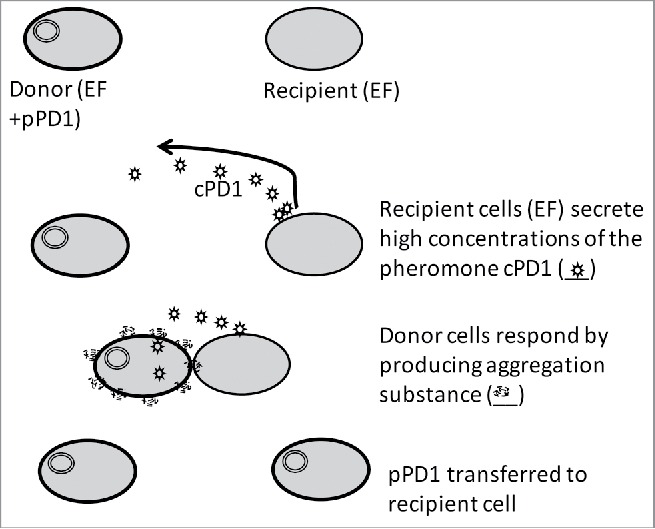
Conjugative transfer of pPD1 in E. faecalis. The plasmid transfer of pPD1 is initiated by the donor cells (EF+pPD1) responding to the secreted pheromone cPD1 produced by the recipient cells (EF). This response triggers the expression of genes involved in the conjugative process in the donor cells. Aggregation substance stabilizes the interaction and facilitates the transfer of the pPD1 plasmid from donor cells to the recipient cells. Figure modified from Bennett and Dunny (2010)33.
Bac-21 has been characterized as a circular bacteriocin produced by E. faecalis strains and is similar to a previously characterized bacteriocin, AS-48.19 The circular bacteriocins are a group of ribosomally synthesized antimicrobial peptides linked by a covalent N- and C-terminus to form a circular peptide backbone. Plasmid pMB2 harbors the locus encoding AS-48, which consists of 10 genes (as-48A, B, C, C1, D, D1, E, F, G and H).20,21 Plasmid pPD1 harbors the bac-21 operon that includes 9 annotated genes (bacA, B, C, D, E, F G, H and I).6,13 It is clear that with the exception of the bacteriocin precursor itself (BacA), most genes in the bacteriocin operon and the proteins they encode have undergone some divergence in sequence.6 However, no data is available to indicate whether these changes have functional consequences. The physical structure of the final Bac-21 bacteriocin product has not yet been determined. Although the bacteriocin-encoding genes give rise to identical amino acid sequences, in light of the sequence divergence found in the remainder of the bacteriocin-producing operon it is possible that Bac-21 may undergo different post-translational modifications than AS-48. Since there is ambiguity surrounding functional consequences of sequence divergence in these 2 operons, we refer them separately as AS-48 and Bac-21. AS-48 was the first circular bacteriocin for which the secondary and tertiary structure has been deciphered.22 Initial studies on AS-48 identified an N to C-terminal peptide bond linking the N-terminal methionine (Met1) to the C-terminal tryptophan (Trp70), resulting in a unique a circular structure that is characteristic of these bacteriocins.22 Like AS-48, mature Bac-21 peptide is predicted to have 70 amino acids residues derived from 105-aminoacid prepeptide.22 In vitro, Bac-21 is secreted, and its activity is detected in liquid cultures of exponential as well as stationary-phase cells, indicating that it is constitutively produced (data not shown). In our study, we identified that Bac-21-producing EF exhibited bacteriocin activity in vitro against many multidrug-resistant clinical isolates of E. faecium and E. faecalis.6
Colonization
Numerous scientific studies have focused on the application of bacteriocins in food and packaging industries, since most Gram-positive bacteria including Listeria are highly susceptible to circular bacteriocins.23 However, little is known about the actual contribution of bacteriocins to the producing strain in its natural ecosystems such as the mammalian GI tract, where EF exists as a commensal. To understand the mechanisms enterococci use to colonize the GI tract, we developed a model that allows mouse intestinal colonization by laboratory strains of EF without the need for antibiotic treatment, thereby modeling commensal colonization rather than invasion resulting from antibiotic disruption of intestinal homeostasis.6 Delivering a marked strain of EF in drinking water ad libitum for 2 weeks enabled us to establish long-term colonization with EF in an unperturbed mouse intestinal environment. Although the strain (OG1) used in our study is originally derived from an oral human isolate, this does not preclude its ability to colonize the GI tract.24 The oral cavity is of course contiguous with and a part of the gastrointestinal tract, and it is therefore not surprising that bacterial traits enabling gut colonization may also promote colonization of the oral cavity.
It is clear that introducing most bacteria (pathogens and commensals/probiotics) into the mouse gut through oral gavage, as is commonly done, does not result in long-term colonization, even when carried out repeatedly.25-27 When we gavaged mice with ∼109 CFU of EF, we observed variable colonization levels that lasted for 2-3 d. However, when we fed mice for a long period of time (2 weeks) by introducing the bacteria in their drinking water, we observed stable colonization of EF in the GI tract extending up to at least 11 weeks. In addition, comparative genome analysis after whole-genome sequencing of EF isolated from the intestine after 4 weeks of colonization revealed no evidence for new mobile genetic elements or spontaneous mutations that could contribute to their success in adapting to mouse GI tract (unpublished data). This suggests that bacteria adapt to the intestinal environment through changes in the gene expression and this adaptation phenomenon is supported by prolonged exposure to EF through the drinking water. Continuous dosing of mice with bacteria through their drinking water may provide greater opportunity for non-indigenous strains of enterococci to establish a niche by competing with others members of the microbiota for resources. Additionally, certain host factors or immune responses elicited by EF during its presence in the GI tract could also contribute to its adaptation. Our model may offer a new means of investigating the bacterial mechanisms that underlie GI tract colonization.
Using this model, we assessed the role of the bacteriocin encoding conjugative plasmid pPD1 on enterococcal dynamics in the mouse gut. Interestingly, the strain carrying pPD1 exhibited enhanced ability to colonize via outcompeting susceptible strains of enterococci and occupying the entire enterococcal niche. Deletion of bacA and bacB genes in pPD1 disabled the ability of EF (EF+pPD1:: ΔbacAB) to produce Bac-21 and eliminated any colonization benefit to the host strain. Moreover, carriage of the mutant pPD1 (pPD1:: ΔbacAB) impaired long-term persistence in the GI tract. It is likely that deletion in bacAB reduces bacterial fitness in a highly competitive environment through the excessive metabolic burden of maintaining and duplicating a large and non-advantageous plasmid. Since Bac-21 has been shown to have a relatively broad spectrum of antibacterial activity, we investigated the effect of EF+pPD1 on other bacterial populations in the GI environment. Comparative analysis of 16S rDNA obtained from high-throughput pyrosequencing of cecal DNA obtained from control, EF and EF+pPD1 colonized groups of mice was performed. We found no significant differences in composition between the ceca of control mice and those colonized by EF indicating that colonization of mice with laboratory strains of EF does not substantially alter intestinal microbial ecology. However, comparison of the cecal composition of EF and EF+pPD1 revealed a significant decrease due to Bac-21 effect on the presence of Gram-negative, Mucispirillum species that belong to the phylum Deferribacteres. We speculate that this effect on the microbiota, specifically on Mucispirillum species, is more likely secondary because Gram-positive bacteriocins such as Bac-21 have low efficacy against Gram-negative bacteria. It seems likely that the primary mechanism by which pPD1 enhances colonization is through the elimination of competing enterococcal strains. Alternatively, it is possible that Bac-21 could affect Mucispirillum dynamics indirectly through a direct undefined effect on host gene expression.
Competition
We investigated pPD1-mediated enterococcal competition in the gut more carefully, through competitive colonization studies using differentially marked enterococcal strains. Introducing EF-S (EF lacking pPD1, hence susceptible to Bac-21) and EF+pPD1 at various levels (1:1, 1:9 and 9:1) in drinking water showcased the ability of EF+pPD1 to outcompete Bac-21 susceptible EF-S, again suggesting that harboring pPD1 provides a competitive advantage for EF in the intestinal tract. Similar competitive experiments using EF+pPD1:: ΔbacAB and EF-S did not affect EF-S survival in the GI tract, indicating that competitive ability is indeed due to the Bac-21 activity against the susceptible enterococci. Ectopic expression of bacABCDE in EF+pPD1:: ΔbacAB restored bacteriocin activity (but not in plasmid-free EF) indicating that the distal part of the bac operon (bacFGHI) is necessary for bacteriocin expression and that the bacAB in-frame deletion did not result in any polar effects on the expression of the distal part of the bacteriocin determinant. EF+pPD1:: ΔbacAB bacABCDE+ was able to colonize the GI tract and its ability to compete was restored to the levels seen with the intact pPD1. To investigate if the colonization and competitive advantage are unique to pPD1 and its determinant Bac-21, we tested the effect of another pheromone-inducible plasmid, pCF10, in EF colonization of the intestinal tract. This plasmid does not encode a bacteriocin determinant. Unlike pPD1, pCF10 did not provide a competition and colonization advantage to outcompete the indigenous enterococci. These findings support our hypothesis that bacteriocins like Bac-21 can drive the competition between closely related bacterial species in a competitive environment.
Conjugation
The pPD1 plasmid transfer is initiated in response to a small signal peptide cPD1 - the pheromone constitutively secreted by the recipient bacteria in vitro (Fig. 1).28,29 The occurrence and effect of this unique mechanism of horizontal gene transfer in an intestinal environment has been reported for similar pheromone-responsive plasmids in a few studies.29,30,31,32 Our experimental strategy to study the pPD1 associated competitive benefit also allowed us to examine whether there was transfer of the pPD1 plasmid along with its bacteriocin trait to non-producing enterococci in the GI tract via conjugation. Collectively, these experiments revealed that pPD1 enhances EF competition in the GI tract by Bac-21-mediated killing or through the transfer of functional pPD1 plasmid to other non Bac-21 producing enterococcal strains via conjugation thereby increasing the population of bacteriocin producers in the GI tract. In our study, we also identified transfer of pPD1 to indigenous gut enterococci, both in vivo and in vitro, at a variable frequency. Conjugation is associated with cell-to-cell contact, usually facilitated by the aggregation factor produced by the donor in response to the recipient's pheromone.29 The evidence for conjugation, in addition to the potent and specific antimicrobial effect of Bac-21 on susceptible enterococci, suggests that enterococci (both exogenous and indigenous) are physically closely associated in a similar niche in an intestinal environment.
Therapeutic potential
In our investigation we unexpectedly observed that EF+pPD1:: ΔbacAB is defective in conjugation. The mechanism behind the effect of the ΔbacAB mutation on pPD1 conjugation is currently not known. Complementation of ΔbacAB allele ectopically restored bac-21 production, colonization and competition, but not conjugation. Using this complementing strain (therapeutic strain- EF+pPD1:: ΔbacAB bacA-E+) that can produce Bac-21 but is defective in conjugation, we assessed its ability to outcompete vancomycin-resistant E. faecalis (V583) in mice (Fig. 2). This strategy eliminated the risk for the transfer of bacteriocin production to the multidrug resistant strain of enterococci, V583. Using our colonization model, we colonized mice with V583, followed by administration of the therapeutic strain. Compared to the control group, V583 levels in the challenged mice were below the detection limit in most of the animals, effectively clearing or suppressing colonization by this multidrug-resistant E. faecalis strain. In addition, bacteriocin traits were not transferred to the V583. These results demonstrate that introducing a conjugation impaired Bac-21 producing strain into the GI tract can successfully reduce V583 colonization in the GI tract without profound effect on the microbiota, unlike conventional antibiotic treatment strategies (Fig. 2). This study provides critical proof-of-concept that bacteriocin-expressing enterococci are capable of eliminating other antibiotic-resistant enterococci from the GI tract, which can be one important component of a novel therapeutic strategy. However, more work is necessary to fully realize the potential of this therapeutic strategy. For example, stable colonization by a bacteriocin-producing EF strain (after decolonization of the antibiotic-resistant target) may allow the bacteriocin producer to acquire new antibiotic resistant traits from other community members, rendering the bacteriocin producer just as problematic as the original target. Hence, future research on the bacteriocin mode of action and the molecular mechanisms that Bac-21 producing commensal EF use to identify and establish a successful niche for intestinal colonization will be critical for the development of bacteriocin-delivery strains that are unable to establish stable, long-term colonization of the gut but are nevertheless capable of efficiently decolonizing targeted antibiotic-resistant enterococci.
Figure 2.
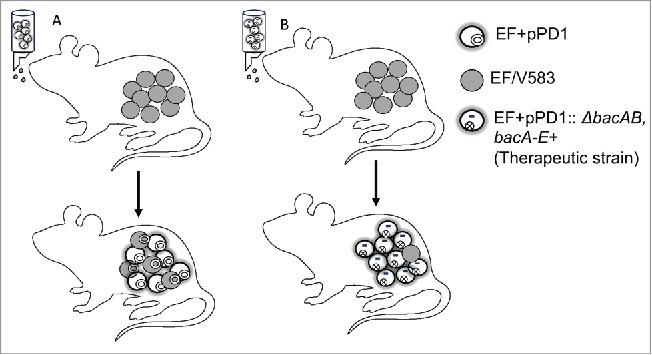
Bac-21 produced by EF could contribute to its colonization and clearance of non-pPD1 strains. (A) Bac-21 enhances the colonization of EF+pPD1, by serving as a killing peptide and facilitating the competition with the non-pPD1 strains of enterococci. Conjugation driven by the pheromone response ensures that only the pPD1 harboring population attains immunity in the presence of Bac-21. (B) Engineering a strain that is defective in conjugation but effective in producing Bac-21 through an ectopic locus in EF+pPD1:: ΔbacAB, bacA-E+ (therapeutic strain) enhances the colonization ability of the Bac-21 producers in eliminating the susceptible strains (EF or V583) without conjugal transfer of bacteriocin traits.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].McBride SM, Fischetti VA, Leblanc DJ, Moellering RC Jr, Gilmore MS. Genetic diversity among Enterococcus faecalis. PloS one 2007; 2:e582; PMID:17611618; http://dx.doi.org/ 10.1371/journal.pone.0000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009; 155:1749-57; PMID:19383684; http://dx.doi.org/ 10.1099/mic.0.026385-0 [DOI] [PubMed] [Google Scholar]
- [3].Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol 2000; 21:510-5; PMID:10968716; http://dx.doi.org/ 10.1086/501795 [DOI] [PubMed] [Google Scholar]
- [4].Shepard BD, Gilmore MS. Antibiotic-resistant enterococci: the mechanisms and dynamics of drug introduction and resistance. Microbes and Infection / Institut Pasteur 2002; 4:215-24; PMID:11880055; http://dx.doi.org/ 10.1016/S1286-4579(01)01530-1 [DOI] [PubMed] [Google Scholar]
- [5].Broaders E, Gahan CG, Marchesi JR. Mobile genetic elements of the human gastrointestinal tract: potential for spread of antibiotic resistance genes. Gut microbes 2013; 4:271-80; PMID:23651955; http://dx.doi.org/ 10.4161/gmic.24627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, Cao Y, Bousounis P, Kristich CJ, Salzman NH. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 2015; 526:719-22; PMID:26479034; http://dx.doi.org/ 10.1038/nature15524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dobson A, Cotter PD, Ross RP, Hill C. Bacteriocin production: a probiotic trait? Appl Environ Microbiol 2012; 78:1-6; PMID:22038602; http://dx.doi.org/ 10.1128/AEM.05576-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev 1995; 59:171-200; PMID:7603408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Maqueda M, Sánchez-Hidalgo M, Fernández M, Montalbán-López M, Valdivia E, Martínez-Bueno M. Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiol Rev 2008; 32:2-22; PMID:18034824; http://dx.doi.org/ 10.1111/j.1574-6976.2007.00087.x [DOI] [PubMed] [Google Scholar]
- [10].Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol 2010; 8:15-25; PMID:19946288; http://dx.doi.org/ 10.1038/nrmicro2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ingolf F, Nes DBD, Yasuyoshi Ike. Enterococcal Bacteriocins and Antimicrobial Proteins that Contribute to Niche Control BTI - Enterococci: From Commensals to Leading Causes of Drug Resistant Infection In: Michael S Gilmore E-I-C, Clewell Don B, Yasuyoshi Ike, Nathan Shankar (ed). Enterococci: From Commensals to Leading Causes of Drug Resistant Infection. Massachusetts Eye and Ear Infirmary: Boston, 2014. [Google Scholar]
- [12].Nakayama J, Yoshida K, Kobayashi H, Isogai A, Clewell DB, Suzuki A. Cloning and characterization of a region of Enterococcus faecalis plasmid pPD1 encoding pheromone inhibitor (ipd), pheromone sensitivity (traC), and pheromone shutdown (traB) genes. J Bacteriol 1995; 177:5567-73; PMID:7559344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tomita H, Fujimoto S, Tanimoto K, Ike Y. Cloning and genetic and sequence analyses of the bacteriocin 21 determinant encoded on the Enterococcus faecalis pheromone-responsive conjugative plasmid pPD1. J Bacteriol 1997; 179:7843-55; PMID:9401046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yagi Y, Kessler RE, Shaw JH, Lopatin DE, An F, Clewell DB. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol 1983; 129:1207-15; PMID:6411857 [DOI] [PubMed] [Google Scholar]
- [15].Nes IF, Diep DB, Holo H. Bacteriocin Diversity in Streptococcus and Enterococcus. J Bacteriol 2007; 189:1189-98; PMID:17098898; http://dx.doi.org/ 10.1128/JB.01254-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fujimoto S, Tomita H, Wakamatsu E, Tanimoto K, Ike Y. Physical mapping of the conjugative bacteriocin plasmid pPD1 of Enterococcus faecalis and identification of the determinant related to the pheromone response. J Bacteriol 1995; 177:5574-81; PMID:7559345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hirt H. Reinhard Wirth, Albrecht Muscholl Comparative analysis of 18 sex pheromone plasmids from Enterococcus faecalis: detection of a new insertion element on pPD1 and implications for the evolution of this plasmid family. Mol Gen Genet 1996; 252:640-7; PMID:8917306 [DOI] [PubMed] [Google Scholar]
- [18].Tanimoto K, Tomita H, Ike Y. The traA gene of the Enterococcus faecalis conjugative plasmid pPD1 encodes a negative regulator for the pheromone response. Plasmid 1996; 36:55-61; PMID:8938053; http://dx.doi.org/ 10.1006/plas.1996.0032 [DOI] [PubMed] [Google Scholar]
- [19].Gonzalez C, Langdon GM, Bruix M, Gálvez A, Valdivia E, Maqueda M, Rico M. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc Natl Acad Sci U S A 2000; 97:11221-6; PMID:11005847; http://dx.doi.org/ 10.1073/pnas.210301097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martinez-Bueno M, Galvez A, Valdivia E, Maqueda M. A transferable plasmid associated with AS-48 production in Enterococcus faecalis. J Bacteriol 1990; 172:2817-18; PMID:2110152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cebrian R, Rodríguez-Ruano S, Martínez-Bueno M, Valdivia E, Maqueda M, Montalbán-López M. Analysis of the promoters involved in enterocin AS-48 expression. PloS one 2014; 9:e90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grande Burgos MJ, Pulido RP, Del Carmen Lopez Aguayo M, Galvez A, Lucas R. The Cyclic Antibacterial Peptide Enterocin AS-48: Isolation, Mode of Action, and Possible Food Applications. Int J Mol Sci 2014; 15:22706-27; PMID:25493478; http://dx.doi.org/ 10.3390/ijms151222706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang SC, Lin CH, Sung CT, Fang JY. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 2014; 5:241; PMID:24904554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gold Og Fau-Jordan HV, Jordan Hv Fau-van Houte J, van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Archives Oral Biol 1975; 20:473-477; PMID:80718926714177 [DOI] [PubMed] [Google Scholar]
- [25].Aktas B, De Wolfe TJ, Tandee K, Safdar N, Darien BJ, Steele JL. The Effect of Lactobacillus casei 32G on the Mouse Cecum Microbiota and Innate Immune Response Is Dose and Time Dependent. PloS one 2015; 10:e0145784; PMID:26714177; http://dx.doi.org/ 10.1371/journal.pone.0145784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Watson D, Sleator RD, Hill C, Gahan CG. Enhancing bile tolerance improves survival and persistence of Bifidobacterium and Lactococcus in the murine gastrointestinal tract. BMC Microbiol 2008; 8:1-10; PMID:18173832; http://dx.doi.org/ 10.1186/1471-2180-8-176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].D'Orazio SE. Animal models for oral transmission of Listeria monocytogenes. Front Cell Infect Microbiol 2014; 4:15; PMID:24575393; http://dx.doi.org/ 10.3389/fcimb.2014.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wirth R. The sex pheromone system of Enterococcus faecalis EJB Reviews 1994. Springer Berlin Heidelberg: Berlin, Heidelberg, 1995, pp 117-28. [Google Scholar]
- [29].Wirth R. Sex pheromones and gene transfer in Enterococcus faecalis. Res Microbiol 2000; 151:493-6; PMID:10961465; http://dx.doi.org/ 10.1016/S0923-2508(00)00163-7 [DOI] [PubMed] [Google Scholar]
- [30].Huycke MM, Gilmore MS, Jett BD, Booth JL. Transfer of Pheromone-Inducible Plasmids between Enterococcus faecalis in the Syrian Hamster Gastrointestinal Tract. J Infect Dis 1992; 166:1188-91; PMID:1402034; http://dx.doi.org/ 10.1093/infdis/166.5.1188 [DOI] [PubMed] [Google Scholar]
- [31].Marcinek H, Wirth R, Muscholl-Silberhorn A, Gauer M. Enterococcus faecalis gene transfer under natural conditions in municipal sewage water treatment plants. Appl Environ Microbiol 1998; 64:626-32; PMID:9464401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Licht TR, Laugesen D, Jensen LB, Jacobsen BL. Transfer of the pheromone-inducible plasmid pCF10 among Enterococcus faecalis microorganisms colonizing the intestine of mini-pigs. Appl Environ Microbiol 2002; 68:187-93; PMID:11772626; http://dx.doi.org/ 10.1128/AEM.68.1.187-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bennett RJ, Dunny GM. Analogous telesensing pathways regulate mating and virulence in two opportunistic human pathogens. mBio 2010; 1:e00181-00110; PMID:20827374; http://dx.doi.org/ 10.1128/mBio.00181-10 [DOI] [PMC free article] [PubMed] [Google Scholar]


