Abstract
The potent carcinogen aflatoxin B1 is a weak mutagen but a strong recombinagen in Saccharomyces cerevisiae. Aflatoxin B1 exposure greatly increases frequencies of both heteroallelic recombination and chromosomal translocations. We analyzed the gene expression pattern of diploid cells exposed to aflatoxin B1 using high-density oligonucleotide arrays comprising specific probes for all 6218 open reading frames. Among 183 responsive genes, 46 are involved in either DNA repair or in control of cell growth and division. Inducible growth control genes include those in the TOR signaling pathway and SPO12, whereas PKC1 is downregulated. Eleven of the 15 inducible DNA repair genes, including RAD51, participate in recombination. Survival and translocation frequencies are reduced in the rad51 diploid after aflatoxin B1 exposure. In mec1 checkpoint mutants, aflatoxin B1 exposure does not induce RAD51 expression or increase translocation frequencies; however, when RAD51 is constitutively overexpressed in the mec1 mutant, aflatoxin B1 exposure increased translocation frequencies. Thus the transcriptional profile after aflatoxin B1 exposure may elucidate the genotoxic properties of aflatoxin B1.
INTRODUCTION
The fungal mycotoxin aflatoxin B1 (AFB1) is a potent carcinogen, and low levels of chronic exposure correlate with increased neoplasia, primarily liver cancer, in humans (Hsu et al., 1991; Shen and Ong, 1996; Wogan, 1999) and in many animal species (Eaton and Gallagher, 1994). At the low doses observed in chronic human exposure, the carcinogenic potential of AFB1 is correlated with DNA adduct formation (Bailey, 1994; Buss et al., 1990; Otteneder and Lutz, 1999). As demonstrated by epidemiological studies, a G-to-T transversion in the codon 249 of the p53 gene is often found in AFB1-associated hepatocellular carcinoma (Eaton and Gallagher, 1994). Although mutation in the p53 tumor suppressor gene may be an important etiologic factor in AFB1-induced liver cancer in humans, animal studies suggest that loss of p53 function is not a strict requirement. Other effects of AFB1 or other enhancers of cell proliferation, such as hepatitis B virus infection, are likely required (Eaton and Gallagher, 1994). Further elucidation of the genotoxic effects of AFB1 may thus improve our understanding of its potent carcinogenicity.
AFB1 is a mutagen in Saccharomyces cerevisiae (Sengstag et al., 1996), Escherichia coli, rainbow trout, mice, rat and human cells (reviewed in Smela et al., 2001), and a recombinagen in yeast and in human cells (Stettler and Sengstag, 2001). In yeast, AFB1 can induce mitotic, homologous recombination resulting in heteroallelic gene conversion and translocations (Sengstag et al., 1996). After yeast cells are exposed to low doses of AFB1 in the expected range of human exposure, there is a strong stimulation of recombination but not mutation (unpublished data). In human lymphoblastoid cell line TK6, AFB1 exposure increases heteroallelic recombination at the thymidine kinase locus resulting in loss of heterozygosity (Stettler and Sengstag, 2001). Thus, understanding the molecular basis for the recombinogenicity of AFB1 in yeast may help understand the potent carcinogenicity of AFB1 compared with toxins with similar mutagenicity.
The remarkable recombinogenicity of AFB1 may result from a combination of factors. First, specific AFB1-DNA adducts may enzymatically or spontaneously convert to DNA double-strand breaks, thus directly initiating recombination. The N7 adduct 8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B1 is the major product in vitro (Essigmann et al., 1977) and in vivo (Lin et al., 1977; Croy et al., 1978). The positively charged imidazole ring of the principal DNA adduct promotes depurination, giving rise to an apurinic (AP) site, which can further yield single-strand breaks by β-elimination (Friedberg et al., 1995). Clusters of these single-strand breaks could yield double-strand breaks. Alternatively, mildly alkaline conditions can subsequently result in the formation of a chemically and biologically stable foramidopyrimidine derivative (AFB1-FAPY), which represents a significant product in vivo (Croy and Wogan, 1981). AP sites can be removed by the base excision repair (BER) pathway, and the AFB1-N7-guanine adducts can be removed by the nucleotide excision repair (NER; Leadon et al., 1981). The AFB1-FAPY adduct, however, is a nonrepairable, persistent lesion (Martin and Garner, 1977) that interferes with DNA replication. Such interference could indirectly stimulate recombination (Friedberg et al., 1995) and generate DNA double-strand breaks. However, chromosomal fragments have not been detected by pulse-field electrophoresis after yeast cells were exposed to AFB1 (unpublished data).
Alternatively, exposure to AFB1 could also elicit a stress response in yeast that stimulates more recombination than mutation. We thus investigated the global cellular response to a 4-h. exposure to AFB1. DNA microarrays have been used successfully in yeast to investigate the global transcriptional response after exposure to saline (Posas et al., 2000), methyl methanesulfonate (MMS; Jelinsky and Samson, 1999, Gasch et al., 2000), and ionizing radiation (Gasch et al., 2001). The current mRNA expression analysis shows that a large fraction of the AFB1-induced genes is involved in maintenance of DNA integrity. Because the majority of the transcriptionally upregulated DNA repair genes belong to the NER or recombinational repair (RR) pathway, we exposed the respective rad1 and rad51 repair mutants to AFB1 and measured translocation frequencies. To strengthen the correlation between AFB1-associated recombination and RAD51 induction, we measured AFB1-associated recombination in mec1 checkpoint mutants, defective in the DNA damage inducibility of RAD51, and in mec1 mutants expressing higher basal levels of RAD51. Our data suggest that AFB1 upregulates a recombinational repair pathway that involves RAD51 and RAD1.
MATERIALS AND METHODS
Media and Strains
Standard media, including YM medium (0.76% yeast nitrogen base without amino acids, 2% glucose), YM medium supplemented with appropriate amino acids, and YPD medium (yeast extract, peptone, dextrose) were used for the culture of yeast strains. Amino acids, adenine, and uracil were purchased from Merck (Dietikon, Switzerland), yeast nitrogen base, and bacto agar from Difco (Chemie Brunschwig, Basel, Switzerland).
Yeast strains contain two overlapping his3 fragments on chromosomes II and IV and were derived from YB109 (Fasullo and Dave, 1994). Translocation frequencies were determined by selecting for His+ recombinants that are generated by mitotic recombination between the his3 fragments. (Fasullo and Davis, 1987). YMK2181 (MATa/MATα, ura3-52/ura3-52, his3-Δ200/his3-Δ200, ade2-101/ade2-101, trp1-Δ1/TRP1, gal3-/gal3-, leu2-3112/leu2-3112, GAL1::his3-Δ5′/GAL1::his3-Δ5′, trp1::his3-Δ3′/trp1::his3-Δ3′, leu2-Δ3′, leu2-Δ5′, kanMX4, HOcs), and YB110 (MATa/MATα, ade2-101/ade2-101, ura3-52/ura3-52, his3-Δ200/his3-Δ200, trp1-Δ1/trp1-Δ1, leu2/LEU2, GAL1::his3-Δ5′/GAL1, trp1::his3Δ3′/trp1-Δ1, LYS2/lys2-801; Fasullo and Dave, 1994) have been previously used to measure DNA damage-associated translocation. YB150 (rad1) and YB195 (rad51) are identical to YB110 (Rad+) except for the rad51 and rad1 disruptions, respectively. We replaced the ade2-101 allele in YB109 and with ade2-n (YB318) and the ade2-101 allele in YA102 with ade2-a (YB336) by two-step gene replacement using the plasmid pKH9 (Huang and Symington, 1994).
mec1 checkpoint mutants that measure AFB1-associated translocations contain either mec1-21 or the mec1 null mutation. The original MATα mec1-21 (YA16) strain is derived from W303 (Sanchez et al., 1996). We backcrossed YA16 10 times with strains in the S288c background (YB163 and FY251 [Dong and Fasullo, 2003] and YB336) to generate meiotic segregants YB316 (MATα ura3-52 his3-Δ200, trp1-Δ1, ade2-a, mec1-21) and YB314 (MATα ura3-52 his3-Δ200, trp1Δ-1, ADE2, mec1-21) by tetrad dissections. YB318 was crossed with YB314 to generate the meiotic segregant YB319 (MATa-inc, ura3-52, his3-Δ200, ade2-n, trp1-Δ1, leu2, lys2, GAL1::his3-Δ5′, trp1::his3-Δ3′, mec1-21). YB325 (MATa/MATα, ade2-a/ade2-n, ura3-52/ura3-52, his3-Δ200/his3-Δ200, trp1-Δ1/trp1-Δ1, leu2/LEU2, GAL1::his3-Δ5′/GAL1, trp1::his3-Δ3′/trp1-Δ1, lys3–801/lys2-801, mec1-21/mec1-21) was then used to measure translocations and heteroallelic recombination in the mec1-21 background. To measure translocations in the mec1 null mutant, we first introduced the sml1::kanMX allele in YB318 and YB315 by PCR-mediated gene replacement (Goldstein and McCusker, 1999) to make YB320 and YB317, respectively, because lethality conferred by mec1 deletions is suppressed by sml1 mutations (Zhao et al., 1998). YB323 (MATa/MATα, ade2-a/ade2-n, ura3-52/ura3-52, his3-Δ200/his3-Δ200, trp1-Δ1/trp1-Δ1, leu2/LEU2, GAL1::his3-Δ5′/GAL1, sml1::kanMX/sml1::kanMX, trp1::his3Δ3′/trp1-Δ1, lys2–801/lys2–801) was then derived by a diploid cross of YB320 and YB317. The mec1Δ::TRP1 allele (Zhao et al., 2000) was introduced into YB320 and YB317 to make YB321 and YB322, respectively. YB324 (MATa/MATα, ade2-a/ade2-n, ura3-52/ura3-52, his3-Δ200/his3-Δ200, trp1-Δ1/trp1-Δ1, leu2/LEU2, GAL1::his3-Δ5′/GAL1, sml1::kanMX/sml1::kanMX, trp1::his3Δ3′/trp1-Δ1, lys2–801/lys2–801, mec1Δ::TRP1/mec1Δ::TRP1) was then derived by a diploid cross. To overexpress RAD51 in the mec1 mutants, pR51.3 (Leu+), containing RAD51 on a 2 μ plasmid, was introduced into YB325 (Sung and Stratton, 1996).
The 2 μ URA3 plasmids pMK637 (this work) or pSB229 (Eugster et al., 1992), containing hCYP1A2+hOR and hCYP1A1+hOR cDNAs, respectively, or the LEU2 plasmid pCS512 (Sengstag et al., 1996), containing hCYP1A1+hOR cDNAs, were first introduced into yeast strains by DNA transformation to metabolically activate the AFB1 and benzo-(a)-pyrene-7,8-dihydrodiol (BaP-DHD; Klebe et al., 1983). The 2 μ URA3 plasmid pCS316, containing the hCYP1A1+hOR cDNA in the opposite orientation as in pSB229 (Eugster et al., 1992), was introduced into YB110, YB324, and YB335 to measure AFB1-associated translocations in mec1 checkpoint mutants. pMK637 was introduced into the strain YMK2181 to measure AFB1 related-changes in gene expression using the oligonucleotide arrays. pCS512 was introduced into YB150 and the plasmid pSB229 was introduced into YB195 and YB110 to measure chromosomal translocation frequency and drug killing after exposure to ethyl methanesulfonate (EMS), AFB1, and BaP-DHD.
Exposure of Yeast Strains to DNA-damaging Agents
In brief, exponentially growing yeast cells were collected by centrifugation and resuspended in 0.1 M sodium phosphate buffer (pH 7.5); the final cell density was 4 × 108 cells/ml. To measure the stimulation of recombination, 1 ml of the cells in 0.1 M sodium phosphate buffer (pH 7.5) was exposed to chemicals for 4 h. at 30°C in a rotary shaker. The cells were then pelleted in a clinical centrifuge, washed, and diluted in supplemented minimal medium. To measure the net frequencies of recombination, the spontaneous frequencies were subtracted from the DNA damage–associated frequency. To measure AFB1-associated changes in gene expression, 2 ml of cells in 0.1 M sodium phosphate buffer was exposed to 25 μM AFB1 for 4 h at 30°C in a rotary shaker. Cells were then centrifuged and resuspended in the appropriate buffers to extract nucleic acids.
Preparation of Nucleic Acids for Oligonucleotide Arrays and Hybridization
After AFB1 exposure, cells were washed once, resuspended in 0.5 ml RLT buffer (Qiagen GmbH, Hilden, Germany) supplemented with 1% mercaptoethanol (Riedel-deHaën, Hannover, Germany) and transferred to a glass tube. Acid-washed glass beads (Ø 0.45–0.55 mm, Merck, Darmstadt, Germany) were added up to the meniscus and the cells were disrupted by heavy vortexing three times for 3 min. After addition of 3.3 ml RLT buffer, the lysate was recovered with a glass capillary. Total RNA was isolated using the RNeasy Midi Kit (Qiagen AG, Basel, Switzerland) according to the manufacturer's protocol. RNA quality was assessed on an agarose gel. Poly(A)+ RNA was amplified and biotin-labeled as follows. Starting with 20 μg total RNA, double-stranded cDNA was constructed using the GibcoBRL Superscript choice system (Life Technologies AG, Basel, Switzerland) and a T7-(T)24 primer to introduce a T7 promoter. Double-stranded cDNA was purified by three successive phenol:chloroform:isoamyl alcohol extractions and a subsequent alcohol precipitation. Phase-Lock Gel (5 Prime to 3 Prime, Boulder, CO) was used for all organic extractions to increase recovery. Using ∼0.2–0.5 μg cDNA as a template, a biotin-labeled riboprobe was synthesized with the help of the T7 Megascrip system (Ambion, Austin, TX) and two biotin-labeled nucleotides (Bio-11-CTP and Bio-16-UTP, Enzo Diagnostics, Farmingdale, NY), which replaced one third of the provided CTP and UTP. The 6-h in vitro transcription reaction yielded ∼50 μg cRNA, which was purified by RNA affinity resin (RNeasy spin columns, Qiagen). An aliquot was separated on a 0.8% agarose gel to check sample integrity. Subsequently, 40 μg of the transcript were used to hybridize a set of four commercially available oligonucleotide expression arrays (GeneChip Ye6100 arrays, Affymetrix, Santa Clara, CA) comprising a total of more than 260,000 oligonucleotides complementary to 6218 yeast open reading frames (ORFs). The biotinylated cRNA samples were fragmented to increase hybridization efficiency and specificity and to reduce potential problems caused by nucleic acid secondary structure (Wodicka et al., 1997). Chip hybridization, washing, and staining with a streptavidin-phycoerythrin conjugate were performed using Affymetrix instrumentation according to the company's recommended protocols. The arrays were read at 7.5 μm with a confocal scanner (Molecular Dynamics, Sunnyvale, CA) and analyzed with GENECHIP software, version 3.0. A threshold of 20 arbitrary fluorescence units was assigned to any gene with a calculated expression level below 20, because discrimination of mRNA levels in this low range could not be performed. Chip hybridization and mRNA quality were verified with controls on the arrays consisting of 3′, middle, and 5′ regions of housekeeping genes (actin, SPT15, SRB4) and marker oligonucleotides at the corners, edges, and in the middle of the array (Wodicka et al., 1997; unpublished data).
Statistical Analysis of the AFB1/DMSO Data Set
mRNA levels were expressed as the average difference of hybridization signals, measured as fluorescence intensity, between perfect match and central-mismatch oligonucleotide probe sets (Wodicka et al., 1997), and supplemented with an absent/present call generated by the Affymetrix software. Data from different chips were normalized using the parameter of total chip signal. We calculated the mean of the average differences of two chips each of AFB1 (AFB1+) and solvent (AFB1–) exposed cells. Only ORFs deviating <40% of this mean value (purity ≥ 0.6) were used for further analysis; 5630 ORFs fulfilled this criterion. The data sets were then imported into a MS Excel spreadsheet for further calculations and logical operations.
Preparation of RNA for Quantitative PCR Analysis
RNA was extracted from control cells, and cells were exposed to AFB1 (Shirra et al., 2001). RNA quality was assessed on a 0.8% agarose gel. DNaseI (0.05 U/ml, BD Biosciences, San Diego, CA) was added to ensure that no DNA was present in the extraction and after digestion at 37°C for 30 min, was inactivated in 1 mM EDTA (pH. 8.0). After extraction in phenol:chloroform:isoamyl alcohol (25:24:1, pH 4.5) and chloroform extraction, the aqueous layer was precipitated in 0.2 M NaOAc, 70% EtOH. The RNA pellet was then resuspended in TrisEDTA. One milligram of RNA was used for the reverse transcription reaction (first-strand cDNA synthesis), using a protocol described in the reverse transcription system kit (Promega, Madison, WI). cDNA was measured in a iCycler (Bio-Rad, Richmond, CA) by quantitative PCR (QPCR) using the IQ Green SYBR supermix kit (Bio-Rad). Cycle conditions included denaturation at 95°C, followed by 35 cycles of 95°C denaturation, 57°C reannealing, and 72°C reaction; a 95°C denaturation step; and a 55°C reannealing step. Rad51 cDNA was measured using oligos 5′-CAACTTGGGCGACCACTT G-3′ and 5′-AAAGGCTGGCCGACCAAT-3′. Act1 cDNA was measured using oligos 5′-CCACCAATCCAGACGGAGACT-3′ and 5′-GCCGAAAGAATG CAAAAG GA-3′. Rad1 cDNA was measured using 5′-CTAATTGTGCCTCATCGACCAA-3′ and 5′-GGATGCCAATAAACCGTCAGTATC-3′.
Measurements of DNA Damage–associated Recombination Frequencies in Checkpoint and rad Mutants and in Wild Type
We measured the frequency AFB1, EMS, and BaP-DHD–associated translocations and drug toxicity in the rad mutants, YB195pSB229 (rad51) and YB150pCS512 (rad1); checkpoint mutants, YB324pCS316 (sml1, mec1) and YB325pCS316 (mec1); and the Rad+ proficient strain YB110pCS316, as previously described (Sengstag et al., 1996). YB195, YB150, YB324, and YB325 transformants were grown in YM His-Ade-Trp-Lys and YB110 transformants in YM His-Ade-Trp. After exposure to chemical agents, cells were resuspended to a density of 8 × 108 cells/ml, 100–250 μl was plated directly on YM Ade-Ura-Trp-Leu-Lys to select for His+ recombinants, and the appropriate dilution was plated on YPD to measure viability. Selection plates were incubated at 30°C, and the colonies were counted after 7 days.
Chemicals
Benzo-(a)-pyrene-7,8-dihydrodiol (BaP-DHD; Midwest Research Institute, Kansas City, MO) and aflatoxin B1 (AFB1, Fluka, Buchs, Switzerland) were dissolved in DMSO. Ethyl-methane-sulfonate (EMS) was obtained from Eastman Kodak (Rochester, NY). DNA modifying enzymes were obtained from New England Biolabs, Inc. (Beverly, MA), 5-fluoroorotic acid (FOA) from Toronto Research Chemicals Inc. (Toronto, Ontario, Canada) and zymolyase was purchased from Seikagaku Corp. (Tokyo, Japan).
RESULTS
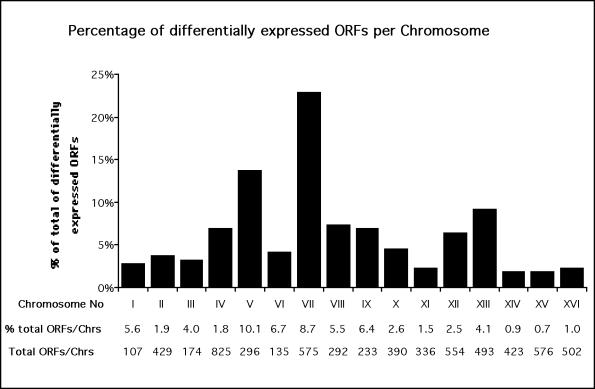
Genes responsive to AFB1 treatment were identified through parallel analysis of the mRNA expression profiles. Cells from strain YMK2181pMK637 were treated for 4 h with either 25 μM AFB1, the solvent DMSO, or water. Poly(A)+ RNAs were amplified and labeled to make biotin-labeled cRNA probes. After hybridization to the chip arrays, the biotinylated probes were fluorescently labeled and the chips were read in a specially designed confocal scanning fluorescence microscope. The quantitative image analysis was based on the average of the differences between the perfect match oligonucleotide and the corresponding central-mismatch oligonucleotide so that nonspecific and background contributions could be eliminated. Each experiment was done in duplicate. After normalization of the data, the fold change in the transcriptional expression of each ORF was calculated using the AFB1 exposed (AFB1 +) and control (AFB1–) data sets. Differences in hybridization intensity between the same ORFs are proportional to changes in transcript levels, and the intensity changes >2.0-fold are both significant and accurate, according to previous studies (Wodicka et al., 1997). Comparison of the data sets of 0.4% DMSO and H2O-treated cells identified one ORF whose expression was influenced by the solvent 0.4% DMSO; 478 specific ORFs exhibited greater than twofold change in expression due to AFB1 exposure. Chromosomal distribution of the responsive ORFs is depicted in Figure 1. Nearly one fourth of all the responsive genes are located on chromosome VII, where they represent 8.7% of the ORFs.
Figure 1.
Distribution of AFB1 responsive genes on the different chromosomes. Bars indicate the percentage of the total number of ORFs showing a ≥3-fold altered expression level; the roman numerals indicate the chromosome number. Information about the total ORF number of each chromosome (total ORFs/chrs) were retrieved from the MIPS database (Mewes et al., 1997) and used to calculate the percentage of transcriptionally responsive ORFs per total number ORFs on each chromosome (% total ORFs/chrs). Data are means of two independent experiments.
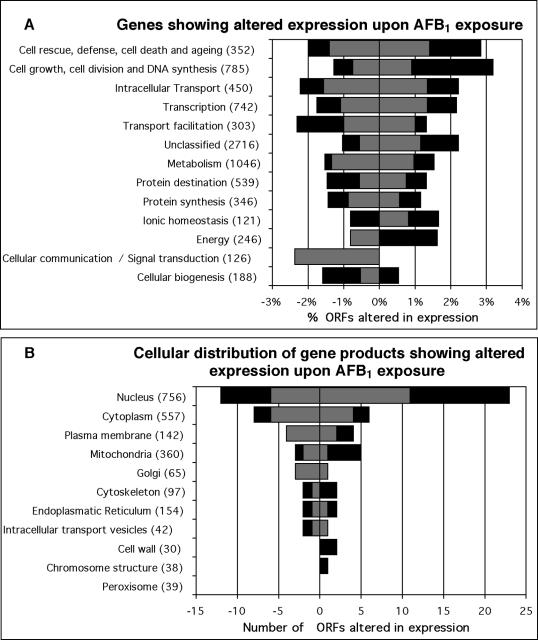
Of 6218 ORFs, 183 (2.9%) showed at least a threefold change in transcript levels after AFB1 exposure. One hundred seventeen were upregulated; the strongest induction was 35.1-fold (YEL068C; Table 1). Of the 66 genes that were downregulated, the strongest repression was 14.1-fold (YDR306C; Table 2). Most of the products of the responsive genes are located in the nucleus (79%; Figure 2). To gain an overview of the transcriptional response to AFB1, the ORFs were assigned to functional categories according to the MIPS database (Munich Information Center for Protein Sequences; Mewes et al., 1997). The category of cell growth, cell division, and DNA synthesis contains 30 AFB1-responsive genes, the most number of any category. The second most numerous is the metabolism category, which contains 28 AFB1-responsive genes. However, comparing the percentage of responsive genes in each respective category, the category of cell rescue, defense, cell death, and aging contains the largest percentage of AFB1-responsive genes (4.8%; Figure 2). A more detailed view is provided by the analysis of the subcategories (Figure 3). Thus, there are genes in several functional categories whose expression is either induced or repressed after AFB1 exposure.
Table 1.
Yeast genes induced at least 3-fold upon AFB1 exposure compared to control (DMSO)
| Intensityd
|
|||||||
|---|---|---|---|---|---|---|---|
| Database IDa | Geneb | Ratioc | DMSO | AFB1 | Functione | ||
| Metabolism | |||||||
| ypl123c | 0 | 28.7 | 22 | 632 | Ribonuclease of the T2 family | ||
| yer170w* | ADK2 | 5.1 | 20 | 102 | Adenylate kinase, mitochondrial | ||
| ynl264c | 0 | 4.9 | 20 | 97 | Involved in lipid biosynthesis and multidrug resistance, similarity to Sec14p | ||
| ymr205c* | PFK2 | 4.1 | 20 | 81 | 6-phosphofructokinase, beta subunit | ||
| ymr217w | GUA1 | 3.7 | 20 | 74 | GMP synthase (glutamine-hydrolysing) | ||
| ylr056w | ERG3 | 3.7 | 36 | 133 | C-5 sterol desaturase | ||
| yhr003c | 0 | 3.5 | 20 | 69 | Similarity to molybdopterin-converting factor homolog YKL027w | ||
| ygr170w | PSD2 | 3.4 | 20 | 67 | Phosphatidylserine decarboxylase 2 | ||
| yil085c* | KTR7 | 3.4 | 20 | 67 | Putative mannosyltransferase of the KRE2 family | ||
| yil134w* | FLX1 | 3.4 | 20 | 67 | FAD carrier protein (MCF), mitochondrial | ||
| ygr287c | 0 | 3.3 | 20 | 66 | Similarity to maltase | ||
| ygr286c | BIO2 | 3.2 | 20 | 63 | Biotin synthetase | ||
| yll061w* | 0 | 3.1 | 33 | 102 | High affinity s-methylmethionine permease, similarity to Gap1p and other amino acid permeases | ||
| ydl049c | KNH1 | 3.1 | 21 | 64 | Functional homolog of KRE9 | ||
| Energy | |||||||
| yhr039c | MSC7 | 4.9 | 20 | 97 | H+-transporting ATPase V0 domain 13-kDa subunit, vacuolar | ||
| ygl119w* | ABC1 | 4.2 | 20 | 83 | Ubiquinol-cytochrome-c reductase complex assembly protein | ||
| ymr205c* | PFK2 | 4.1 | 20 | 81 | 6-phosphofructokinase, beta subunit | ||
| Cell growth, cell division, and DNA Synthesis | |||||||
| yer149c | PEA2 | 19.6 | 20 | 391 | Involved in oriented growth toward mating partner | ||
| yhr152w | SPO12 | 8.5 | 20 | 169 | Sporulation protein required for chromosome division in meiosis I | ||
| ygr152c | RSR1 | 8.2 | 20 | 163 | GTP-binding protein of the ras superfamily | ||
| yer095w* | RAD51 | 7.7 | 29 | 224 | DNA repair protein | ||
| ygr140w | CBF2 | 6.6 | 20 | 132 | Kinetochore protein complex CBF3, 110-kDa subunit | ||
| yer016w* | BIM1 | 6.2 | 20 | 124 | Associated with microtubules, required for a cell cycle check point | ||
| ycr089w | FIG2 | 5.5 | 20 | 109 | Involved in mating induction | ||
| yer170w* | ADK2 | 5.1 | 20 | 102 | Adenylate kinase, mitochondrial | ||
| yer111c* | SWI4 | 5.1 | 20 | 101 | Transcription factor | ||
| ykl079w* | SMY1 | 5.0 | 20 | 99 | Kinesin-related protein | ||
| ydl102w* | CDC2 | 5.0 | 21 | 104 | DNA-directed DNA polymerase delta, catalytic 125-kDa subunit | ||
| ygl043w* | DST1 | 4.6 | 20 | 91 | TFIIS (transcription elongation factor); DNA strand transfer protein catalyzing homologous DNA strand exchange | ||
| yar007c | RFA1 | 4.6 | 20 | 91 | DNA replication factor A, 69-kDa subunit, binds ssDNA | ||
| ybr114w* | RAD16 | 4.5 | 38 | 169 | Nucleotide excision repair protein | ||
| ymr167w | MLH1 | 4.1 | 20 | 82 | DNA mismatch repair protein | ||
| yer122c* | GLO3 | 4.1 | 20 | 82 | Zinc finger protein | ||
| ygr041w | BUD9 | 4.0 | 20 | 79 | Budding protein | ||
| yhr135c* | YCK1 | 3.8 | 120 | 452 | Casein kinase I isoform | ||
| yhl024w | NOS1 | 3.7 | 20 | 74 | Required for sporulation and formation of meiotic spindle | ||
| ygl086w | MAD1 | 3.7 | 20 | 73 | Spindle assembly checkpoint protein; required for cell cycle delay in response to impaired kinetochore function | ||
| yhr066w | SSF1 | 3.4 | 21 | 71 | Mating protein | ||
| yjl098w | SAP185 | 3.3 | 20 | 66 | Sit4p-associating protein | ||
| yfr036w* | CDC26 | 3.1 | 35 | 108 | Anaphase-promoting complex (cyclosome) subunit | ||
| yer125w* | RSP5 | 3.1 | 40 | 123 | Hect domain E3 ubiquitin-protein ligase | ||
| Transcription | |||||||
| yer111c* | SWI4 | 5.1 | 20 | 101 | Transcription factor | ||
| ykl139w | CTK1 | 4.7 | 20 | 93 | Carboxy-terminal domain (CTD) kinase, alpha subunit | ||
| ygl043w* | DST1 | 4.6 | 20 | 91 | TFIIS (transcription elongation factor) | ||
| yer171w* | RAD3 | 4.6 | 20 | 91 | DNA helicase | ||
| yer122c* | GLO3 | 4.1 | 20 | 82 | Zinc finger protein | ||
| ygr249w* | MGA1 | 3.9 | 20 | 78 | Similarity to heat shock transcription factors | ||
| yer146w | LSM5 | 3.8 | 20 | 75 | Similarity to human snRNP E | ||
| ynl251c | NRD1 | 3.7 | 20 | 74 | Involved in regulation of nuclear pre-mRNA abundance | ||
| ygr246c | BRF1 | 3.5 | 20 | 70 | TFIIIB subunit, 70 kDa | ||
| ymr112c | MED11 | 3.5 | 20 | 69 | Mediator complex subunit | ||
| ygl172w | NUP49 | 3.3 | 20 | 66 | Nuclear pore protein | ||
| ygl092w* | NUP145 | 3.3 | 20 | 65 | Nuclear pore protein | ||
| ygl237c* | HAP2 | 3.2 | 48 | 154 | CCAAT-binding factor subunit | ||
| yil130w | 0 | 3.1 | 20 | 62 | Similarity to Put3p and to hypothetical protein YJL206c | ||
| Protein synthesis | |||||||
| ygr094w* | VAS1 | 8.1 | 20 | 161 | Valyl-tRNA synthetase | ||
| yhr020w | 0 | 3.3 | 20 | 66 | Similarity to prolyl-tRNA synthetases; putative class II tRNA synthetase | ||
| Protein destination | |||||||
| yer098w | UBP9 | 7.4 | 20 | 148 | Ubiquitin carboxyl-terminal hydrolase | ||
| ylr163c | MAS1 | 5.0 | 20 | 99 | Mitochondrial processing peptidase | ||
| ygl119w* | ABC1 | 4.2 | 20 | 83 | Ubiquinol-cytochrome-c reductase complex assembly protein | ||
| yil085c* | KTR7 | 3.4 | 20 | 67 | Putative alpha-1,2-mannosyltransferase | ||
| ymr264w | CUE1 | 3.2 | 20 | 64 | Involved in ubiquitination and degradation at the ER surface | ||
| yfr036w* | CDC26 | 3.1 | 35 | 108 | Subunit of anaphase-promoting complex (cyclosome) | ||
| yer125w* | RSP5 | 3.1 | 40 | 123 | Hect domain E3 ubiquitin-protein ligase | ||
| Transport facilitation | |||||||
| yjr040w* | GEF1 | 5.0 | 20 | 99 | Voltage-gated chloride channel protein | ||
| yil134w* | FLX1 | 3.4 | 20 | 67 | FAD carrier protein (MCF), mitochondrial | ||
| yhr175w* | CTR2 | 3.1 | 20 | 62 | Copper transport protein | ||
| yll061w* | 0 | 3.1 | 33 | 102 | Similarity to amino acid transport protein Gap1p, MFS | ||
| Intracellular transport | |||||||
| ygr257c | 0 | 6.3 | 20 | 126 | Similarity to members of the mitochondrial carrier family | ||
| yjr040w* | GEF1 | 5.0 | 20 | 99 | Voltage-gated chloride channel protein | ||
| ykl079w* | SMY1 | 5.0 | 20 | 99 | Kinesin-related protein | ||
| yor034c | AKR2 | 4.6 | 25 | 116 | Involved in constitutive endocytosis of Ste3p | ||
| yhr135c* | YCK1 | 3.8 | 120 | 452 | Casein kinase I isoform | ||
| ygl137w | SEC27 | 3.4 | 20 | 68 | Coatomer complex beta′ chain (beta′-cop) of secretory pathway vesicles | ||
| yil134w* | FLX1 | 3.4 | 20 | 67 | FAD carrier protein (MCF), mitochondrial | ||
| ygl172w* | NUP49 | 3.3 | 20 | 66 | Nuclear pore protein | ||
| ygl092w* | NUP145 | 3.3 | 20 | 65 | Nuclear pore protein | ||
| yhr175w* | CTR2 | 3.1 | 20 | 62 | Copper transport protein | ||
| Cellular biogenesis | |||||||
| yer016w* | BIM1 | 6.2 | 20 | 124 | Associated with microtubules | ||
| Cell rescue, defense, cell death, and aging | |||||||
| yer095w* | RAD51 | 7.7 | 29 | 224 | DNA repair protein | ||
| yer171w* | RAD3 | 4.6 | 20 | 91 | DNA helicase | ||
| ybr114w* | RAD16 | 4.5 | 38 | 169 | Nucleotide excision repair protein | ||
| ydl102w* | CDC2 | 5.0 | 21 | 104 | DNA polymerase delta, catalytic 125-kDa subunit | ||
| ygr138c* | 0 | 4.1 | 20 | 81 | Member of major facilitator superfamily (MFS) multidrug-resistance protein family | ||
| ygr249w* | MGA1 | 3.9 | 20 | 78 | Similarity to heat shock transcription factors | ||
| yhr135c* | YCK1 | 3.8 | 120 | 452 | Casein kinase I isoform | ||
| yor009w | 0 | 3.7 | 53 | 195 | Similarity to Tir1p and Tir2p | ||
| yer143w | DDI1 | 3.6 | 20 | 71 | Induced in response to DNA alkylation damage | ||
| yer125w* | RSP5 | 3.1 | 40 | 123 | Hect domain E3 ubiquitin-protein ligase | ||
| Ionic homeostasis | |||||||
| yjr040w* | GEF1 | 5.0 | 20 | 99 | Voltage-gated chloride channel protein | ||
| yhr175w* | CTR2 | 3.1 | 20 | 62 | Copper transport protein | ||
| Classification not yet clear-cut | |||||||
| yel055c | POL5 | 6.2 | 20 | 124 | DNA polymerase V | ||
| yar050w* | FLO1 | 4.4 | 20 | 88 | Flocculin, cell wall protein involved in flocculation | ||
| ycl068c | 0 | 3.1 | 20 | 61 | Similarity to the N-terminal third of Bud5p | ||
| ygl179c | 0 | 3.0 | 20 | 60 | Serine/threonine protein kinase with similarity to Elm1p and Kin82p | ||
| Unclassified | |||||||
| yel068c | 0 | 35.1 | 20 | 701 | Protein of unknown function | ||
| ygr107w | 0 | 17.6 | 20 | 351 | Protein of unknown function | ||
| ygr164w | 0 | 16.0 | 20 | 320 | Similarity to Hansenula wingei mitochondrial site-specific nuclease Pir | ||
| ygr247w | 0 | 15.4 | 60 | 923 | Protein of unknown function | ||
| ygr153w | 0 | 10.5 | 20 | 209 | Protein of unknown function | ||
| ygr176w | 0 | 10.4 | 31 | 321 | Protein of unknown function | ||
| yfl012w | 0 | 9.9 | 20 | 197 | Protein of unknown function | ||
| yel025c | 0 | 8.1 | 20 | 161 | Protein of unknown function | ||
| ygr203w | 0 | 6.7 | 20 | 134 | Similarity to X. laevis protein-tyrosin-phosphatase Cdc homolog 2 and to hypothetical protein YPR200c | ||
| yhr217c | 0 | 6.1 | 20 | 121 | Similarity to subtelomeric encoded YDR544c | ||
| yer189w | 0 | 5.9 | 20 | 117 | Similarity to subtelomerically-encoded proteins including Yil177p, Yhl049p, and Yjl225p | ||
| yil102c | 0 | 5.4 | 20 | 107 | Similarity to YIL014c-a | ||
| ydr149c | 0 | 5.0 | 20 | 100 | No annotation | ||
| yal037w | 0 | 4.6 | 21 | 97 | Similarity to GTP-binding proteins | ||
| yer038c | 0 | 4.6 | 20 | 92 | Protein of unknown function | ||
| yor059c | 0 | 4.5 | 55 | 245 | Similarity to YGL144c | ||
| yjl100w | 0 | 4.4 | 20 | 88 | Similarity to hypothetical C. elegans protein C56A3.8 | ||
| ygr150c | 0 | 4.4 | 20 | 87 | Similarity to Yjl083p | ||
| yil007c | 0 | 4.4 | 20 | 87 | Similarity to human proteosomal modulator subunit p27 | ||
| yer037w | 0 | 4.3 | 23 | 99 | Similarity to hypothetical protein YGL224c | ||
| ygr081c | 0 | 4.3 | 22 | 95 | Similarity to chicken myosin heavy chain Pir | ||
| ygl246c | 0 | 4.1 | 20 | 82 | Similarity to C. elegans dom-3 protein | ||
| yfl060c | SNO3 | 4.1 | 20 | 81 | Member of stationary phase-induced gene family | ||
| ydl105w | QRI2 | 4.1 | 20 | 81 | Protein of unknown function | ||
| ygl102c | 0 | 3.9 | 28 | 110 | Protein of unknown function | ||
| ylr181c | 0 | 3.8 | 20 | 76 | Protein of unknown function | ||
| yil040w | 0 | 3.8 | 20 | 75 | Similarity to T. brucei NADH | ||
| yer104w | 0 | 3.8 | 20 | 75 | Protein of unknown function | ||
| yjl104w | 0 | 3.7 | 24 | 89 | Similarity to C. elegans hypothetical protein F45G2.c | ||
| ybr013c | 0 | 3.7 | 20 | 74 | Protein of unknown function | ||
| ygl131c | 0 | 3.7 | 22 | 81 | Similarity to S. pombe hypothetical protein C3H1.12C | ||
| ygr126w | 0 | 3.6 | 20 | 72 | Similarity to hypothetical protein YPR156c | ||
| yjl109c | 0 | 3.6 | 20 | 72 | Similarity to ATPase Drs2p | ||
| yjr038c | 0 | 3.6 | 20 | 71 | Protein of unknown function | ||
| ygl079w | 0 | 3.5 | 83 | 290 | Protein of unknown function | ||
| yil019w | 0 | 3.4 | 20 | 67 | Has potential coiled-coil region, similarity to S. pombe hypothetical protein SPAC3F10 | ||
| ygl057c | 0 | 3.3 | 25 | 82 | Protein of unknown function | ||
| yel057c | 0 | 3.2 | 40 | 129 | Protein of unknown function | ||
| ylr003c | 0 | 3.2 | 20 | 64 | Protein of unknown function | ||
| ygl133w | 0 | 3.1 | 20 | 62 | Similarity to hypothetical protein YPL216w | ||
| ypl146c | 0 | 3.1 | 20 | 62 | Similarity to myosin heavy chain proteins | ||
| ycr013c | 0 | 3.1 | 20 | 62 | Similarity to M. leprae B1496_F1_41 protein | ||
| ymr255w | 0 | 3.1 | 123 | 380 | Protein of unknown function | ||
| ybr250w | 0 | 3.1 | 20 | 61 | Protein of unknown function | ||
| yfr013w | 0 | 3.1 | 20 | 61 | Similarity to YOL017w | ||
| ydl039c | 0 | 3.0 | 73 | 221 | Protein of unknown function | ||
| yfr024c | 0 | 3.0 | 20 | 60 | Similarity to Ysc84p, Rvs167p, Abp1p, and Sla1p | ||
| ykr088c | 0 | 3.0 | 20 | 60 | Similarity to B. subtilis spore germination protein II | ||
Indicates ORF number, and the asterisk (*) indicates ORFs that fall into multiple categories.
0 indicates no designation.
Ratio is respective to cells treated without toxin.
Hybridization signal given in arbitrary units of fluorescence studies.
Description of gene function according to the Yeast Protein Database.
Table 2.
Yeast genes repressed at least 3-fold upon AFB1 exposure compared to control (DMSO)
| Intensityd
|
|||||
|---|---|---|---|---|---|
| Database IDa | Geneb | Ratioc | DMSO | AFB1 | Functione |
| Metabolism | |||||
| yil154c* | IMP2 | -6.4 | 127 | 20 | Involved in control of mitochondrial sugar utilization |
| ykl174c* | 0 | -4.1 | 82 | 20 | Similarity to choline transport protein Hnm1p |
| yer052c | HOM3 | -3.9 | 77 | 20 | L-aspartate 4-P-transferase |
| yil116w | HIS5 | -3.9 | 77 | 20 | Histidinol-phosphate aminotransferase |
| yer061c* | CEM1 | -3.8 | 76 | 20 | Beta-keto-acyl-ACP synthase, mitochondrial |
| yjl068c | 0 | -3.8 | 75 | 20 | Similarity to human esterase D |
| ymr296c | LCB1 | -3.7 | 74 | 20 | Serine C-palmitoyl transferase subunit |
| ygr124w | ASN2 | -3.6 | 71 | 20 | Asparagine synthetase |
| yhr092c* | HXT4 | -3.5 | 69 | 20 | Moderate- to low-affinity glucose transporter, MFS |
| ydr242w | AMD2 | -3.4 | 87 | 26 | Amidase |
| ygl186c* | 0 | -3.3 | 103 | 31 | Member of the purine/cytosine permease family, MFS |
| yml070w* | DAK1 | -3.3 | 76 | 23 | Putative dihydroxyacetone kinase |
| ylr240w* | VPS34 | -3.3 | 65 | 20 | Phosphatidylinositol 3-kinase |
| yhr106w | TRR2 | -3.2 | 63 | 20 | Thioredoxin reductase |
| yer061c* | CEM1 | -3.8 | 76 | 20 | Beta-keto-acyl-ACP synthase, mitochondrial |
| Cell growth, cell division, and DNA synthesis | |||||
| yhr165c* | PRP8 | -5.2 | 104 | 20 | U5 snRNP protein, pre-mRNA splicing factor |
| yel032w | MCM3 | -3.9 | 77 | 20 | Replication initiation protein |
| yil047c* | SYG1 | -3.9 | 77 | 20 | Member of the major facilitator superfamily (MFS) |
| ybl105c* | PKC1 | -3.9 | 77 | 20 | Serine/threonine protein kinase |
| ycr088w | ABP1 | -3.7 | 74 | 20 | Actin-binding protein |
| ygl073w* | HSF1 | -3.7 | 73 | 20 | Heat shock transcription factor |
| Transcription | |||||
| ylr357w* | RSC2 | -5.2 | 104 | 20 | Component of abundant chromatin remodeling complex |
| yhr165c* | PRP8 | -5.2 | 104 | 20 | U5 snRNP protein, pre-mRNA splicing factor |
| ygl013c* | PDR1 | -3.7 | 74 | 20 | Transcription factor related to Pdr3p |
| ygl073w* | HSF1 | -3.7 | 73 | 20 | Heat shock transcription factor |
| ycl031c* | RRP7 | -3.2 | 96 | 30 | Involved in pre-rRNA processing and ribosome assembly |
| ymr219w | ESC1 | -3.1 | 279 | 91 | Establishes silent chromatin |
| ybr237w* | PRP5 | -3.0 | 73 | 24 | Pre-mRNA processing RNA-helicase |
| yjr017c | ESS1 | -3.0 | 136 | 45 | Processing/termination factor 1 |
| Protein synthesis | |||||
| yer117w | RPL23B | -3.6 | 83 | 23 | Ribosomal protein L23.e |
| ycl031c* | RRP7 | -3.2 | 96 | 30 | Involved in pre-rRNA processing and ribosome assembly |
| Protein destination | |||||
| ymr197c* | VTI1 | -12.2 | 243 | 20 | V-SNARE: involved in Golgi retrograde protein traffic |
| ybr283c | SSH1 | -8.6 | 171 | 20 | Involved in co-translational pathway of protein transport |
| ylr121c | YPS4 | -4.6 | 185 | 40 | Yapsin 4, Gpi-anchored aspartyl protease |
| ygr028w* | MSP1 | -4.6 | 91 | 20 | Intra-mitochondrial sorting protein, ATPase |
| ycl031c* | RRP7 | -3.2 | 96 | 30 | Involved in pre-rRNA processing and ribosome assembly |
| ybr237w* | PRP5 | -3.0 | 73 | 24 | Pre-mRNA processing RNA-helicase |
| ybr201w | DER1 | -3.0 | 60 | 20 | Involved in protein degradation in the ER |
| Transport facilitation | |||||
| yil088c | 0 | -4.8 | 96 | 20 | Similarity to members of the major facilitator superfamily (MFS) |
| yll055w | 0 | -4.5 | 107 | 24 | Similarity to Dal5p and members of the allantoate permease family, MFS |
| ykl174c* | 0 | -4.1 | 82 | 20 | Similarity to choline transport protein Hnm1p |
| yhr092c* | HXT4 | -3.5 | 69 | 20 | Moderate- to low-affinity glucose transporter, MFS |
| ygl186c* | 0 | -3.3 | 103 | 31 | Member of the purine/cytosine permease family, MFS |
| Intracellular transport | |||||
| ymr197c* | VTI1 | -12.2 | 243 | 20 | V-SNARE: involved in Golgi retrograde protein traffic |
| yil115c | NUP159 | -5.2 | 103 | 20 | Nuclear pore protein |
| ygr028w* | MSP1 | -4.6 | 91 | 20 | Intra-mitochondrial sorting protein, ATPase |
| ymr183c | SSO2 | -3.8 | 76 | 20 | Syntaxin (T-SNARE) |
| yhr092c* | HXT4 | -3.5 | 69 | 20 | Moderate- to low-affinity glucose transporter, MFS |
| ylr240w* | VPS34 | -3.3 | 65 | 20 | Phosphatidylinositol 3-kinase |
| ydr246w | TRS23 | -3.2 | 131 | 41 | Involved in targeting and fusion of ER to golgi transport vesicles |
| yhr156c | 0 | -3.2 | 64 | 20 | Weak similarity to mouse kinesin KIF3B |
| ycr032w | BPH1 | -3.2 | 63 | 20 | Probably involved in acetic acid export |
| Cellular biogenesis | |||||
| yil154c* | IMP2 | -6.4 | 127 | 20 | Involved in control of mitochondrial sugar utilization |
| ylr357w* | RSC2 | -5.2 | 104 | 20 | Component of abundant chromatin remodeling complex |
| Cellular communication/signal transduction | |||||
| yil047c* | SYG1 | -3.9 | 77 | 20 | Member of the major facilitator superfamily (MFS) |
| ybl105c* | PKC1 | -3.9 | 77 | 20 | Serine/threonine protein kinase |
| ylr240w* | VPS34 | -3.3 | 65 | 20 | Phosphatidylinositol 3-kinase |
| Cell rescue, defense, cell death and aging | |||||
| ymr173w | DDR48 | -4.9 | 98 | 20 | Heat shock protein, ATPase, Chaperon |
| yhl046c | 0 | -3.9 | 78 | 20 | Similarity to members of the Srp1p/Tip1p family |
| ybl105c* | PKC1 | -3.9 | 77 | 20 | Serine/threonine protein kinase |
| ygl013c* | PDR1 | -3.7 | 74 | 20 | Transcription factor |
| ygl073w* | HSF1 | -3.7 | 73 | 20 | Heat shock transcription factor |
| yml070w* | DAK1 | -3.3 | 76 | 23 | Putative dihydroxy acetone kinase |
| Unclassified | |||||
| ydr306c | 0 | -13.1 | 281 | 20 | Contains an f-box, weak similarity to S. pombe hypothetical protein SPAC6F6 |
| ypl247c | 0 | -5.4 | 262 | 49 | Similarity to human HAN11 protein and petunia an11 protein |
| yal031c | FUN21 | -5.2 | 103 | 20 | Protein of unknown function |
| ygr102c | 0 | -5.1 | 102 | 20 | Protein of unknown function |
| ymr115w | 0 | -4.6 | 91 | 20 | Similarity to YKL133c |
| yfl043c | 0 | -4.4 | 87 | 20 | No annotation |
| ydr229w | 0 | -4.2 | 137 | 33 | Protein of unknown function, possible coiled-coil protein |
| yil087c | 0 | -4.0 | 121 | 30 | Protein of unknown function |
| yil077c | 0 | -4.0 | 79 | 20 | Protein of unknown function |
| yml067c | 0 | -3.6 | 72 | 20 | Similarity to YAL042w |
| ybr137w | 0 | -3.5 | 84 | 24 | Protein of unknown function |
| ydl076c | 0 | -3.5 | 77 | 22 | Protein of unknown function |
| ygl045w | 0 | -3.5 | 69 | 20 | Protein of unknown function |
| yhr090c | NBN1 | -3.4 | 68 | 20 | Protein with effect on bem and rad phenotypes, similarity to YOR064c, YNL097c |
| ydr128w | 0 | -3.3 | 66 | 20 | Similarity to Sec27p, YMR131c and human retinoblastoma-binding protein |
| ykl204w | 0 | -3.3 | 66 | 20 | Protein of unknown function, probable purine nucleotide-binding protein |
| ygl082w | 0 | -3.3 | 69 | 21 | Similarity to hypothetical protein YPL191c |
| ypl170w | 0 | -3.2 | 85 | 27 | Similarity to C. elegans LIM homeobox protein |
| ypr063c | 0 | -3.2 | 63 | 20 | Protein of unknown function |
| ygl005c | 0 | -3.1 | 61 | 20 | Similarity to X. laevis kinesin-related protein Eg5 |
| ymr184w | 0 | -3.0 | 60 | 20 | Protein of unknown function |
| ycl044c | 0 | -3.0 | 60 | 20 | Protein of unknown function |
Indicates ORF number, and the asterisk (*) indicates ORFs that fall into multiple categories.
0 indicates no designation.
Ratio is respective to cells treated without toxin.
Hybridization signal given in arbitrary units of fluorescence.
Description of gene function according to the Yeast Protein Database.
Figure 2.
Functional (A) and cellular (B) classification of AFB1 responsive ORFs. Indicated are transcripts levels altered more than threefold (gray bars) and fourfold (black bars) after 4-h treatment with 25 μM AFB1. The amount of ORFs in the cellular distribution (B) is given as absolute numbers, and the ORFs per functional category (A) are given as percent of total genes assigned to the respective category. Categories are derived from the MIPS database (Mewes et al., 1997). Note that some ORFs fall into multiple categories. Values are means of two independent experiments.
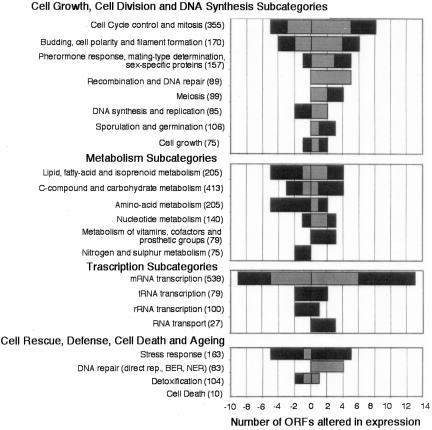
Figure 3.
Assignments of AFB1 responsive yeast genes to subcategories. Gray bars indicate ORFs showing an over threefold, black bars over fourfold change in expression. Categories and subcategories are derived from the MIPS database (Mewes et al., 1997). Values are means of two independent experiments.
Because the genotoxicity of AFB1 may result from AFB1-induced DNA damage, we identified AFB1-inducible DNA repair genes. Of 109 genes involved in DNA damage repair according to the Yeast Protein Database (Payne and Garrels, 1997), 15 (14%) were upregulated (RAD51, CDC2(POL3), DST1, RAD3, RSP5, RFA1, RAD16, MLH1, MMS21, DIN7, MET18, HPR5, RFA2, MSH6, RAD1) and 3 were repressed (DDR48, SIR4, DNL4) at least twofold. Furthermore, of the 16 genes assigned to specific repair pathways, 11 genes (69%) function in recombinational repair, 7 genes (44%) in nucleotide excision repair, and 4 genes function in both pathways. Only 2 of the repressed genes are not in either pathway but function in nonhomologous end joining (NHEJ; Table 3). Analysis of cell cycle periodicity of the repair genes showed that changes in expression levels after AFB1 exposure are not simply caused by changes in cell cycle progression (Keller-Seitz, 2001). Furthermore, 44 genes exhibiting at least a twofold or greater change in expression are involved in damage signaling, stress response, or cell cycle control (Table 4). Thus, AFB1 exposure induces DNA repair genes in NER, MMR, and recombinational DNA repair.
Table 3.
AFB1-responsive DNA repair genes showing a 2-fold or greater change in expression
| Gene namea
|
Intensityc
|
Other pathwaysf
|
||||||
|---|---|---|---|---|---|---|---|---|
| ORF | Ratiob | DMSO | AFB1 | NERd | RRe | Functiong | ||
| RAD51‡ | YER095W | 7.7 | 29 | 224 | + | Stimulates pairing and strand-exchange between homologous ssDNA and dsDNA, functionally similar to E. coli recA protein | ||
| POL3 | YDL102W | 5.0 | 21 | 104 | + | BER, MMR, TLS | DNA polymerase delta large subunit | |
| DST1 | YGL043W | 4.6 | 20 | 91 | + | TFIIS, DNA strand transfer protein catalyzing homologous DNA strand exchange | ||
| RAD3* | YER171W | 4.6 | 20 | 91 | + | + | DNA helicase, component of TFIIH | |
| RFA1† | YAR007C | 4.6 | 20 | 91 | + | + | DNA replication factor A, 69-kDa subunit | |
| RAD16*† | YBR114W | 4.5 | 38 | 169 | + | DNA helicase involved in G2 repair of inactive genes, member of the Snf2 (Swi2) protein family, recognizes transition between paired and unpaired DNA strands | ||
| MLH1 | YMR167W | 4.1 | 20 | 82 | + | MMR | MMR protein and homolog of E. coli mutL, shows anti-recombinase activity | |
| MMS21 | YEL019C | 2.9 | 30 | 87 | + | Involved in DNA repair | ||
| MET18* | YIL128W | 2.5 | 20 | 49 | + | Involved in NER and RNA polymerase II transcription | ||
| HPR5‡# | YJL092W | 2.4 | 59 | 140 | + | TLS, NHEJ | DNA helicase involved in DNA repair; suppressor of RAD6 and RAD18, has anti-recombinase function | |
| RFA2† | YNL312W | 2.2 | 22 | 48 | + | + | DNA replication factor A, 36-kDa subunit | |
| MSH6 | YDR097C | 2.2 | 20 | 43 | + | MMR | Part of DNA mismatch binding factor, involved in repair of single base mismatches | |
| RAD1*† | YPL022W | 2.1 | 20 | 41 | + | + | MMR | Homolog of human XP-F and mammalian ERCC-4 protein, acts in different recombination pathway than Rad52p |
| MLH3 | YPL164C | 2.0 | 20 | 40 | + | MMR | Interacts with Mlh1p and functions with Msh3p to suppress homologous recombination | |
| SIR4 | YDR227W | -2.1 | 47 | 22 | NHEJ | Silencing regulatory and DNA-repair coiled-coil protein | ||
| DNL4 | YOR005C | -2.0 | 42 | 21 | NHEJ | ATP-dependent DNA ligase IV; involved in nonhomologous end joining | ||
Assignment to specific repair pathways according to Friedberg et al. (1995) and the Yeast Protein Database (YPD). The symbols *, #, ‡ and † indicate membership in the RAD3, RAD6, RAD52 epistasis groups and NER repairosome, respectively.
Ratio is respective to cells treated without toxin.
Hybridization signal is given in arbitrary units of fluorescence.
Nucleotide excision repair.
Recombination repair.
Other repair pathways include base excision repair (BER), mismatch repair (MMR), translesion synthesis (TLS) and non-homologous end-joining (NHEJ).
Description of gene function according to the Yeast Protein Database.
Table 4.
AFB1-responsive genes involved in damage signaling, stress response, and cell cycle control.
| Intensityc
|
|||||
|---|---|---|---|---|---|
| Gene name | ORFa | Ratiob | DMSO | AFB1 | Functiond |
| SPO12 | YHR152W | 8.5 | 20 | 169 | Sporulation protein |
| RSR1 | YGR152C | 8.2 | 20 | 163 | GTP-binding protein of the ras superfamily |
| CBF2 | YGR140W | 6.6 | 20 | 132 | Subunit A of CBF3 kinetochore complex, required for cell cycle arrest at anaphase |
| BIM1 | YER016W | 6.2 | 20 | 124 | Microtubules-associated protein |
| SW14 | YER111C | 5.1 | 20 | 101 | Transcription factor that participates in the SBF complex (Swi4p—Swi6p) for regulation at the cell cycle box (CCB) element |
| CTK1 | YKL139W | 4.7 | 20 | 93 | Putative TOR downstream target, cyclin-dependent protein kinase that phosphorylates c-terminal domain of RNA polymerase II large subunit |
| GLO3 | YER122C | 4.1 | 20 | 82 | GAP involved in transition from stationary to proliferative phase |
| MGA1 | YGR249W | 3.9 | 20 | 78 | Similarity to heat shock transcription factor |
| YCK1 | YHR135C | 3.8 | 120 | 452 | Casein kinase I isoform, 41% similarity to Hrr25p |
| ? | YOR009W | 3.7 | 53 | 195 | Similarity to members of the PAU1 family, has stress-induced proteins SRP1/TIP1 family signature |
| MAD1 | YGL086W | 3.7 | 20 | 73 | Involved in TOR signaling, DNA damage-induced recombination, required for cell cycle delay in response to impaired kinetochore function |
| DDI1 | YER143W | 3.6 | 20 | 71 | Induced in response to DNA alkylation damage, gene contains a cis-acting element that regulates expression of MAG1 |
| SAP185 | YJL098W | 3.3 | 20 | 66 | Phosphatase Sit4p-associating protein, role in cell cycle control |
| CDC26 | YFR036W | 3.1 | 35 | 108 | Subunit of anaphase-promoting complex, heat inducible |
| RSP5 | YER125W | 3.1 | 40 | 123 | Ubiquitin-protein ligase, functions in the OLE1 activation pathway |
| CLB1 | YGR108W | 2.9 | 20 | 57 | Cyclin, G2/M-specific |
| BIK1 | YCL029C | 2.7 | 22 | 59 | Microtubule-associated protein required for microtubule function during mitosis and mating, interacts with Bim1p and Bub3p |
| RNH1 | YMR234w | 2.6 | 20 | 52 | Ribonuclease H, endonuclease that degrades RNA in RNA-DNA hybrids |
| DIN7 | YDR263C | 2.5 | 20 | 50 | DNA-damage inducible protein, production increases during meiosis at about the time of recombination, has similarity to human XPG protein (DNA-repair protein complementing XP-G cells) related to xeroderma pigmentosum group G and Cockayne's syndrome |
| PAK1 | YER129W | 2.4 | 20 | 48 | DNA polymerase alpha suppressing protein kinase |
| TOR1 | YJR066W | 2.3 | 20 | 45 | PI-3 kinase homolog, influences cell growth, DNA damage-induced recombination, G1-S checkpoint genes, potential targets: RPS6A+B, PHO85 |
| PTK1 | YKL198C | 2.2 | 35 | 78 | Serine/threonine protein kinase, acts in TOR signalling pathway |
| CTK3 | YML112W | 2.2 | 20 | 44 | C-terminal domain (CTD) kinase gamma subunit, associates with Ctk1p and Ctk2p |
| SPO16 | YHR153C | 2.2 | 20 | 43 | Sporulation protein |
| TOR2 | YKL203C | 2.1 | 29 | 61 | PI-4 kinase, involved in cell growth, DNA damage induced recombination, G1-S progression, related to Tor1p |
| PCL10 | YGL134W | 2.1 | 30 | 62 | Cyclin like protein interacting with Pho85p, putative TOR downstream target |
| PRP8 | YHR165C | -5.2 | 104 | 20 | U5 snRNA-associated splicing factor, component of the spliceosome |
| DDR48 | YMR173W | -4.9 | 98 | 20 | Stress protein induced by heat shock, DNA damage, or osmotic stress |
| ? | YHL046C | -3.9 | 78 | 20 | Similarity to members of the PAU1 family, repressed by TUP1 |
| MCM3 | YEL032W | -3.9 | 77 | 20 | Acts at ARS elements to initiate replication; member of the MCM/P1 family, mutant shows hyper-rec phenotype |
| PKC1 | YBL105C | -3.9 | 77 | 20 | Protein kinase C, regulates map kinase cascade involved in regulating cell wall metabolism, mutant shows hyper-rec phenotype |
| HSF1 | YGL073W | -3.7 | 73 | 20 | Heat shock transcription factor, induces DDR48 |
| DAK1 | YML070W | -3.3 | 76 | 23 | Dihydroxyacetone kinase, induced in high salt |
| VPS34 | YLR240W | -3.3 | 65 | 20 | PI-3 kinase, required for vacuolar protein sorting, activated by protein kinase Vps15p |
| PHO85 | YPL031C | -3.0 | 59 | 20 | Putative TOR downstream target, cyclin-dependent protein kinase that interacts with Pho80p-like cyclins to regulate phosphate pathway |
| TEC1 | YBR083W | -3.0 | 59 | 20 | Ty transcription activator, induces DDR48 and SPO12 |
| SPT6 | YGR116W | -2.7 | 54 | 20 | Transcription elongation protein involved in chromatin structure that influences expression of many genes, mutant shows hyper-rec phenotype |
| BCK2 | YER167W | -2.6 | 52 | 20 | Involved in the SIT4 pathway for CLN1-3 activation and in suppression of lethality due to mutations in the protein kinase C pathway |
| GAT1 | YFL021W | -2.6 | 51 | 20 | Involved in TOR signalling, gata zinc finger transcription factor that plays a supplemental role to Gln3p |
| TAF90 | YBR198C | -2.5 | 49 | 20 | TFIID and SAGA subunit, required for activated transcription by RNA polymerase II |
| SIT4 | YDL047w | -2.3 | 46 | 20 | Serine/threonine phosphatase involved in cell cycle regulation; member of the PPP family of protein phosphatases and related to PP2a phosphatase |
| SIR4 | YDR227W | -2.1 | 47 | 22 | Silencing regulatory and DNA-repair coiled-coil protein |
| FUN30 | YAL019W | -2.1 | 48 | 23 | Similarity to helicases of the Snf2 (Swi2) protein family, recognizes transition between paired and unpaired DNA strands |
ORFs showing a 2 or more fold change in expression are listed. Information on gene function and regulation was obtained from the sources indicated.
Indicates ORF number.
Ratio is respective to cells treated without toxin.
Hybridization signal is given in arbitrary units of fluorescence.
Description of gene function according to the Yeast Protein Database.
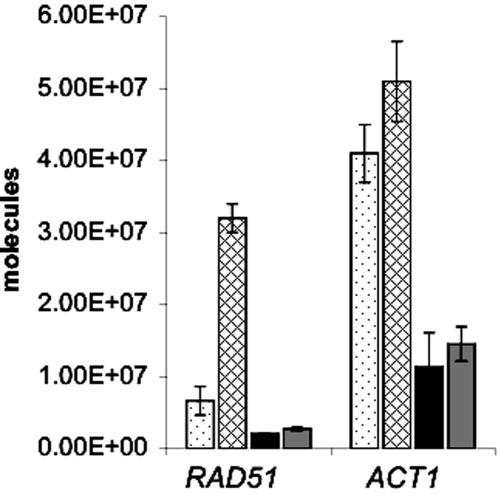
AFB1-inducible repair genes that function in multiple recombination pathways include RAD51 (7.7-fold increased) and RAD1 (2.1-fold increased). We confirmed that RAD51 RNA increases after AFB1 exposure by QPCR, using actin RNA as a control (Figure 4). YB110 (pCS316) was exposed to 25 μm AFB1 for 4 h, and RNA was extracted for QPCR. The amount of RAD51 RNA increased fivefold in YB110 cells treated with AFB1, whereas actin RNA did not significantly increase (Figure 4). However, we found that the amount of RAD1 mRNA increased less than twofold (unpublished data). The differences between the QPCR results and the microarray results are likely due to the greater sensitivity of the microarrays (Etienne et al., 2004).
Figure 4.
Quantitative RT-PCR of RAD51 and ACT1 RNA in MEC1 (YB110 pCS316) and mec1 (YB324 pCS316) strains after cells were exposed to 25 μM AFB1. RNA was extracted from cells after a 4-h exposure. The vertical axis indicates the number of RNA molecules per 10 ng of total RNA. Three independent experiments were performed. Dot-filled bars represent untreated wild-type (MEC1) cells. Diagonal-filled bars represent AFB1-treated wild-type cells. Black bars represent untreated mec1Δ cells, and gray bars represent AFB1-treated mec1Δ cells. mec1-21 (YB325 pCS316) cells gave similar results as the mec1 null mutant.
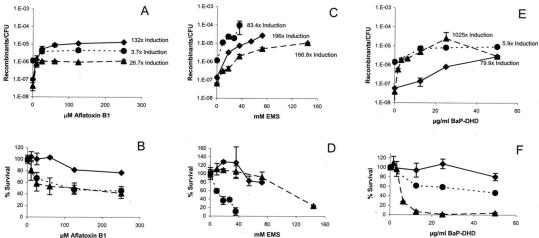
Although we did not prove that the induced expression of the NER genes and RAD51 is necessary for AFB1-associated recombination, we did use rad1 (YB150 pCS512) and rad51 mutant (YB195 pSB229) yeast strains to determine whether RAD1 or RAD51 function in AFB1-associated recombination and lethality (Figure 5). We measured the frequencies of AFB1-associated translocations by selecting for His+ recombinants as previously described (Fasullo and Davis, 1987). Besides AFB1, the carcinogen benzo-(a)-pyrene-7,8-dihydrodiol (BaP-DHD) and the mutagen ethyl methanesulfonate (EMS) were also tested. Although the viability of both mutant strains is slightly decreased after exposure to AFB1, the rad51 strain is hypersensitive to EMS and the rad1 strain is hypersensitive to BaP-DHD (Figure 5). Compared with the wild-type (Rad+) strain, the frequency of AFB1-associated translocations was slightly decreased in both the rad51 and rad1 mutants, whereas the frequency of EMS-associated recombination increased in the rad51 strain and the frequency of BaP-DHD–associated translocations was significantly higher in the rad1 mutant (Figure 5). At EMS concentrations greater than 40 mM, EMS-associated recombination could not be measured because of the extreme EMS toxicity. Because the frequencies of spontaneous recombination are (1.4 ± 0.4) × 10–7 and (3.8 ± 1.8) × 10–8 in the wild-type and rad1 strains, respectively, and low compared with the DNA damage–associated frequencies, the DNA damage–associated frequencies are similar to the net recombination frequencies. In the rad51 mutant the spontaneous frequency (avg.) was (1.3 ± 0.2) × 10–6, and thus the net recombination frequencies (avg.) for the highest level of AFB1-associated, EMS-associated, and BaP-DHD–associated translocations were 3.4 × 10–6, 96 × 10–6, 7.7 × 10–6, respectively. The highest net AFB1-associated frequency is still ∼25% lower in the rad51 diploid than in wild-type, whereas the highest net BaP-DHD–associated frequency was about threefold higher in the rad51 diploid than in wild type. These results indicate that RAD51 and RAD1 function in AFB1-associated recombination, whereas RAD51 and RAD1 suppress EMS-associated and BaP-DHD–associated recombination, respectively.
Figure 5.
Induction of recombination and drug killing by AFB1, EMS, and BaP-DHD in wild-type (♦), rad1 (▴), and rad51 (♦) yeast strains. The S. cerevisiae strains YB150pCS512 (rad1), YB195pSB229 (rad51), and the repair proficient strain YB110pSB229 (wt) all express hCYP1A1 and hOR for metabolic activation of the toxic compounds. Recombination frequency was determined as His+ recombinant/colony forming unit (CFU) and plotted against drug concentration. Survival percentage was calculated as the ratio of CFU after drug treatment to CFU before drug treatment multiplied by 100%. The ratio of the recombination frequencies obtained after drug exposure and before drug exposure, or fold induction, is indicated for selective drug concentrations. The means and standard deviations (bars) were calculated from two to five independent experiments. (A) and (B) Recombination frequencies and % survival after exposure to AFB1. (C) and (D) Recombination frequencies and % survival after exposure to EMS. (E) and (F) Recombination frequencies and % survival after exposure to BaP-DHD.
To further understand the correlation between RAD51 expression and AFB1-associated recombination, we measured translocation frequencies in mec1 checkpoint strains. mec1 checkpoint mutants are deficient in RAD51 induction after MMS and x-ray exposure (Gasch et al., 2001). We found that the mec1-21 (YB325) and mec1Δ::TRP1 (YB324) mutants cannot induce RAD51 levels after exposure to 25 μM AFB1 (Figure 4). The translocation frequency increased 26-fold after wild type (YB110 pCS316) was exposed to 25 μM AFB1, consistent with previous studies (Sengstag et al., 1996). We found no significant increase in translocation frequencies after both the mec1 deletion mutant (YB324 pCS316) and the mec1-21 mutant (YB325 pCS316) were exposed to 25 μM AFB1 (Table 5). However, because the sml1 null mutant exhibited a decrease in AFB1-associated recombination, the decrease in AFB1-associated recombination in the mec1 null mutant could be partially conferred by the sml1 mutation. To increase Rad51 in the mec1-21 strain (YB325), we introduced the 2 μ LEU2 plasmid (pR51.3; Sung and Stratton, 1996) containing RAD51 expressed from a strong constitutive PGK (phosphoglycerol kinase) promoter by selecting for Leu+ transformants. By QPCR, we found that the basal level of RAD51 RNA before and after AFB1 exposure in the Leu+ transformants was the same and more than a thousand fold higher than the basal level of RAD51 RNA in mec1-21 (YB325), whereas there was no change in ACT1 RNA levels (unpublished data). In YB325 (pR51.3) cells, translocation frequencies increased ninefold after AFB1 exposure. RAD51 overexpression in mec1-21 also increased lethality after AFB1 exposure, suggesting that other detrimental recombination events may also be generated. These data indicate that an increase in RAD51 expression can enhance AFB1-associated recombination in a strain deficient in the DNA damage inducibility of RAD51.
Table 5.
Translocation frequencies in wild-type, mec1, and mec1 (pR51.3) strains
| Translocation frequency (× 107)c
|
||||
|---|---|---|---|---|
| Genotype (strain)a | Survival (%)b | Spontaneousc | AFB1-associatedd | Fold increasee |
| MEC1 (YB110 pCS316) | 86 | 2.7 ± 1.2 | 69 ± 14 | 26 |
| mec1-21 (YB325 pCS316) | 80 | 24 ± 4 | 42 ± 14 | 1.8 |
| sml1::kanMX (YB323 pCS316) | 94 | 2.3 ± 1 | 10 ± 5 | 4.4 |
| mec1::TRP1 sml1::kanMX (YB324 pCS316) | 100 | 12 ± 9 | 15 ± 7 | 1.2 |
| mec1-21 (YB325 pCS316, pR51.3) | 55 | 49 ± 12 | 440 ± 250 | 9 |
See text for complete genotype.
CFU after 25 μm AFB1 exposure/CFU before exposure × 100%.
His+ recombinants/total CFU.
His+ recombinants after AFB1 exposure/total CFU after AFB1 exposure.
AFB1-associated translocation frequency/spontaneous translocation frequency.
DISCUSSION
AFB1 is a strong recombinagen but weak mutagen in S. cerevisiae. To elucidate the genotoxic properties of AFB1, we investigated the global transcriptional response of a diploid yeast strain after exposure to AFB1 using high-density oligonucleotide arrays. We determined the expression pattern of 6218 ORFs representing the entire yeast genome. Other studies have determined the global transcriptional responses after exposure to MMS and ionizing radiation; however, these studies have used haploid strains, rendering it difficult to compare data. Nonetheless, our results demonstrate that AFB1 exposure elicits a complex transcriptional response pattern. A large fraction of the responsive genes are involved in cell rescue, cell cycle control, and DNA repair; this latter category included genes involved in both excision and recombinational repair. Subsequent comparison of survival and translocation frequencies in rad51 and rad1 mutants after exposure to AFB1, EMS, and BaP-DHD indicate that the stimulation of recombination by different carcinogens requires different DNA repair genes.
The gene expression patterns reveal that AFB1-induced transcripts are not evenly distributed among yeast chromosomes (Figure 1). The differences ranged from ≥3-fold induction of 10% of ORFs on chromosome V to only 0.7% on chromosome XV. On the level of individual ORFs, a subset of genes that exhibit significant change in expression are closely linked; these include RAD3 (YER171W, 4.6-fold increased), which is located between ADK2 (YER170W, 5.1-fold increased) and BRR2 (YER172C, 2.2-fold increased), and RAD51 (YER095W, 7.7-fold increased), that is located close to UBP9 (YER098W, 7.4-fold increased) and SWI4 (YER111C, 5.1-fold increased). The linked ORFs are on different DNA strands (Goffeau et al., 1996), suggesting that changes in chromosome structure may alter expression of multiple genes. Hence, our data suggest that some DNA damage responsive genes might be organized as clusters in coregulated chromosomal regions.
The complex response pattern caused by AFB1 reflects the broad range of toxic effects in the cell; however, the pattern of gene expression after exposure to AFB1 does not reflect a general stress response or a general response to DNA damaging agents, such as alkylating agents (Jelinsky and Samson, 1999). Five stress response genes, DDR48, PAI3, YML131W, YKL100C, and YNL116W, that are upregulated after MMS exposure (Jelinsky and Samson, 1999) and saline stress conditions (Posas et al., 2000) are not induced after exposure to AFB1. The only gene assigned to the general stress response that was induced with MMS (17.8-fold) and AFB1 (2.6-fold) was the DNA damage-inducible gene DDI1. It is unlikely that the AFB1 solvent DMSO triggered the general stress response, because the only difference between the gene expression patterns after exposure to DMSO and H2O was the DMSO-dependent induction of YML131W. In addition, RNR1, which is generally induced after exposure alkylating agents and UV (Elledge and Davis, 1990), was not induced after exposure to AFB1. We therefore suggest that the gene expression pattern of the cells exposed to AFB1 did not result from a general stress response but from specific AFB1-induced toxicity, such as AFB1-induced DNA damage.
The gene expression patterns may provide further insights into the recombinogencity of AFB1. Many of the responsive genes are directly or indirectly involved in recombination (reviewed in Aguilera et al., 2000; Nicholson et al., 2000). Several are also induced after diploid cells are exposed to γ rays; these include RAD51, SRS2, RFA1, RFA2, and MSH6 (Mercier et al., 2001). Among the AFB1-inducible DNA repair genes, RAD51 (7.7-fold increased) exhibited the strongest induction. The proteins encoded by the AFB1-inducible genes RFA1 and RFA2 are subunits of the replication factor A (RPA) and promote Rad51-stimulated DNA pairing and strand exchange in vitro (Sung, 1994) by removing secondary DNA structures (Sung and Robberson, 1995). Interestingly, numerous genes of the NER pathway were induced after AFB1 exposure; these included RAD1, RAD3, RAD16, and MET18. RAD1 functions in several RAD51-independent mitotic recombination events (Davies et al., 1995; Saparbaev et al., 1996; Paques and Haber, 1999; Aguilera et al., 2000; Haber, 2000; Nicholson et al., 2000) as well as in the spontaneous generation of homology-directed translocations (Fasullo et al., 1998).
Several genes that are upregulated after AFB1 exposure also function to decrease particular recombination events. For example, the Hpr5/Srs2 helicase is suggested to function as an antirecombinase preventing excessive and aberrant RAD51-mediated recombination events (Klein, 2000). In addition, some genes of the MMR pathway, including MLH1, MLH3, and MSH6, were induced, and Mlh1 and Mlh3 can both reduce recombination between repeated sequences containing mismatches (Nicholson et al., 2000). Although the induction of these genes may seem contradictory to the notion that AFB1 stimulates recombination, HPR5/SRS2 is also upregulated in meiosis during which higher levels of heteroallelic and ectopic recombination occur. Mitotic, heteroallelic recombination is not decreased in mismatch repair mutants (Saparbaev et al., 1996), and msh2 mutants do not exhibit decreased mitotic recombination between his3 fragments positioned on different chromosomes (unpublished data). Yeast Mlh1 interacts with Sgs1, a protein encoded by a human BLM homologue (Foury, 1997), and may be involved in some aspect of general recombination (Pedrazzi et al., 2001). We also speculate that the induction of the mismatch repair proteins may contribute to the weak mutagenicity of AFB1. Thus, the upregulation of MSH6 and HPR5 is consistent with the genotoxic properties of AFB1.
Besides genes encoding DNA repair functions, genes involved in damage signaling, stress response, and cell cycle progression were also upregulated after AFB1 exposure. Overexpression of SPO12, which was strongly induced (8.5-fold) after AFB1 exposure, is thought to reduce cyclin-dependent kinase activity and trigger exit from mitosis (Shah et al., 2001). The observation that 9 (TOR1, TOR2, CTK1, MAD1, PTK1, PCL10, VPS34, PHO85, GAT1) of 43 genes displaying at least twofold change in expression have functions in TOR (target of rapamycin) signaling suggests a role of this pathway in response to AFB1 toxicity. Genes involved in TOR signaling, including VPS34 (phosphatidylinositol 3-kinase), are involved in regulatory mechanisms modulating protein synthesis and degradation and are important for promoting growth (Keith and Schreiber, 1995; Thomas and Hall, 1997; Dennis et al., 1999). The significance of these genes may be further elucidated when specific cell cycle checkpoints are identified that are triggered by AFB1 exposure.
The downregulation of some genes may also function to increase the recombinogenicity of AFB1-induced DNA lesions. For example, the gene encoding protein kinase C (PKC1) is downregulated (3.9-fold decreased); null pkc1 mutants are inviable and arrest during S phase, whereas other mutations in PKC1 confer a hyper-recombinogenic phenotype (Huang and Symington, 1994). Mutations in two other AFB1 downregulated signaling genes, MCM3 and SPT6, also confer hyper-recombinogenic phenotypes (Aguilera et al., 2000). These data provide further evidence that the downregulation as well as the upregulation of specific genes may contribute to the recombinogenic cellular response to AFB1.
The mechanism by which the AFB1-induced changes in gene expression increase recombination is unknown. However, these changes may aid in identifying genes that contribute to the genotoxicity of AFB1, compared with other DNA-damaging agents. For example, AFB1-associated recombination depends on the function of several AFB1-inducible genes, such as RAD1 and RAD51. We demonstrated that higher levels of RAD51 message correlates with higher frequencies of AFB1-associated translocations in checkpoint mutants deficient in RAD51 induction. In mammalian cells overexpression of RAD51 also increases the frequency of chromosomal rearrangements and translocations (Richardson et al., 2004). Thus, an increase in RAD51 levels in particular mammalian or yeast cells may increase recombination. Considering that mutations in upstream regulatory regions of other DNA damage-inducible genes, such as RAD54, do not confer a decrease in either radiation resistance or recombination (Cole and Mortimer, 1989), further experiments are necessary to understand whether RAD51 induction per se is required for AFB1-associated translocations.
We had previously observed that rad51 diploid mutants exhibit 30- and 10-fold higher frequencies of translocations after x-ray and UV exposure, respectively (Fasullo et al., 2001), whereas, rad1 mutants exhibit decreased frequencies of x-ray–associated translocations (Fasullo et al., 1998), compared with wild type. Higher frequencies of x-ray–associated translocations were detected in rad51 mutants even when survival was low (Fasullo et al., 2001). Thus, it is unlikely that AFB1-induced lethality caused the recombination defect. Most His+ recombinants generated in the rad51 mutant contained nonreciprocal translocations; whereas the majority of translocations identified after AFB1 and UV exposure in wild-type strains are reciprocal translocations. Nonreciprocal translocations may occur by break-induced replication (BIR) when a DNA polymerase replicates past a single-strand nick or when chromosomal fragments are inherited in subsequent divisions (Fasullo et al., 1998). Considering that we are unable to detect chromosomal fragments after AFB1 exposure, we speculate that AFB1 lesions do not trigger replication fork collapse.
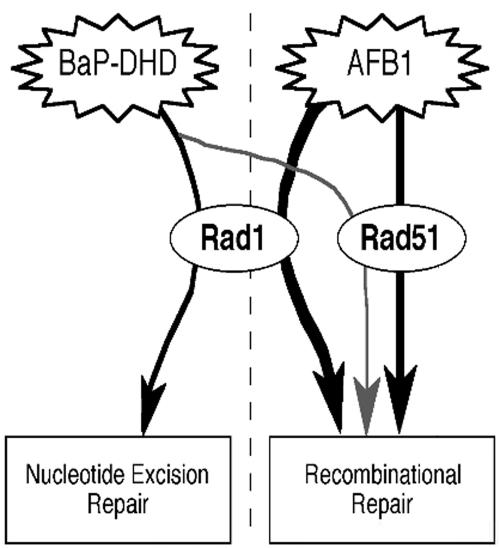
RAD1 and RAD51 play a different function in EMS or BaP-DHD–associated recombination (Figure 6). Although DNA damage generated by alkylating agents, such as EMS, is mainly repaired by BER (Friedberg et al., 1995), DNA damage that results from agents that form bulky adducts, such as BaP-DHD, is mainly repaired by NER (Hess et al., 1997). rad1 mutants are more sensitive to AFB1 than to EMS and are extremely sensitive to BaP-DHD; this suggests that NER is not the main pathway or is redundant in the repair of AFB1 lesions. We speculate that AFB1-induced lesions may require RAD1 to either initiate or process a recombination intermediate; RAD1-dependent recombination pathways have been extensively demonstrated by different groups (for review see Aguilera et al., 2000; Haber, 2000; Sung et al., 2000) and may participate in crossing-over (Symington et al., 2000). Interestingly, BaP-DHD is more recombinogenic in rad1 mutants than in wild-type strains, suggesting that more recombination events occur when the bulky adduct is not excised.
Figure 6.
Role of RAD1 and RAD51 in the different repair pathways for DNA damage caused by AFB1, EMS, and BaP-DHD. Models are derived from the results obtained with rad1 and rad51 mutants. The repair of AFB1-induced DNA damage involves both RAD1 and RAD51, and RAD1 is required for recombinational repair of AFB1-induced DNA damage. RAD1 is required for the excision of BaP-DHD–induced DNA adducts, and rad1 mutants exhibit higher frequencies of BaP-DHD–associated translocations, possibly due to the persistence of BaP-DHD–induced DNA adducts.
Similarly, rad51 mutants are more sensitive to EMS than to AFB1, indicating that RAD51 is involved in repair of AFB1 lesions, but more important in the repair of EMS lesions. The stimulation of recombination by particular alkylating agents, such as EMS, is dependent on cell division, suggesting that EMS-associated recombination occurs after the DNA polymerase encounters the unrepaired lesion (Galli and Schiestl, 1999; Aguilera et al., 2000). The hypersensitivity of the rad51 strain to EMS likely results from the rad51 defect in double-strand break repair; we speculate that double-strand breaks could be generated during BER if a DNA polymerase transverses a single-strand nick or gap and could stimulate a BIR mechanism. Additional experiments would be necessary to demonstrate that BER is a mechanism for generating more EMS-induced translocations in rad51 mutants.
DNA damage and the subsequent repair are thought to account largely for the carcinogenicity of AFB1. Recombinogenicity of a genotoxin could be pivotal in carcinogenesis as demonstrated by negative results in several mutagenesis tests (Schiestl, 1989; Galli and Schiestl, 1995, 1998). Results shown here help elucidate mechanisms by which changes in gene expression contribute to the genotoxicity of a compound. It will be interesting to investigate whether AFB1 also changes the gene expression of orthologous genes in mammalian cells.
Acknowledgments
We thank B. Weibel and H. Chen for excellent technical support. This work was supported by Grant 0-200-96 from the Swiss Federal Institute of Technology Zurich to C.S. and by Grant CA70105 to M.T.F. We thank Cinzia Cera for carefully reading this manuscript and Fumin Tong for advice concerning QPCR.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E04–05–0375. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–05–0375.
References
- Aguilera, A., Chavez, S., and Malagon, F. (2000). Mitotic recombination in yeast: elements controlling its incidence. Yeast, 16, 731–754. [DOI] [PubMed] [Google Scholar]
- Bailey, G.S. (1994). Role of aflatoxin-DNA adducts in the cancer process. In: The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance, ed. D.L. Eaton and J.D. Groopmann, San Diego: Academic Press, 137–148.
- Buss, P., Caviezel, M., and Lutz, W.K. (1990). Linear dose-response relationship for DNA adducts in rat liver from chronic exposure to aflatoxin B1. Carcinogenesis 11, 2133–2135. [DOI] [PubMed] [Google Scholar]
- Cole, G.M., and Mortimer, R.K. (1989). Failure to induce a DNA repair gene, RAD54, in Saccharomyces cerevisiae does not affect DNA repair or recombination phenotypes. Mol. Cell. Biol. 9, 3314–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy, R.G., Essigmann, J.M., Reinhold, V.N., and Wogan, G.N. (1978). Identification of the principal aflatoxin B1-DNA adduct formed in vivo in rat liver. Proc. Natl. Acad. Sci. USA 75, 1745–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy, R.G., and Wogan, G.N. (1981). Quantitative comparison of covalent aflatoxin-DNA adducts formed in rat and mouse livers and kidneys. J. Natl. Cancer Inst. 66, 761–768. [PubMed] [Google Scholar]
- Davies, A.A., Friedberg, E.C., Tomkinson, A.E., Wood, R.D., and West, S.C. (1995). Role of the Rad1 and Rad10 proteins in nucleotide excision repair and recombination. J. Biol. Chem. 270, 24638–24641. [DOI] [PubMed] [Google Scholar]
- Dennis, P.B., Fumagalli, S., and Thomas, G. (1999). Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr. Opin. Genet. Dev. 9, 49–54. [DOI] [PubMed] [Google Scholar]
- Dong, Z., and Fasullo, M. (2003). Multiple recombination pathways for sister chromatid exchange in Saccharomyces cerevisiae: role of RAD1 and the RAD52 epistasis group genes. Nucleic Acids Res. 10, 2576–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, D.L., and Gallagher, E.P. (1994). Mechanisms of aflatoxin carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 34, 135–172. [DOI] [PubMed] [Google Scholar]
- Elledge, S.J., and Davis, R.W. (1990). Two genes differentially regulated in the cell cycle and by DNA damaging agents encode alternative regulatory subunits of ribonucleotide reductase. Genes Dev. 4, 740–751. [DOI] [PubMed] [Google Scholar]
- Essigmann, J.M., Croy, R.G., Nadzan, A.M., Busby, W.F.J., Reinhold, V.N., Buchi, G., and Wogan, G.N. (1977). Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc. Natl. Acad. Sci. USA 74, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne, W., Meyer, M.H., Peppers, J., and Meyer, R.A. (2004). Comparison of mRNA gene expression by RT-PCR and DNA microarray. Biotechniques 36, 618–626. [DOI] [PubMed] [Google Scholar]
- Eugster, H.P., Bärtsch, S., Würgler, F.E., and Sengstag, C. (1992). Functional co-expression of human oxidoreductase and cytochrome P450 1A1 in Saccharomyces cerevisiae results in increased EROD activity. Biochem. Biophys. Res. Commun. 185, 641–647. [DOI] [PubMed] [Google Scholar]
- Fasullo, M., and Dave, P. (1994). Mating type regulates the radiation-associated stimulation of reciprocal translocation events in Saccharomyces cerevisiae. Mol. Gen. Genet. 243, 63–70. [DOI] [PubMed] [Google Scholar]
- Fasullo, M.T., and Davis, R.W. (1987). Recombination substrates designed to study recombination between unique and repetitive sequences in vivo. Proc. Natl. Acad. Sci. USA 84, 6215–6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasullo, M.T., Bennett, T., AhChing, P., and Koudelik, J. (1998). The Saccharomyces cerevisiae RAD9 checkpoint reduces the DNA damage-associated stimulation of directed reciprocal translocations. Mol. Cell. Biol. 18, 1190–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasullo, M.T., Giallanza, P., Bennett, T., Cera, C., and Dong, Z. (2001). Saccharomyces cerevisiae rad51 mutants are defective in DNA damage-stimulated sister chromatid exchange but exhibit increase rates of homology-directed translocations. Genetics 158, 959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foury, F. (1997). Human genetic diseases: a cross-talk between man and yeast. Gene 195, 1–10. [DOI] [PubMed] [Google Scholar]
- Friedberg, E.C., Walker, G.C., and Siede, W. (1995). DNA Repair and Mutagenesis, Washington, DC: ASM Press.
- Galli, A., and Schiestl, R.H. (1995). Salmonella test positive and negative carcinogens show different effects on intrachromosomal recombination in G2 cell cycle arrested yeast cells. Carcinogenesis 16, 659–663. [DOI] [PubMed] [Google Scholar]
- Galli, A., and Schiestl, R.H. (1998). Effect of Salmonella assay negative and positive carcinogens on intrachromosomal recombination in S-phase arrested yeast cells. Mutat. Res. 419, 53–68. [DOI] [PubMed] [Google Scholar]
- Galli, A., and Schiestl, R.H. (1999). Cell division transforms mutagenic lesions into deletion-recombinagenic lesions in yeast cells. Mutat. Res. 429, 13–26. [DOI] [PubMed] [Google Scholar]
- Gasch, A., Spellman, P., Kao, C., Carmel-Harel, O., Eisen, M., Storz, G., Botstein, D., and Brown, P.O. (2000). Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11, 4241–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch, A., Huang, M., Metzner, S., Botstein, D., Elledge, S., and Brown, P. (2001). Genomic expression responses to DNA-damaging agents and the regulatory role of the yeast ATR homolog Mec1p. Mol. Biol. Cell 12, 2987–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffeau, A. et al. (1996). Life with 6000 genes. Science 274, 563–547. [DOI] [PubMed] [Google Scholar]
- Goldstein, A.L., and McCusker, J.H. (1999). Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15, 1541–1553. [DOI] [PubMed] [Google Scholar]
- Haber, J.E. (2000). Recombination: a frank view of exchanges and vice versa. Curr. Opin. Cell. Biol. 12, 286–292. [DOI] [PubMed] [Google Scholar]
- Hess, M.T., Gunz, D., Luneva, N., Geacintov, N.E., and Naegeli, H. (1997). Base pair conformation-dependent excision of benzo[a]pyrene diol epoxideguanine adducts by human nucleotide excision repair enzymes. Mol. Cell. Biol. 17, 7069–7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, I.C., Metcalf, R.A., Sun, T., Welsh, J.A., Wang, N.J., and Harris, C.C. (1991). Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature 350, 427–428. [DOI] [PubMed] [Google Scholar]
- Huang, K., and Symington, L. (1994). Mutation of the gene encoding protein kinase C1 stimulates mitotic recombination in Saccharomyces cerevisiae. Mol. Cell. Biol. 14, 6039–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinsky, S.A., and Samson, L.D. (1999). Global response of Saccharomyces cerevisiae to an alkylating agent. Proc. Natl. Acad. Sci. USA 96, 1486–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith, C.T., and Schreiber, S.L. (1995). PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science 270, 50–51. [DOI] [PubMed] [Google Scholar]
- Keller-Seitz, M. (2001). PhD thesis no. 14321. Swiss Federal Institute of Technology (ETH).
- Klebe, R.J., Harriss, J.V., Sharp, Z.D., and Douglas, M.G. (1983). A general method for polyethylene-glycol-induced genetic transformation of bacteria and yeast. Gene 25, 333–341. [DOI] [PubMed] [Google Scholar]
- Klein, H.L. (2000). A radical solution to death. Nat. Genet. 25, 132–134. [DOI] [PubMed] [Google Scholar]
- Leadon, S.A., Tyrrell, R.M., and Cerutti, P.A. (1981). Excision repair of aflatoxin B1-DNA adducts in human fibroblasts. Cancer Res. 41, 5125–5129. [PubMed] [Google Scholar]
- Lin, J.-K., Miller, J.A., and Miller, E.C. (1977). 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions in rat liver in vivo. Cancer Res. 37, 4430–4438. [PubMed] [Google Scholar]
- Martin, C.N., and Garner, R.C. (1977). Aflatoxin B1-oxide generated by chemical or enzymatic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature 267, 863–865. [DOI] [PubMed] [Google Scholar]
- Mercier, G., Denis, Y., Marc, P., Picard, L., and Dutriex, M. (2001). Transcriptional induction of repair genes during slowing of replication in irradiated Saccharomyces cerevisiae. Mutat. Res. 487, 157–172. [DOI] [PubMed] [Google Scholar]
- Mewes, H.W., Albermann, K., Heumann, K., Liebl, S., and Pfeiffer, F. (1997). MIPS: a database for protein sequences, homology data and yeast genome information. Nucleic Acids Res. 25, 28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A., Hendrix, M., Jinks-Robertson, S., and Crouse, G.F. (2000). Regulation of mitotic homeologous recombination in yeast. Functions of mismatch repair and nucleotide excision repair genes. Genetics 154, 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otteneder, M., and Lutz, W.K. (1999). Correlation of DNA adduct levels with tumor incidence: carcinogenic potency of DNA adducts. Mutat. Res. 424, 237–247. [DOI] [PubMed] [Google Scholar]
- Paques, F., and Haber, J.E. (1999). Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Res. 63, 349–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, W.E., and Garrels, J.I. (1997). Yeast Protein database (YPD): a database for the complete proteome of Saccharomyces cerevisiae. Nucleic Acids Res. 25, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzi, G., et al. (2001). Direct association of Bloom's syndrome gene product with the human mismatch repair protein. MLH1. Nucleic Acids Res. 29, 4378–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas, F., Chambers, J.R., Heyman, J.A., Hoeffler, J.P., de Nadal, E., and Arino, J. (2000). The transcriptional response of yeast to saline stress. J. Biol. Chem. 275, 17249–17255. [DOI] [PubMed] [Google Scholar]
- Richardson, C., Stark, J.M., Ommundsen, M., and Jasin, M. (2004). Rad51 overexpression promotes alternative double-strand break repair pathways and genome instability. Oncogene 23, 546–553. [DOI] [PubMed] [Google Scholar]
- Sanchez, Y., Desany, B.A., Jones, W.J., Liu, Q., Wang, B., and Elledge, S.J. (1996). Regulation of RAD53 by the ATM-like kinases MEC1 and TEL1 in yeast cell cycle checkpoint pathways. Science 27, 357–360. [DOI] [PubMed] [Google Scholar]
- Saparbaev, M., Prakash, L., and Prakash, S. (1996). Requirement of mismatch repair genes MSH2 and MSH3 in the RAD1-RAD10 pathway of mitotic recombination in Saccharomyces cerevisiae. Genetics 142, 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R.H. (1989). Nonmutagenic carcinogens induce intrachromosomal recombination in yeast. Nature 337, 285–288. [DOI] [PubMed] [Google Scholar]
- Sengstag, C., Weibel, B., and Fasullo, M. (1996). Genotoxicity of aflatoxin B1: evidence for a recombination-mediated mechanism in Saccharomyces cerevisiae. Cancer Res. 56, 5457–5465. [PMC free article] [PubMed] [Google Scholar]
- Shah, R. Jensen, S., Frenz., L. M., Johnson, A.L., and Johnston, L.H. (2001). The Spo12 protein of Saccharomyces cerevisiae: regulator of mitotic exit whose cell cycle-dependent degradation is mediated by the anaphase-promoting complex. Genetics 159, 965–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, H.M., and Ong, C.N. (1996). Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat. Res. Rev. Genet. Toxicol. 366, 23–44. [DOI] [PubMed] [Google Scholar]
- Shirra, M.K., Patton-Vogt, J., Ulrich, A., Liuta-Tehlivets, O., Kohlwein, S.D., Henry, S.A., and Arndt, K.M. (2001). Inhibition of acetyl coenzyme A carboxylase activity restores expression of the INO1 gene in a snf1 mutant strain of Saccharomyces cerevisiae. Mol. Cell. Biol. 21, 5710–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smela, M.E., Currier, S.S., Bailey, E.A., and Essigmann, J.M. (2001). The chemistry and biology of aflatoxin B 1, from mutational spectrometry to carcinogenesis. Carcinogenesis 22, 535–545. [DOI] [PubMed] [Google Scholar]
- Stettler, P., and Sengstag, C. (2001). Liver carcinogen aflatoxin B1 as an inducer of mitotic recombination in a human cell line. Mol. Carcinog. 31, 125–138. [DOI] [PubMed] [Google Scholar]
- Sung, P. (1994). Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science, 265, 1241–1243. [DOI] [PubMed] [Google Scholar]
- Sung, P., and Robberson, D.L. (1995). DNA strand exchange mediated by a RAD51-ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82, 453–461. [DOI] [PubMed] [Google Scholar]
- Sung, P., and Stratton, S.A. (1996). Yeast Rad51 recombinase mediates polar DNA strand exchange in the absence of ATP hydrolysis. J. Biol. Chem. 271, 27983–27986. [DOI] [PubMed] [Google Scholar]
- Sung, P., Trujillo, K.M,. and Van Komen, S. (2000). Recombination factors of Saccharomyces cerevisiae. Mutat. Res. 451, 257–275. [DOI] [PubMed] [Google Scholar]
- Symington, L., Kang, L., and Moreau, S. (2000). Alteration of gene conversion tract length and associated crossing over during plasmid gap repair in nuclease-deficient strains of Saccharomyces cerevisiae. Nucleic Acids Res. 28, 4649–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G., and Hall, M.N. (1997). TOR signaling and control of cell growth. Curr. Opin. Cell Biol. 9, 782–787. [DOI] [PubMed] [Google Scholar]
- Wodicka, L., Dong, H., Mittmann, M., Ho, M.H., and Lockhart, D.J. (1997). Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat. Biotechnol. 15, 1359–1367. [DOI] [PubMed] [Google Scholar]
- Wogan, G.N. (1999). Aflatoxin as a human carcinogen. Hepatology 30, 573–575. [DOI] [PubMed] [Google Scholar]
- Zhao, X., Muller, E.G., and Rothstein, R. (1998). A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2, 329–340. [DOI] [PubMed] [Google Scholar]
- Zhao, X., Georgieva, B., Chabes, A., Domkin, V., Ippel, J.H., Schleucher, J., Wijmenga, S., Thelander, L. (2000). Mutational and structural analyses of the ribonucleotide reductase inhibitor Sml1 define its Rnr1 interaction domain whose inactivation allows suppression of mec1 and rad53 lethality. Mol. Cell. Biol. 20, 9076–9083. [DOI] [PMC free article] [PubMed] [Google Scholar]