Abstract
Spodoptera exigua, which feeds on various crops worldwide, has natural enemies that are susceptible to the insecticides used against S. exigua. We investigate the toxicity and residue risk of 9 insecticides on the development of H. axyridis, C. sinica, S. manilae and T. remus. S. manilae and T. remus adults were sensitive to all 9 insecticides (LC50 less than 2.75 mg a.i. liter−1), while H. axyridis and C. sinica adults were less sensitive (LC50 between 6 × 10−5 mg a.i. liter−1 and 78.95 mg a.i. liter−1). Emamectin benzoate, spinosad, indoxacarb, alpha-cypermethrin, chlorfenapyr and chlorantraniliprole showed no toxicity on H. axyridis, C. sinica, S. manilae and T. remus pupae with the recommended field concentrations. The risk analysis indicated that chlorantraniliprole is harmless to larvae of four natural enemies and adult of H. axyridis, C. sinica and S. manilae. Emamectin benzoate and spinosad had higher safety to the development of H. axyridis, C. sinica, S. manilae and T. remus with the risk duration less than 4d. Indoxacarb, tebufenozide, chlorfenapyr, methomyl, alpha-cypermethrin and chlorpyrifos showed dangerously toxic and long risk duration on S. manilae and T. remus adults.
The beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), is a polyphagous insect feeding on various agricultural crops including vegetables, cotton, and ornamentals1. They have spread rapidly in the tropical and sub-tropical regions, due to their migratory habit and world-wide distribution of available host plants2,3. The use of insecticides remains the major strategy in dealing with S. exigua as it is quick, cost-effective and effective. However, lethal and sub-lethal effects of broad-spectrum insecticides may impact beneficial species4,5. Misuse of insecticides might account for pest outbreaks because extensive and intensive insecticides applied to target pests and may accelerate resistance development. Another important factor is that insecticides may indiscriminately kill natural enemies. In fact, natural enemies tend to be more susceptible to insecticides than pests6,7.
Insecticides and biological control are two important management strategies. Integrating these two strategies is important for the success of any management program8. Insecticides should be only used when necessary and be least disruptive to biological control9. Knowing the impact of insecticides on natural enemies is essential for integration of these two strategies10. It is essential to understand the dynamics of integrating prudently used chemical pesticides along with biological control organisms for the effective implementation of an IPM strategy8,10.
Harmonia axyridis (Pallas), (Coleoptera: Coccinellidae) and Chrysoperla sinica (Tjeder), (Neuroptera: Chrysopidae) are predator with a wide host range, attacking aphids, eggs and larvae of Lepidoptera including S. exigua, the two predators are an important component of natural and agricultural habitats11,12. Snelleniua manilae (Ashmead) and Telenomus remus Nixon (Hymenoptera: Scelionidae) are larvae and egg parasitoid, respectively, both S. manilae adults and T. remus adults have control ability against S. exigua13,14,15,16. Two predators and two parasitoids have potential as biological agents for S. exigua suppression in the field.
However, insecticide use can greatly suppress the populations of natural enemies, lowering their biological control potential17,18. Presently, relatively little is known about the susceptibility of four natural enemies to currently popular insecticides, which are being investigated as potential alternatives to organophosphate insecticides. Information on the relative toxicity of various insecticides to four natural enemies H. axyridis, C. sinica, S. manilae and T. remus can aid in the development of an integrated pest management strategy for S. exigua.
Results
Toxicity to three H. axyridis developmental life stages
The LC50 values were significantly different for different developmental life stages. For H. axyridis larvae, The LC50 values of selected insecticides to T. nubilale ranged from 0.015 to 13.31 mg a.i. liter−1. The order of toxicity (high-low) for the 9 insecticides was as follows: methomyl > indoxacarb > tebufenozide > alpha-cypermethrin > chlorpyrifos > chlorantraniliprole, spinosad ≥ chlorfenapyr, emamectin benzoate (LC50 values with overlapping the 95% confidence intervals were classified as having the same level of toxicity) (Table 1). For H. axyridis pupae there was no significant lethal effect at the recommended field application rate of the insecticides except chlorpyrifos, tebufenozide and methomyl (LC50: 527.68, 350.08 and 314.75 mg a.i. liter−1, respectively) (Table 3). For adult H. axyridis, chlorpyrifos displayed the highest toxicity (LC50: 0.04 mg a.i. liter−1 at 24 h). Tebufenozide, chlorfenapyr and alpha-cypermethrin displayed higher toxicity (LC50 between 0.48 and 1.91 mg a.i. liter−1, respectively). Spinosad, indoxacarb, methomyl, chlorantraniliprole and emamectin benzoate had slower toxicity (LC50 between 16.3 and 22.52 mg a.i. liter−1) (Table 1).
Table 1. Toxicity of insecticides on H. axyridis, C. sinica and T. remus larvae and H. axyridis, C. sinica, S. manilae and T. remus adult.
| Natural enemy | Insecticides | Slope ± SE | LC50 (mg a.i. liter−1) | 95% confidence limits | RQa | Categoryb | |
|---|---|---|---|---|---|---|---|
| H. axyridis | Larvae | emamectin benzoate | 1.68 ± 0.20 | 13.31 | 10.75~16.86 | 0.17 | 1 |
| spinosad | 1.63 ± 0.20 | 6.11 | 4.90~7.92 | 2.45 | 1 | ||
| indoxacarb | 2.06 ± 0.23 | 0.033 | 0.027~0.040 | 681.82 | 2 | ||
| chlorpyrifos | 2.20 ± 0.25 | 2.06 | 1.72~2.56 | 291.26 | 2 | ||
| alpha-cypermethrin | 1.89 ± 0.21 | 0.61 | 0.50~0.75 | 36.89 | 1 | ||
| tebufenozide | 1.79 ± 0.21 | 0.13 | 0.11~0.16 | 1442.31 | 2 | ||
| chlorfenapyr | 2.18 ± 0.23 | 11.07 | 9.30~13.38 | 6.78 | 1 | ||
| chlorantraniliprole | 2.54 ± 0.28 | 4.36 | 3.69~5.30 | 5.16 | 1 | ||
| methomyl | 2.06 ± 0.23 | 0.015 | 0.012~0.018 | 17500 | 3 | ||
| Adult | emamectin benzoate | 1.87 ± 0.21 | 78.95 | 64.74~99.04 | 0.028 | 1 | |
| spinosad | 2.03 ± 0.22 | 32.26 | 26.77~39.86 | 0.46 | 1 | ||
| indoxacarb | 1.43 ± 0.19 | 43.1 | 33.18~55.16 | 0.52 | 1 | ||
| chlorpyrifos | 2.38 ± 0.24 | 0.04 | 0.034~0.048 | 15000 | 3 | ||
| alpha-cypermethrin | 1.71 ± 0.21 | 1.91 | 1.53~2.51 | 11.78 | 1 | ||
| tebufenozide | 1.37 ± 0.19 | 0.48 | 0.37~0.68 | 390.63 | 2 | ||
| chlorfenapyr | 1.40 ± 0.20 | 0.98 | 0.75~1.39 | 76.53 | 2 | ||
| chlorantraniliprole | 1.93 ± 0.21 | 57.88 | 47.78~70.69 | 0.39 | 1 | ||
| methomyl | 2.01 ± 0.21 | 51.12 | 42.39~61.75 | 5.13 | 1 | ||
| C. sinica | Larvae | emamectin benzoate | >3.33 | <1 | 1 | ||
| spinosad | >22.22 | <1 | 1 | ||||
| indoxacarb | >33.33 | <1 | 1 | ||||
| chlorpyrifos | >888.89 | <1 | 1 | ||||
| alpha-cypermethrin | >33.33 | <1 | 1 | ||||
| tebufenozide | >277.78 | <1 | 1 | ||||
| chlorfenapyr | >111.11 | <1 | 1 | ||||
| chlorantraniliprole | >33.33 | <1 | 1 | ||||
| methomyl | >388.89 | <1 | 1 | ||||
| Adult | emamectin benzoate | 1.94 ± 0.21 | 0.24 | 0.20~0.29 | 9.38 | 1 | |
| spinosad | 1.55 ± 0.19 | 22.77 | 17.91~28.71 | 0.66 | 1 | ||
| indoxacarb | 2.12 ± 0.22 | 0.029 | 0.025~0.035 | 775.86 | 2 | ||
| chlorpyrifos | 1.71 ± 0.20 | 0.63 | 0.51~0.78 | 952.38 | 2 | ||
| alpha-cypermethrin | 1.72 ± 0.20 | 1.81 × 10−3 | 1.44 × 10−3 ~2.24 × × 10−3 | 12430.9 | 3 | ||
| tebufenozide | 1.87 ± 0.21 | 34.16 | 27.92~43.31 | 5.49 | 1 | ||
| chlorfenapyr | 1.90 ± 0.21 | 6.00 × 10−5 | 5.00 × 10−5~7.00 × 10−5 | 1.25 × 106 | 3 | ||
| chlorantraniliprole | 1.98 ± 0.21 | 29.09 | 24.11~35.35 | 0.77 | 1 | ||
| methomyl | 1.94 ± 0.21 | 2.40 × 10−3 | 1.97 × 10−3 ~2.90 × 10−3 | 1.33 × 105 | 3 | ||
| S. manilae | Adult | emamectin benzoate | 2.45 ± 0.24 | 3.00 × 10−4 | 2.60 × 10−4~3.60 × 10−4 | 7500 | 3 |
| spinosad | 2.51 ± 0.24 | 5.76 × 10−3 | 4.92 × 10−3~6.78 × 10−3 | 2604.17 | 3 | ||
| indoxacarb | 0.98 ± 0.14 | 0.084 | 0.052~0.17 | 267.86 | 2 | ||
| chlorpyrifos | 2.33 ± 0.24 | 0.071 | 0.060~0.085 | 8450.7 | 3 | ||
| alpha-cypermethrin | 2.55 ± 0.26 | 3.20 × 10−5 | 2.73 × 10−5~3.79 × 10−5 | 7.03 × 105 | 3 | ||
| tebufenozide | 1.18 ± 0.13 | 9.35 × 10−5 | 6.78 × 10−5~1.28 × 10−4 | 2.01 × 106 | 3 | ||
| chlorfenapyr | 2.15 ± 0.22 | 1.05 × 10−3 | 8.80 × 10−4~1.26 × 10−3 | 71428.6 | 3 | ||
| chlorantraniliprole | 2.09 ± 0.22 | 2.75 | 2.30~3.35 | 8.18 | 1 | ||
| methomyl | 2.61 ± 0.28 | 4.81 × 10−6 | 4.10 × 10−6~5.76 × 10−6 | 5.46 × 107 | 3 | ||
| T. remus | Larvae | emamectin benzoate | >3.33 | <1 | 1 | ||
| spinosad | >22.22 | <1 | 1 | ||||
| indoxacarb | >33.33 | <1 | 1 | ||||
| chlorpyrifos | >888.89 | <1 | 1 | ||||
| alpha-cypermethrin | >33.33 | <1 | 1 | ||||
| tebufenozide | >277.78 | <1 | 1 | ||||
| chlorfenapyr | >111.11 | <1 | 1 | ||||
| chlorantraniliprole | >33.33 | <1 | 1 | ||||
| methomyl | >388.89 | <1 | 1 | ||||
| Adult | emamectin benzoate | <1 × 10−9 | >2.25 × 109 | 3 | |||
| spinosad | <1 × 10−7 | >1.50 × 108 | 3 | ||||
| indoxacarb | <1 × 10−9 | >2.25 × 1010 | 3 | ||||
| chlorpyrifos | <1 × 10−9 | >6.00 × 1011 | 3 | ||||
| alpha-cypermethrin | <1 × 10−12 | >2.25 × 1013 | 3 | ||||
| tebufenozide | <1 × 10−10 | >1.88 × 1012 | 3 | ||||
| chlorfenapyr | <1 × 10−6 | >7.50 × 107 | 3 | ||||
| chlorantraniliprole | <1 × 10−5 | >2.25 × 106 | 3 | ||||
| methomyl | <1 × 10−7 | >2.63 × 109 | 3 |
RQa, risk quotient = recommended field rate (g a.i. ha−1)/LC50 of H. axyridis, C. sinica and T. remus larvae and H. axyridis, C. sinica, S. manilae and T. remus adult (mg mg a.i. liter−1).
Categoryb, 1: safe; 2: slightly to moderately toxic; 3: dangerously toxic.
Table 3. Toxicity of insecticides on S. manilae larvae.
| Insecticides | Concentration (mg a.i. liter−1) | Survival Rate (±SD) (%) of S. exigua (48 h) | Pupation rate (±SD) (%) | Emergence rate (±SD) (%) |
|---|---|---|---|---|
| emamectin benzoate | 0.022 | 41.33 ± 2.96 | 94.84 ± 2.60 a | 94.41 ± 2.83 a |
| spinosad | 0.97 | 51.33 ± 2.96 | 93.86 ± 3.41 a | 95.40 ± 2.31 a |
| indoxacarb | 0.15 | 37.67 ± 2.33 | 94.44 ± 5.56 a | 97.22 ± 2.78 a |
| chlorpyrifos | 0.063 | 44.33 ± 4.67 | 100 ± 0.0 a | 95.35 ± 2.36 a |
| alpha-cypermethrin | 1.21 | 37.67 ± 3.93 | 94.87 ± 5.13 a | 97.22 ± 2.78 a |
| tebufenozide | 112.23 | 52.33 ± 2.91 | 94.00 ± 3.40 a | 97.78 ± 2.22 a |
| chlorfenapyr | 7.56 | 44.33 ± 2.96 | 95.21 ± 2.41 a | 97.62 ± 2.38 a |
| chlorantraniliprole | 0.103 | 36.67 ± 2.03 | 94.19 ± 2.91 a | 96.97 ± 3.03 a |
| methomyl | 48.32 | 38.00 ± 1.00 | 94.19 ± 2.91 a | 96.97 ± 3.03 a |
| control | 98.00 ± 1.00 | 94.33 ± 1.33 a | 98.81 ± 1.19 a |
The data in the table are mean ± SE, and means followed by the same letter are not significantly different; LSD, P < 0.05.
Toxicity to three C. sinica developmental life stages
The insecticides didn’t show lethal effect to C. sinica larvae 24 h after treatment at the recommended field application concentrations except chlorpyrifos (LC50: 185.67 mg a.i. liter−1) and methomyl (LC50: 162.08 mg a.i. liter−1) (Table 1), and the percentage emergence rate of C. sinica pupae at the recommended field rate of the insecticides was not significantly different from untreated C. sinica pupae (F = 0.65, d.f. = 9, P = 0.74) (Table 2). For C. sinica adult, the order of toxicity (high-low) of the 9 insecticides was as follows: chlorfenapyr > alpha-cypermethrin, methomyl > indoxacarb > emamectin benzoate > chlorpyrifos > spinosad, chlorantraniliprole ≥ tebufenozide. Chlorfenapyr displayed the highest toxicity (LC50: 6 × 10−5 mg a.i. liter−1), while tebufenozide had the lowest toxicity (LC50: 29.09 mg a.i. liter−1) (Table 1).
Table 2. Toxicity of insecticides on pupae of H. axyridis, C. sinica, S. manilae and T. remus.
| Insecticides | The recommended field rate (mg a.i. liter−1) | Emergence rate (%) |
|||
|---|---|---|---|---|---|
| H. axyridis (±SD) (%) | C. sinica (±SD) (%) | S. manila (±SD) (%) | T. remus (±SD) (%) | ||
| emamectin benzoate | 3.33 | 94.44 ± 2.22 a | 93.33 ± 1.93 a | 96.67 ± 1.93 a | 95.56 ± 2.94 a |
| spinosad | 22.22 | 92.22 ± 1.11 a | 94.44 ± 1.11 a | 97.78 ± 1.11 a | 93.33 ± 1.93 a |
| indoxacarb | 33.33 | 95.56 ± 1.11 a | 94.44 ± 2.93 a | 96.67 ± 1.93 a | 94.44 ± 1.11 a |
| chlorpyrifos | 888.89 | LC50: 527.68 mg·L−1 | 93.33 ± 1.93 a | 95.56 ± 1.11 a | 96.67 ± 3.33 a |
| alpha-cypermethrin | 33.33 | 97.78 ± 1.11 a | 97.78 ± 1.11 a | 97.78 ± 1.11 a | 94.45 ± 4.01 a |
| tebufenozide | 277.78 | LC50: 350.08 mg·L−1 | 95.56 ± 1.11 a | 95.56 ± 1.11 a | 94.45 ± 2.22 a |
| chlorfenapyr | 111.11 | 94.44 ± 2.94 a | 93.33 ± 3.33 a | 95.55 ± 2.22 a | 94.44 ± 1.11 a |
| chlorantraniliprole | 33.33 | 94.44 ± 1.11 a | 92.22 ± 1.11 a | 96.67 ± 1.93 a | 96.67 ± 1.93 a |
| methomyl | 388.89 | LC50: 314.75 mg·L−1 | 93.33 ± 1.93 a | 95.56 ± 1.11 a | 95.56 ± 4.44 a |
| control | 95.56 ± 1.11 a | 94.44 ± 1.11 a | 96.67 ± 1.93 a | 95.56 ± 1.11 a | |
The data in the table are mean ± SE, and those in the same column followed by same letters are not significantly different (P < 0.05).
Toxicity to three S. manilae developmental life stages
Concentrations treated parasitized S. exigua larvae were based on the LC50 value for S. exigua. The percentage pupation rate of S. manilae larvae by treated parasitized S. exigua larvae was not significantly different from untreated S. manilae larvae (F = 0.28, d.f. = 9, P = 0.97) (Table 3). The percentage emergence rate of S. manilae pupae at the recommended field rate of the insecticides was not significantly different from untreated S. manilae pupae (F = 0.30, d.f. = 9, P = 0.97) (Table 2). All insecticides were toxic to S. manilae adults 24 h post-treatment (LC50 values between 1 × 10−5 and 2.75 mg a.i. liter−1). The order of toxicity (high-low) of the 9 insecticides was as follows: methomyl > alpha-cypermethrin > tebufenozide > emamectin benzoate > spinosad > chlorfenapyr > indoxacarb, chlorpyrifos > chlorantraniliprole (Table 1).
Toxicity to three T. remus developmental life stages
None of the insecticides affected T. remus larvae survival (Table 1). The percentage emergence rate of T. remus pupae at the recommended field rate of the insecticides was not significantly different from untreated T. remus pupae (F = 0.16, d.f. = 9, P = 0.99) (Table 2). All insecticides were toxic to T. remus adults 24 h post-treatment (LC50 values less than 1 × 10−5 mg a.i. liter−1).
Risk assessment of insecticides on H. axyridis, C. sinica and T. remus larvae and H. axyridis, C. sinica, S. manilae and T. remus adult
The classification of the 9 insecticides based on risk quotient values in presented in Table 1. All tested insecticides were safe for larvae of C. sinica and T. remus with risk quotients less than 1. Emamectin benzoate, spinosad, alpha-cypermethrin, chlorfenapyr and chlorantraniliprole were safe for H. axyridis larvae (risk quotients, 0.17–36.89), while emamectin benzoate, spinosad, indoxacarb, alpha-cypermethrin, chlorantraniliprole and methomyl were safe for H. axyridis adult (risk quotients, 0.028–5.13). However, methomyl (risk quotient, 17500.00) and chlorpyrifos (risk quotient, 15000.00) was considered dangerous to H. axyridis larvae and adult, respectively, whereas chlorpyrifos, indoxacarb and tebufenozide were slightly to moderately toxic to H. axyridis larvae (risk quotients, 291.26–1442.31), and chlorfenapyr and tebufenozide were slightly to moderately toxic to H. axyridis adult (risk quotients, 76.53–390.63). For S. manilae and T. remus adult, all tested insecticides were dangerously toxic, except indoxacarb (risk quotient, 267.86) which was slightly to moderately toxic, and chlorantraniliprole (risk quotient, 8.18) was safe to S. manilae adult. For C. sinica adult, emamectin benzoate, spinosad, tebufenozide and chlorantraniliprole were safe with risk quotients of 0.66–5.49, indoxacarb and chlorpyrifos (risk quotients, 775.86 and 952.38, respectively) were slightly to moderately toxic, whereas alpha-cypermethrin, chlorfenapyr and methomyl (risk quotients, 12430.94–1.33 × 105) were dangerously toxic.
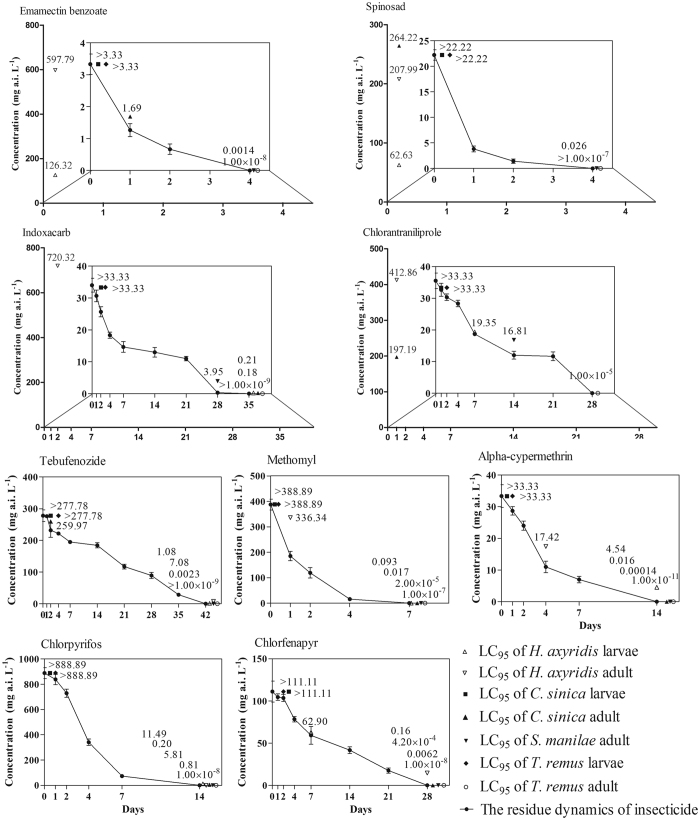
The comparison of LC95 values of 9 insecticides to H. axyridis, C. sinica and T. remus larvae and H. axyridis, C. sinica, S. manilae and T. remus adult with their field recommended rates is shown in Fig. 1. For H. axyridis larvae, the LC95 value of emamectin benzoate and spinosad were distinctly higher than its recommended field concentrations, indicating that these insecticide is harmless to the H. axyridis larvae. However, the LC95 value of tebufenozide was still lower than its residues of the recommended field concentrations occurring 35d after treatment, indicating that tebufenozide had the longest risk duration, followed by indoxacarb (28d), alpha-cypermethrin (7d), chlorpyrifos (7d), methomyl (4d), chlorfenapyr (4d) and chlorantraniliprole (4d). For adult H. axyridis, emamectin benzoate, spinosad, indoxacarb and chlorantraniliprole were harmless with the LC95 value higher than their recommended field concentrations. Similar to H. axyridis larvae, tebufenozide had the longest risk duration (35d) to H. axyridis adult, followed by chlorfenapyr (21d), chlorpyrifos (7d), alpha-cypermethrin (2d) and methomyl (1d). For C. sinica and T. remus larvae, the LC95 of 9 insecticides were higher than their recommended field concentrations, indicating that the 9 insecticides are harmless to them. For adult S. manilae and T. remus, the LC95 value of all 9 insecticides were significantly lower than their recommended field concentrations, indicating that these insecticides would be harmful to them. Meanwhile, tebufenozide had the longest risk duration to S. manilae and T. remus adult (35d), followed by chlorfenapyr (21d), chlorpyrifos (7d), alpha-cypermethrin (7d), methomyl (4d) spinosad (2d) and emamectin benzoate (2d), and the risk duration of chlorantraniliprole and indoxacarb on S. manilae adult (7d and 21d, respectively) shorter than T. remus adult (21d and 28d, respectively).
Figure 1. Comparison of LC95 value of 9 insecticides to H. axyridis, C. sinica and T. remus larvae and H. axyridis, C. sinica, S. manilae and T. remus adult with the residues of the field recommend concentration.
Discussion
Insecticides may kill natural enemies because of their common physiology9. Insecticide evaluations on natural enemies should include not only acute toxicity but also residual toxicity19. Under laboratory conditions, we tested the toxicity and residue risk of 9 insecticides to the development of H. axyridis, C. sinica, S. manilae and T. remus. Of the 9 insecticides tested, Indoxacarb, tebufenozide, chlorfenapyr, methomyl, alpha-cypermethrin and chlorpyrifos showed dangerously toxic and long risk duration on S. manilae and T. remus adults, similar situation was also observed on chlorpyrifos to H. axyridis adults and chlorfenapyr, methomyl and alpha-cypermethrin to C. sinica adults. therefore, these six insecticides are not suitable for the control of S. exigua.
Chlorantraniliprole, the first commercial anthranilic diamide insecticide, is a potent and selective activator of insect ryanodine receptor (RyRs) that are critical for muscle contraction20,21. Activation of the ryanodine receptors in insects affects uncontrolled release of calcium from internal stores in the sarcoplasmic reticulum, causing unregulated release of internal calcium in the cell and leading to feeding cessation, lethargy, muscle paralysis, and ultimately death of the insect21. Among the insecticides evaluated, chlorantraniliprole was dangerously toxic and had a long residual (>21d) activity on T. remus adults, however, the insecticide was safe to larvae and adult of H. axyridis, C. sinica and S. manilae. Brugger et al.22 reported that chlorantraniliprole had selectivity to the beneficial parasitoid wasps Aphidius rhopalosiphi, Trichogramma dendrolimi, Trichogramma chilonis, Trichogramma pretiosum, Aphelinus mali, Dolichogenidea tasmanica and Diadegma semiclausum22. Its use for S. exigua IPM is feasible, and it should be selected according to the target species of the four natural enemies.
Emamectin-benzoate and spinosad were safe to the three life stages of H. axyridis, C. sinica, S. manilae and T. remus and larvae and pupae of S. manilae and T. remus. Though emamectin-benzoate and spinosad were dangerously toxic to adults of S. manilae and T. remus, these two insecticides had short risk duration. This may be caused by emamectin-benzoate and spinosad can penetrate leaf tissues by translaminar movement23. It also is important that, Emamectin-benzoate and spinosad are safe to mammals and harmless to other enemies24,25,26,27. Therefore, these two insecticides are suitable candidates for suppressing outbreaks of S. exigua.
Our study, C. sinica, S. manilae and T. remus pupae, survived the recommended field rate, probably because the pupae were shielded from insecticide contact by the cocoon. The results indicate that the insecticides can be applied during the pupae of the natural enemies, as discussed previously28,29. All the insecticides were non-toxic to T. remus larvae in our study, maybe T. remus larvae were shielded from insecticide contact. Toxicity of the insecticides to S. manilae larvae was likely due to direct host death, because the percentage pupation rate of S. manilae larvae by treated parasitized S. exigua larvae was not significantly different from untreated S. manilae larvae. In the present study, most of the S. exigua were killed directly by insecticides so it was not possible to distinguish whether S. manilae larvae died directly or because their hosts S. exigua larvae were killed.
Release of domesticated natural enemies to control insect has become an important tactic for the management of insect pests in many agricultural crops. Biological control has been increasingly used in crop protection over the past 30 years, with the production of biological control agents also increasing, with more than 130 species of predators and parasitoids on the market in 200030. Laboratory experiment show that S. manilae and T. remus have good control effect against S. exigua13,31,32. The outlook for the success of parasitoids S. manilae and T. remus against S. exigua in crops is now more positive13. Due to their strong toxic on S. manilae and T. remus adults, 8 out of 9 of the insecticides (emamectin-benzoate, spinosad, indoxacarb, tebufenozide, methomyl, alpha-cypermethrin, chlorpyrifos and chlorfenapyr) as measured in this study should be applied with great caution if releasing adults of S. manilae and all tested insecticides in this study should be used with great caution when releasing adults of T. remus.
The extensive use of insecticides often promotes the development of insect resistance. At present, S. exigua have developed high levels of resistance to emamectin benzoate, cypermethrin and chlorpyrifos33. Therefore, the high-efficacy insecticides with minimal impact on natural enemies should be used as alternatives. This study showed important results that will help pest managers to choose the best insecticides to be applied, because products with the lowest impact on biological control agents are the most appropriate for use in IPM programs. However, the impact of insecticides on natural enemies is complex, which requires systematic study to determine sublethal effects on the biology, physiology, and behavior of four natural enemies populations.
Methods
Insects culture
H. axyridis adults and C. sinica adults were obtained from a Brassica oleracea L. var. capitata L field in Tai’an, China. After collection, they were stored separately in plastic insect boxes (23 cm long × 15 cm wide × 9 cm high) with 20–30 adults per box, and maintained under laboratory conditions of 27 ± 1 °C and a photoperiod of 16:8 h (L:D). Both were provided an ad libitum supply of live cotton aphids, Aphis gossypii Glover, (Homoptera, Aphididae) on cotton leaves, and water-soaked cotton ball was supplied as a water supplement. The boxes lined with filter paper disks. The boxes containing adults were checked daily for oviposition. If eggs were found, the adults were transferred to new plastic insect boxes (23 cm long × 15 cm wide × 9 cm high) provided with A. gossypii and water. The boxes containing eggs were checked daily for hatch. After the eggs had hatched and the larvae dispersed from the egg clusters, members of the F1 generation were placed individually into separate glass scintillation vials (20 mm diameter, 70 mm high), and reared to the desired developmental life stages. Glass-vial bioassay toxicity tests were performed using 3d old larvae, 3d pupae and 5d adults of H. axyridis and C. sinica.
All S. manilae and the T. remus populations were provided by the College of Natural Resources and Environment, South China Agricultural University, Guangzhou, China. S. manilae adults were stored in plastic insect boxes (23 cm long × 15 cm wide × 9 cm high) with 50–60 adults per box, and T. remus were stored in 30 mL glass test tubes with 200–300 adults per tube. Both were provided with honey solution and maintained at 27 ± 1 °C with a photoperiod of 12:12 h (L: D). 5 pairs of S. manilae adults were introduced into plastic insect boxes with 2nd instar 100–150 S. exigua larvae. 100–200 T. remus adults were introduced into a glass test tube containing S. exigua 2000–3000 eggs. Parasitized S. exigua larvae or eggs were maintained under laboratory conditions of 27 ± 1 °C, 60–75% relative humidity and photoperiod of 12:12 h (L: D). 3d old larvae and 3d old pupae of S. manilae and T. remus were used in the insect-dip method, and 5d old S. manilae and T. remus adults were used in the glass-vial bioassay.
Insecticides
Nine insecticides were selected because of their use for control of S. exigua: emamectin benzoate (90%; Nanjing Redsun, China); spinosad (90%; Dow AgroSciences, China); indoxacarb (94%; DuPont, China); chlorpyrifos (97%; Dow AgroSciences, China); alpha-cypermethrin (99%; Shandong Dacheng Pesticide, China); tebufenozide (95%; Dow AgroSciences, China); chlorfenapyr (94.5%; BASF Aktiengesellschaft, China); chlorantraniliprole (95.3%; DuPont, China); methomyl (98%; Jiangsu Changlong Chemicals, China).
Toxicity bioassays
The glass-vial bioassay34 was used to determine the toxicity of the insecticides to adults of S. manilae. Each insecticide was applied by pipetting 0.5 mL insecticide dissolved in acetone (analytical reagent, purity ≥99.7%) into each 22 mL glass scintillation vial (20 mm diameter, 70 mm height). Serial dilutions were used to obtain desired concentrations. Each vial was rolled for several minutes until an even layer of insecticide dried on the inner surface. Control treatment vials only received 0.5 mL of acetone. Vials were used the same day they were coated with the insecticides. All 9 insecticides were used the same method. Ten S. manilae adults were transferred into one vial then the vial was sealed with a layer of gauze. There were three (n = 3) replications were used for each rate of insecticide. After 1 h of exposure, the adults were transferred into insecticide-free vials and supplied with 10% honey solution. After 24 h, the number of dead adult S. manilae were counted, and the dose response (LC50) was calculated for each insecticide. This procedure was used for H. axyridis adults, C. sinica adults, H. axyridis larval, C. sinica larval and T. remus adults, however only 2 H. axyridis or C. sinica adults, 1 H. axyridis or C. sinica larva, and 10 T. remus adults were used per vial.
Insectcide toxicity to S. manilae and T. remus larvae was tested by the insect-dip method. A 100 ml stock solution [diluted with 5% (v/v) acetone in a water solution mixed uniformly with 5% (v/v) Tween-80] was prepared for each insecticide. Serial dilutions were used to obtain desired concentrations. 3d old S. manilae and T. remus larvae with their host were dipped for 3 s in an insecticide solution, placed on filter paper, and then individuals were transferred to separate untreated glass scintillation vials, 1 individual was used per vial. For control test, individuals were dipped in distilled water containing 5% acetone. The parasitized S. exigua larvae and parasitized S. exigua eggs were used to test the insectcide toxicity (direct) to S. manilae and T. remus larvae. The pupation rate and emergence rate of S. manilae and the emergence rate of T. remus were computed after two week to enable them to reach adulthood. The insecticide effects on S. manilae, T. remus, H. axyridis and C. sinica pupae was determined using the insect-dip method. A 100 ml stock solution [diluted with 5% (v/v) acetone in water] was prepared for each insecticide with the recommended field rate (emamectin benzoate: 3.33 mg a.i. liter−1; spinosad: 22.22 mg a.i. liter−1; indoxacarb: 33.33 mg a.i. liter−1; chlorpyrifos: 888.89 mg a.i. liter−1; alpha-cypermethrin: 33.33 mg a.i. liter−1; tebufenozide: 277.78 mg a.i. liter−1; Chlorfenapyr: 111.11 mg a.i. liter−1; chlorantraniliprole: 33.33 mg a.i. liter−1; methomy: 388.89 mg a.i. liter−1), the recommended field rate was obtained from the e-Pesticide Manual of ICA, MOA, China (http://www.ny100.cn/). Pupae of H. axyridis, pupae of T. remus with S. exigua eggshells, pupae with cocoon of S. manilae and C. sinica were dipped for 3 s in an insecticide solution, placed on filter paper, and then individuals were transferred to separate untreated glass scintillation vials, 10 individuals were used per vial. For control test, individuals were dipped in distilled water containing 5% acetone. The emergence rate of S. manilae and T. remus were computed after one week to enable them to reach adulthood.
All bioassays had 3 replications of 6–9 different insecticide concentrations, and each replication of each concentration included 20 individuals.
Residue determination
A 100 ml stock solution [diluted with 5% (v/v) acetone in a water solution mixed uniformly with 5% (v/v) Tween-80] was prepared for each insecticide with the recommended field rate. Three pots of cabbage at adult plant stage with leaves blade (ca. 10.0 × 7.0 cm) were grouped and sprayed with insecticide until the plants were completely saturated with the solution. Treated plants were placed outside the greenhouse. All nine insecticides were tested for residue toxicity. Cabbage leaves (ca. 10.0 × 7.0 cm) were collected 0, 1, 2, 4, 7, 14, 21, 28, 35 and 42d post insecticide treatment, rinsing 4 times with Acetone, 10 ml each time, after concentrated in a blowing instrument at 40 °C, added methanol to 1 ml. The concentration of insecticide residue was determined by high performance liquid chromatography (HPLC), using a 5 um Hypersil C18 250*4.6 mm reversed phase column (Diamonsil, America). The mobile phases were methanol:acetonitrile:water (45: 50:5, v/v/v) for emamectin-benzoate and spinosad, methanol:water (80:20, v/v) for indoxacarb, tebufenozide and chlorantraniliprole, methanol:water (90:10, v/v) for chlorpyrifos, alpha-cypermethrin and chlorfenapyr, methanol:water (80:20, v/v) for indoxacarb and methanol:water (50:50, v/v) for methomyl, respectively. The detections were performed at 245 nm for emamectin-benzoate, at 252 nm for spinosad, at 234 nm for indoxacarb, at 289 nm for chlorpyrifos, at 230 nm for alpha-cypermethrin, at 240 nm for tebufenozide, at 261 nm for chlorfenapyr, at 264 nm for chlorantraniliprole and at 234 nm for methomyl. The flow rate was 0.8 ml/min. Ten μl of test solution was injected into the HPLC system.
The residue dynamics calculated by the residues/retention of water on cabbage leaves. The residues measured by HPLC = D × E × F/G × 1 ml. D is the tested insecticides volume; E is the peak area of the tested insecticides; F is the standard sample concentration; G is the peak area of the standard sample.
Statistical analysis
LC50 and LC95 values and slopes were determined by probit analysis using the SPSS program. Survival, mortality, pupation and emergence rates were subjected to arcsine transformation and subsequently analyzed by one-way ANOVA. Means were separated by using Tukey’s Student range test (HSD) at P = 0.05 (SPSS13.0 (SPSS Inc, Chicago, USA)).
Additional Information
How to cite this article: Liu, Y. et al. Toxicity of nine insecticides on four natural enemies of Spodoptera exigua. Sci. Rep. 6, 39060; doi: 10.1038/srep39060 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank Zaifu Xu, Yali Tang (College of Natural Resources and Environment, South China Agricultural University) for their contributions to the S. manilae and the T. remus populations provided. This work was supported by a grant from The National Key Research and Development Program of China (2016YFD0200501).
Footnotes
Author Contributions M.W. and L.Y.Q. conceived and designed research. L.Y.Q. and L.X.Y. performed the experiments. M.W., L.Y.Q. and Z.C. analyzed data and wrote the manuscript. All authors read and approved the manuscript.
References
- Smagghe G. et al. Toxicity and kinetics of methoxyfenozide in greenhouse-selected Spodoptera exigua (Lepidoptera: Noctuidae). Pest Manage. Sci. 59, 1203–1209 (2003). [DOI] [PubMed] [Google Scholar]
- Metcalf R. L. & Metcalf R. A. Destructive and useful insects : 5th ed. McGraw-Hill, New York (1992). [Google Scholar]
- Zheng X. L., Cong X. P., Wang X. P. & Lei C. L. A review of geographic distribution, overwintering and migration in Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). J. Entomol. Res. Soc. 13, 39–48 (2011). [Google Scholar]
- Wang Y. H. et al. Susceptibility of adult Trichogramma nubilale (Hymenoptera: Trichogrammatidae) to selected insecticides with different modes of action. Crop Prot. 34, 76–82 (2012). [Google Scholar]
- Fogel M. N., Schneider M. I., Desneux N., González B. & Ronco A. E. Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 22, 1063–1071 (2013). [DOI] [PubMed] [Google Scholar]
- Bacci L. et al. Toxicity of insecticides to the sweetpotato whitefly (Hemiptera: Aleyrodidae) and its natural enemies. Pest Manage. Sci. 63, 699–706 (2007). [DOI] [PubMed] [Google Scholar]
- Preetha G., Stanley J., Suresh S. & Samiyappan R. Risk assessment of insecticides used in rice on miridbug, Cyrtorhinus lividipennis Reuter, the important predator of brown planthopper, Nilaparvata lugens (Stal). Chemosphere 80, 498–503 (2010). [DOI] [PubMed] [Google Scholar]
- Wright D. J. & Verkert R. H. J. Integration of chemical and biological control systems for arthropods; evaluation in a multitrophic context. Pest Manage. Sci. 44, 207–218 (1995). [Google Scholar]
- Wang H. Y. et al. Assessment of the impact of insecticides on Anagrus nilaparvatae (Pang et Wang) (Hymenoprera: Mymanidae), an egg parasitoid of the rice planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Crop Prot. 27, 514–522 (2008). [Google Scholar]
- Greathead D. J. Natural enemies in combination with pesticides for integrated pest management. In Reuveni R. ed Novel Approaches to Integrated Pest Management Lewis Publishers, Boca Raton, FL, USA 183–197 (1995). [Google Scholar]
- Brown P. M. J. et al. The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. Biocontrol 56, 623–641 (2011). [Google Scholar]
- Xu Y. Y., Mou J. Y. & Hu C. Research and application of Chrysoperla sinica (Tjeder). Entomol. Knowl. 36, 313–315 (1999). [Google Scholar]
- Sun J. S. & Huang S. S. Evaluation of potential control ability of Snellenius manilae (Ashmead) against Spodoptera exigua (Hübner). Acta Ecol. Sin. 30, 1494–1499 (2010).
- Si S. Y. et al. Progress in research on prevention and control of beet armyworm, Spodoptera exigua in China. Chin. J. Appl. Entomol. 49, 1432–1438 (2012). [Google Scholar]
- Cave R. D. Biology, ecology and use in pest management of Telenomus remus. Biocontr. News Inform. 21, 21–26 (2000). [Google Scholar]
- Yang Y., Han Y., Fang Z. H. & Xu Z. F. Effect of host egg age and contact time on the parasitic capacity of Telenomus remus (Hymenoptera: Scelionidae). Chin. J. Appl. Entomol. 49, 1490–1495 (2012). [Google Scholar]
- Lu Y. H., Wu K. M., Jiang Y. Y., Guo Y. Y. & Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 487, 362–367 (2012). [DOI] [PubMed] [Google Scholar]
- Wyckhuys K. A. G. et al. Current status and potential of conservation biological control for agriculture in the developing world. Biol. Control 65, 152–167 (2013). [Google Scholar]
- Desneux N., Decourtye A. & Delpuech J. M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (2007). [DOI] [PubMed] [Google Scholar]
- Lahm G. P., Cordova D. & Barry J. D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 17, 4127–4133 (2009). [DOI] [PubMed] [Google Scholar]
- Cordova D. et al. Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Phys. 84, 196–214 (2006). [Google Scholar]
- Brugger K. E. et al. Selectivity of chlorantraniliprole to parasitoid wasps. Pest Manag. Sci. 66, 1075–1081 (2010). [DOI] [PubMed] [Google Scholar]
- Tomlin C. The Pesticide Manual, 13th ed. British Crop Protection Council, Cambridge, UK, 1344 (2003).
- Giraddi R. S. & Gundannavar K. P. Safety of emamectin benzoate, an avermectin derivative to the egg parasitoids, Trichogramma spp. Karnataka J. Agric. Sci. 19, 417–418 (2006). [Google Scholar]
- Xu H. H. Plant chemical protection, pp. 72–115. China Agriculture Press, Beijing (2007). [Google Scholar]
- Wang Y. H. et al. Susceptibility of adult Trichogramma nubilale (Hymenoptera: Trichogrammatidae) to selected insecticides with different modes of action. Crop Prot. 34, 76–82 (2012). [Google Scholar]
- Schoonover J. R. & Larson L. L. Laboratory activity of spinosad on non-target beneficial arthropods, 1994. Arthrop. Manag. Tests 20, 357 (1995). [Google Scholar]
- Gerling D. & Sinai P. Buprofezin effects on two parasitoid species of whitefly (Homoptera:Aleyrodidae). J. Econ. Entomol. 87, 842–846 (1994). [Google Scholar]
- Jones W. A., Ciomperlik M. A. & Wolfenbarger D. A. Lethal and sublethal effects of insecticides on two parasitoids attacking Bemisia argentifolii (Homoptera, Aleyrodidae). Biol. Control 11, 70–76 (1998). [Google Scholar]
- Van Lenteren J. C. Success in biological control of arthropods by augmentation of natural enemies. In Gurr G., Wratten S. eds Measures of Success in Biological Control. Kluwer Acadamic Publishers, Dordrecht, Netherlands, 77–103 (2000). [Google Scholar]
- Murthy K. S., Rao N. S., Rabinfra R. J. & Jalali S. K. Age related parasitisation potential of the eggs parasitoid Telenomus remus (Scelionidae: Hymenoptera) on certain lepidopterous hosts. J. Entomol. Res. 28, 33–36 (2004). [Google Scholar]
- Tang Y. L., Chen K. W. & Xu Z. F. Study on ontogenesis of Telenomus remus Nixon (Hymenoptera: Scelionidae). J. Changjiang Vege. 18, 1–3 (2010). [Google Scholar]
- Che W. N. Shi T. Wu Y. D. & Yang Y. H. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Entomol. 106, 1855–1862 (2013). [DOI] [PubMed] [Google Scholar]
- Snodgrass G. L. Glass-vial bioassay to estimate insecticide resistance in adult tarnished plant bugs (Heteroptera: Miridae). J. Econ. Entomol. 89, 1053–1059 (1996). [Google Scholar]