Abstract
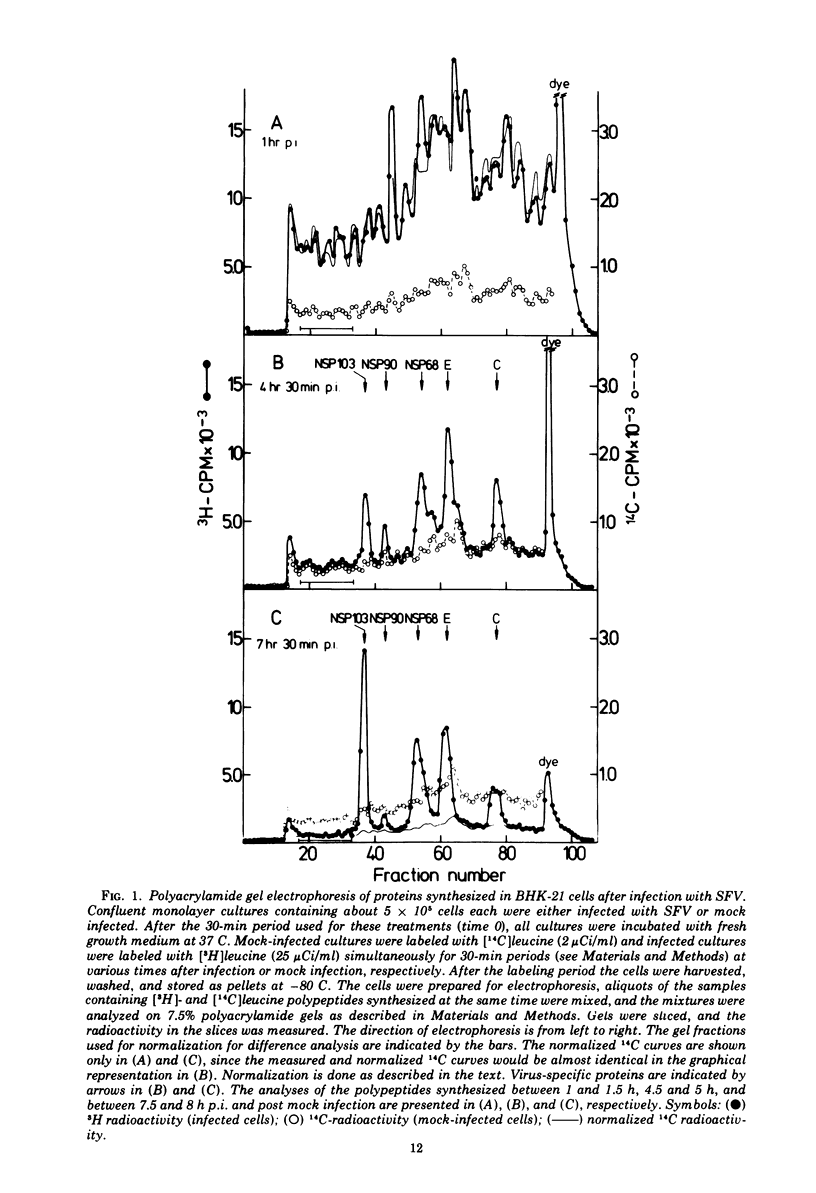
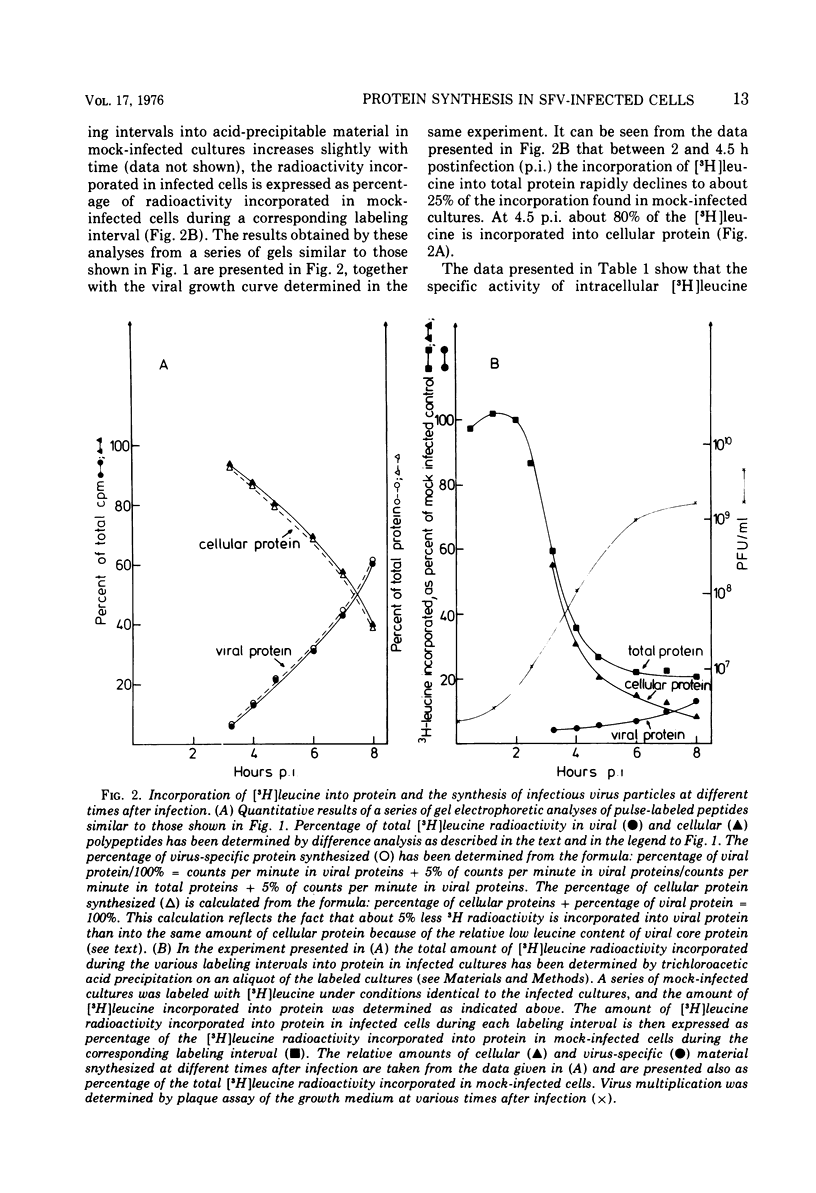
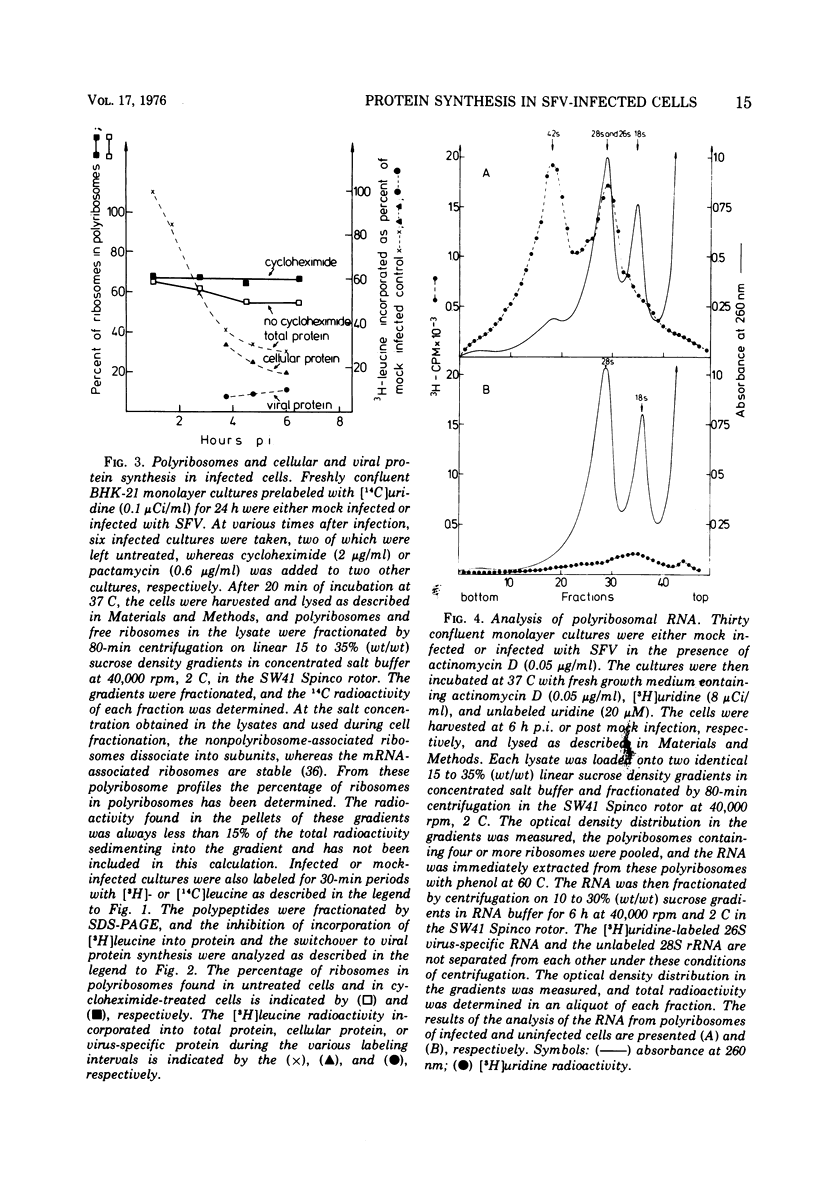
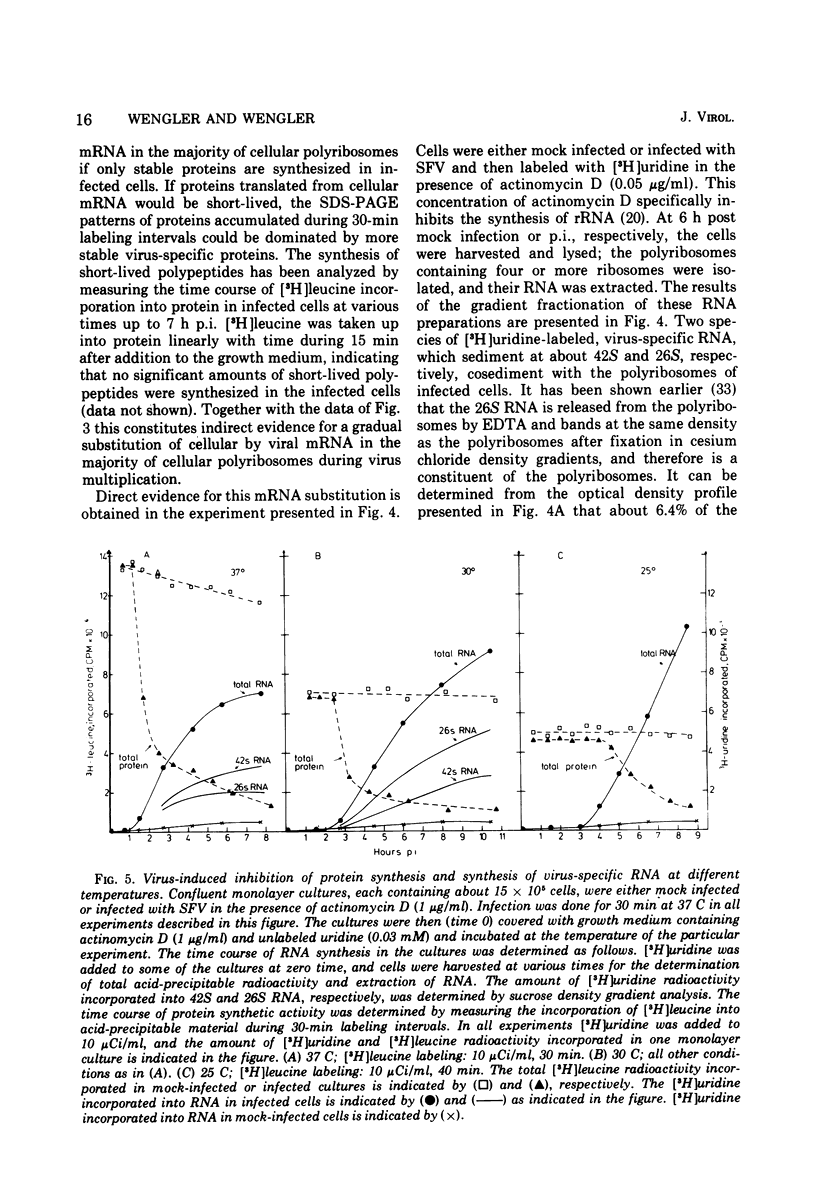
[3H]leucine-labeled proteins synthesized in BHK-21 cells infected with Semliki Forest virus were fractionated by polyacrylamide gel electrophoresis (PAGE). Cellular and virus-specific proteins were identified by difference analysis of the PAGE profiles. The specific activity of intracellular [3H]leucine was determined. Two alterations of protein synthesis, which develop with different time courses, were discerned. (i) In infected cultures an inhibition of overall protein synthesis to about 25% of the protein synthesis in mock-infected cultures develops between about 1 and 4 h postinfection (p.i.). (ii) The relative amount of virus-specific polypeptides versus cellular polypeptides increases after infection. About 80% of the proteins synthesized at 4 h p.i. are cellular proteins. Since significant amounts of nontranslocating ribosomes in polyribosomes were not detected up to 7 h p.i., the inhibition of protein synthesis is not caused by inactivation of about 75% of all polyribosomes but by a decreased protein synthetic activity of the majority of polyribosomes. Indirect evidence indicates that an inhibition of elongation and/or release of protein synthesis develops in infected cells, which is sufficient to account for the observed inhibition of protein synthesis. Inhibition of over-all protein synthesis developed when virus-specific RNA began to accumulate at the maximal rate. This relationship was observed during virus multiplication at 37, 30, and 25 C. A possible mechanism by which synthesis of virus-specific RNA in the cytoplasm could inhibit cellular protein synthesis is discussed. Indirect evidence and analysis of polyribosomal RNA show that the increased synthesis of virus-specific protein is brought about by a substitution of cellular by viral mRNA in the polyribosomes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H., Tamm I. Structural proteins of Semliki Forest virus and its nucleocapsid. Virology. 1970 Jun;41(2):321–329. doi: 10.1016/0042-6822(70)90084-x. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Swanson R., Schlesinger M. J. Effects of different RNAs and components of the cell-free system on in vitro synthesis of Sindbis viral proteins. J Virol. 1974 Sep;14(3):652–663. doi: 10.1128/jvi.14.3.652-663.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- EAGLE H., PIEZ K. A., LEVY M. The intracellular amino acid concentrations required for protein synthesis in cultured human cells. J Biol Chem. 1961 Jul;236:2039–2042. [PubMed] [Google Scholar]
- Ehrenfeld E., Hunt T. Double-stranded poliovirus RNA inhibits initiation of protein synthesis by reticulocyte lysates. Proc Natl Acad Sci U S A. 1971 May;68(5):1075–1078. doi: 10.1073/pnas.68.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Protein synthesis directed by an arbovirus. J Virol. 1968 Jan;2(1):26–32. doi: 10.1128/jvi.2.1.26-32.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Simons K., Renkonen O. Isolation and characterization of the membrane proteins of Semliki Forest virus. Virology. 1974 Oct;61(2):493–504. doi: 10.1016/0042-6822(74)90285-2. [DOI] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaariainen L., Gomatos P. J. A kinetic analysis of the synthesis in BHK 21 cells of RNAs specific for Semliki Forest virus. J Gen Virol. 1969 Sep;5(2):251–265. doi: 10.1099/0022-1317-5-2-251. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I., Burke D. C. Studies on the structural proteins of Semliki Forest virus. J Gen Virol. 1972 Jan;14(1):87–98. doi: 10.1099/0022-1317-14-1-87. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Friedman R. M. Analysis of arbovirus ribonucleic acid forms by polyacrylamide gel electrophoresis. J Virol. 1971 Apr;7(4):504–514. doi: 10.1128/jvi.7.4.504-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey E. H., Hopkins J. W. Molecular weights of some HeLa ribosomal RNA's. J Mol Biol. 1969 Feb 14;39(3):545–550. doi: 10.1016/0022-2836(69)90144-2. [DOI] [PubMed] [Google Scholar]
- Morser M. J., Burke D. C. Cleavage of virus-specified polypeptides in cells infected with Semliki Forest Virus. J Gen Virol. 1974 Mar;22(3):395–409. doi: 10.1099/0022-1317-22-3-395. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Persistent synthesis of 5S RNA when production of 28S and 18S ribosomal RNA is inhibited by low doses of actinomycin D. J Cell Physiol. 1968 Dec;72(3):235–246. doi: 10.1002/jcp.1040720311. [DOI] [PubMed] [Google Scholar]
- Petermann M. L., Pavlovec A. The subunits and structural ribonucleic acids of Jensen sarcoma ribosomes. Biochim Biophys Acta. 1966 Feb 21;114(2):264–276. doi: 10.1016/0005-2787(66)90308-x. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Mathews M. B. Double-stranded RNA as an inhibitor of protein synthesis and as a substrate for a nuclease in extracts of Krebs II ascites cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):225–229. doi: 10.1073/pnas.70.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Stollar V. Effect of double-stranded RNAs from Sindbis virus-infected cells on rabbit reticulocyte and chick embryo cell-free protein synthesis. Biochim Biophys Acta. 1972 Dec 22;287(3):501–513. doi: 10.1016/0005-2787(72)90295-x. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simons K., Keränen S., Käriänen L. Identification of a precursor for one of the Semliki forest virus membrane proteins. FEBS Lett. 1973 Jan 15;29(2):87–91. doi: 10.1016/0014-5793(73)80532-0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Wheeler T., Glanville N., Käriäinen Translation of Semliki-Forest-virus 42-S RNA in a mouse cell free system to give virus-coat proteins. Eur J Biochem. 1974 Nov 1;49(1):101–110. doi: 10.1111/j.1432-1033.1974.tb03815.x. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Sindbis virus infection of chick and hamster cells: synthesis of virus-specific proteins. Virology. 1969 Mar;37(3):367–376. doi: 10.1016/0042-6822(69)90220-7. [DOI] [PubMed] [Google Scholar]
- Taber R., Rekosh D., Baltimore D. Effect of pactamycin on synthesis of poliovirus proteins: a method for genetic mapping. J Virol. 1971 Oct;8(4):395–401. doi: 10.1128/jvi.8.4.395-401.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengler G., Beato M., Hackemack B. A. Translation of 26 S virus-specific RNA from Semliki Forest virus-infected cells in vitro. Virology. 1974 Sep;61(1):120–128. doi: 10.1016/0042-6822(74)90247-5. [DOI] [PubMed] [Google Scholar]
- Wengler G. Comparative studies on polyribosomal, nonpolyribosome-associated and viral 42 S RNA from BHK 21 cells infected with Semliki Forest virus. Virology. 1975 Jun;65(2):601–605. doi: 10.1016/0042-6822(75)90068-9. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Medium hypertonicity and polyribosome structure in Hela cells. The influence of hypertonicity of the growth medium on polyribosomes in Hela cells. Eur J Biochem. 1972 May;27(1):162–173. doi: 10.1111/j.1432-1033.1972.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Studies on the polyribosome-associated RNA in BHK21 cells infected with Semliki Forest virus. Virology. 1974 May;59(1):21–35. doi: 10.1016/0042-6822(74)90202-5. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Zylber E. A., Penman S. The effect of high ionic strength on monomers, polyribosomes, and puromycin-treated polyribosomes. Biochim Biophys Acta. 1970 Mar 19;204(1):221–229. doi: 10.1016/0005-2787(70)90505-8. [DOI] [PubMed] [Google Scholar]



