Abstract
Background
Biliary tract carcinoma (BTC) is a fatal malignancy which aggressiveness contrasts sharply with its relatively mild and late clinical presentation. Novel molecular markers for early diagnosis and precise treatment are urgently needed. The purpose of this study was to evaluate the diagnostic and prognostic value of promoter hypermethylation of the SHOX2 and SEPT9 gene loci in BTC.
Methods
Relative DNA methylation of SHOX2 and SEPT9 was quantified in tumor specimens and matched normal adjacent tissue (NAT) from 71 BTC patients, as well as in plasma samples from an independent prospective cohort of 20 cholangiocarcinoma patients and 100 control patients. Receiver operating characteristic (ROC) curve analyses were performed to probe the diagnostic ability of both methylation markers. DNA methylation was correlated to clinicopathological data and to overall survival.
Results
SHOX2 methylation was significantly higher in tumor tissue than in NAT irrespective of tumor localization (p < 0.001) and correctly identified 71% of BTC specimens with 100% specificity (AUC = 0.918; 95% CI 0.865–0.971). SEPT9 hypermethylation was significantly more frequent in gallbladder carcinomas compared to cholangiocarcinomas (p = 0.01) and was associated with large primary tumors (p = 0.01) as well as age (p = 0.03). Cox proportional hazard analysis confirmed microscopic residual tumor at the surgical margin (R1-resection) as an independent prognostic factor, while SHOX2 and SEPT9 methylation showed no correlation with overall survival. Elevated DNA methylation levels were also found in plasma derived from cholangiocarcinoma patients. SHOX2 and SEPT9 methylation as a marker panel achieved a sensitivity of 45% and a specificity of 99% in differentiating between samples from patients with and without cholangiocarcinoma (AUC = 0.752; 95% CI 0.631–0.873).
Conclusions
SHOX2 and SEPT9 are frequently methylated in biliary tract cancers. Promoter hypermethylation of SHOX2 and SEPT9 may therefore serve as a minimally invasive biomarker supporting diagnosis finding and therapy monitoring in clinical specimens.
Electronic supplementary material
The online version of this article (doi:10.1186/s13148-016-0299-x) contains supplementary material, which is available to authorized users.
Background
Biliary tract cancers (BTC) comprise aggressive neoplasms arising from the epithelial lining of the intra- and extrahepatic bile ducts as well as the gallbladder. Cholangiocarcinoma (CC) and gallbladder carcinoma (GBC) account for only about 3% of all gastrointestinal malignancies [1]. Nonetheless, during the last decades, a steady increase in incidence and mortality rates has been reported for BTC in Europe as well as in the USA [2, 3]. This relatively uncommon malignancy is associated with an overall poor prognosis. In contrast to other cancer entities (e.g., breast, prostate, lymphoma), the 5-year survival rate of BTC has hardly changed during the last years and is not exceeding the 20% threshold [4–6]. Complete surgical resection with negative histological margins is the only potentially curative therapy to date though most patients rapidly develop recurrence even after R0 resection. Clinical symptoms normally appear late and most patients are therefore diagnosed in an advanced stage when a radical surgical resection is no longer possible [7]. Merely palliative treatment can be offered to patients with irresectable BTC [7, 8]. Even high resolution imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) are sometimes inaccurate. Furthermore, advanced endoscopic techniques including endoscopic ultrasonography fine needle aspiration (EUS-FNA) biopsy or brush cytology harvested within endoscopic retrograde cholangiography (ERC) not always lead to conclusive diagnoses [9, 10]. Moreover, established serum biomarkers for BTC like CA 19-9 and CEA have limited sensitivity and specificity in disease detection and response prediction [9, 11].
There is emerging evidence that genetic and epigenetic alterations play a pivotal role during human carcinogenesis [12, 13]. Specific DNA methylation patterns ensure proper gene expression, thereby regulating various fundamental organic processes, such as cellular differentiation and development [14]. Accordingly, aberrant methylation of CpG dinucleotides has been evidenced as a hallmark epigenetic alteration in numerous malignant diseases [14, 15]. Promoter hypermethylation, in particular, has been linked to transcriptional silencing of tumor suppressor genes, thus enabling neoplastic cells to proliferate without restriction [15]. In BTC and its precursor lesions, these epigenetic alterations can be detected with possible valuable clinical implications [16–29].
The short stature homeobox 2 gene (SHOX2) is located on chromosome 3q25 → 3q26.1 [30]. As a transcriptional factor, SHOX2 is known to be involved in various embryonic developmental processes including limb formation and cardiac development [31–33]. Aberrant DNA methylation of SHOX2 has been extensively characterized as a biomarker for the diagnosis of lung cancer. DNA from lung cancer specimens appeared to be significantly higher methylated at the SHOX2 gene locus as compared to morphologically normal adjacent tissue, and a correlation of hypermethylation and gene amplification has been described [34]. Thus, SHOX2 methylation has been suggested as a biomarker in body fluids, e.g., blood plasma, bronchial aspirates, ascites, and pleural effusions [35–41]. Accordingly, a commercially available in vitro diagnostic test kit, the Epi proLung® BL Reflex Assay (Epigenomics AG, Berlin, Germany) has been developed for sensitive assessment of SHOX2 methylation in bronchial aspirates [38]. Recently, increased SHOX2 methylation levels were also detected in metastatic lymph node tissue obtained by endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA) performed in patients with lung cancer, further improving the assessment of nodal status and staging in this entity [42].
Septins are a family of GTP-ase proteins which play a pivotal role in the cell cycle and cytokinesis [43, 44]. SEPT9, a member of the Septin gene family, has been reported to exhibit both oncogenic and tumor suppressive properties in tumorigenesis of solid and hematological malignancies [45]. DNA methylation of SEPT9 has been widely studied in colorectal cancer (CRC). Tumor DNA exhibited significantly higher SEPT9 methylation levels in comparison with matched normal colon epithelium [46, 47]. Hypermethylation of SEPT9 was also present in colon adenomas, thus indicating that this epigenetic alteration is an early event in the adenoma-carcinoma sequence [47]. Methylated SEPT9 has also been detected in plasma samples from colorectal cancer patients, and several studies have proposed its biomarker application for early diagnosis of CRC [46, 48–50]. Furthermore, SEPT9 was found to be frequently methylated in the head and neck and esophageal squamous cell carcinoma, as well as prostate cancer [51–53]. Recently, a panel of four DNA methylation markers including SEPT9 has been reported to aid in the diagnosis of cholangiocarcinoma in tissue as well as biliary brush samples [16].
Methylation status of SHOX2 and SEPT9 and their possible clinical implication has hardly been investigated in BTC. Due to the encouraging results found in other cancer entities, we hypothesize that these epigenetic alterations can serve as biomarkers in cancers arising from the biliary tract. Our long-term goal is to identify novel biomarkers for diagnostic and prognostic purposes to support and individualize treatment of this fatal disease.
Methods
This study was conducted with approval of the Institutional Review Board (IRB) at the University of Bonn, Germany.
Tissue samples
A total of 71 patients who underwent surgical resection for intrahepatic (IHC), perihilar (PHC), or distal cholangiocarcinoma (DC) as well as gallbladder carcinoma (GBC) at the Department of Surgery, University of Bonn, between January 1st, 1990, and December 31st, 2012, were enrolled. Inclusion criteria were (a) histological diagnosis of adenocarcinoma of the biliary tract (BTC); (b) primary resection of the tumor; (c) R0- or R1-resection; and (d) availability of archival formalin-fixed paraffin-embedded tissue blocks (Institute of Pathology, University of Bonn). All BTCs were staged according to the current edition of the American Joint Committee on Cancer (AJCC) cancer staging manual [54]. Relevant demographic and clinicopathological data were catalogued. Information on overall survival was obtained during follow-up visits in the outpatient clinic of our department, or by contacting patients or the general practitioners.
Hematoxylin and eosin slides were routinely prepared from FFPE tissue sections and reviewed under a light microscope. For each patient, an area with the highest tumor cellularity was selected, and a punch biopsy of 1-mm diameter was taken from the respective FFPE block. A second punch biopsy representing morphologically normal adjacent tissue (NAT) was performed.
Plasma samples
Preoperative plasma samples were collected prospectively from an independent cohort of 20 patients suffering from cholangiocarcinoma who underwent surgery between November 2013 and September 2016 at the Department of Surgery, University of Bonn. Tumor staging and documentation of relevant data was performed as mentioned above. Plasma samples from 100 gender- and age-matched patients who did not have a history of a malignant tumor served as controls. Written consent was obtained from all patients.
Preparation of sample DNA
For DNA extraction, bisulfite conversion and subsequent purification of genomic DNA from FFPE tissue biopsies and plasma samples, the innuCONVERT Bisulfite All-In-One Kit as well as the innuCONVERT Bisulfite Body Fluids Kit (Analytik Jena AG, Jena, Germany) were used, respectively. All kits were applied according to the manufacturer’s protocol.
Real-time PCR quantification of SHOX2 and SEPT9 DNA methylation
Relative DNA methylation of the SHOX2 and SEPT9 locus was quantified by a methylation-specific triplex qPCR assay as previously described [39]. Methylation status of the ACTB locus served as the reference. Oligonucleotides used in the assay are summarized in Additional file 1: Table S2.
The SHOX2 Amplicon is a 112 bp sequence and contains 11 CpGs. Four CpGs are included in the detection probe and three in the reverse primer. The SEPT9 Amplicon is 60 bp long and contains five CpGs, which are all covered by the blocking primer. Additionally, three out of five CpGs are covered by the detection probe. Twenty-five nanograms of template DNA was used as template DNA for each single PCR reaction. PCR was carried out using an Applied Biosystems 7500 Fast Real-Time PCR System (Life Technologies Corporation, Carlsbad, CA, USA) and the following temperature profile was applied: 20 min at 95 °C followed by 50 cycles with 2 s at 62 °C, 45 s at 56 °C (each at 100% ramp rate), and 15 s at 95 °C (at 75% ramp rate) [39].
Data analyses
The calibrator and all samples were measured in triplicate, and a mean average of the CT values was calculated. The ∆∆CT method was used to determine a relative methylation value for each valid sample as previously described: ∆∆CTSample = ∆CTSample − ∆CTCalibrator, where ∆CTSample = CTSample/Methylationquantificationassay − CTSample/Totalquantificationassay and ∆CTCalibrator = CTCalibrator/Methylationquantificationassay − CTCalibrator/Totalquantificationassay [55, 56]. Percent methylated reference (PMR) values were calculated applying the following formula: MethylationSample = 100% × 2−∆∆CT [56]. Quantitative SHOX2 and SEPT9 DNA methylation values were dichotomized by setting a methylation cut-off. The cut-off was set in order to reduce the false-positive rate for benign controls to 0% in tissue specimens and to 1% in plasma specimens. All samples with PMR values above the cut-off were classified as positive, while samples with methylation values below the cut-off were classified as negative.
Statistical analyses
Statistical analysis was performed using the SPSS software version 22 (IBM, Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA).
Mann-Whitney U test was applied to test for differences in DNA methylation levels between the two groups. Bonferroni correction was used in case of multiple pairwise comparisons. Pearson’s correlation between SHOX2 and SEPT9 methylation was analyzed. Receiver operating characteristics (ROC) curves were constructed to investigate the ability of DNA hypermethylation to differentiate between samples from BTC patients and samples from patients without BTC. The sum of SHOX2 and SEPT9 DNA methylation values was used to calculate ROC curves for performance evaluation of the combined biomarker panel. The area under the ROC curve (AUC) represents the ability of the test to distinguish between patients with and without BTC.
Overall survival was defined as the time between surgery and death or date of last patient contact and used as primary endpoint for outcome analysis. Univariate and multivariate Cox proportional hazard model was applied to test the association between DNA methylation, clinicopathological parameters, and overall survival. p values refer to Wald test. Two-sided p values <0.05 were considered as statistically significant.
Results
Results from tissue analyses
Patients and tumors
Tissue samples from 71 biliary tract cancers including 42 male (59%) and 29 female (41%) patients with a median age of 63 years (range 36–83) were analyzed (clinical and pathological characteristics are listed in Table 1). Cholangiocarcinomas outnumbered gallbladder carcinomas (n = 54, 76% vs. n = 17, 24%). The majority of cholangiocarcinomas were IHC (n = 25, 35%). Only five tumors (7%) were well differentiated (G1), while the remaining were graded as moderately (G2) or poorly differentiated (G3) (n = 33, 46.5% each). Most BTC were locally advanced and classified as T3 (n = 38, 53.5%). Positive regional lymph nodes were found in 16 patients (22.5%), while intrahepatic metastases were present in six patients upon final histology (M1; 8.5%). Accordingly, the majority of tumors was staged as UICC III (n = 28, 39%). R0-resection was achieved in most of patients (n = 52, 73%).
Table 1.
Clinicopathological data of 71 biliary tract cancer patients included in the tissue study. Associations of clinicopathological data with relative DNA methylation of SHOX2 and SEPT9 in tumor tissue
| SHOX2 methylation | SEPT9 methylation | |||
|---|---|---|---|---|
| Clinicopathological data | n | (%) | ||
| p value (n) | p value (n) | |||
| Tumor location | ||||
| GBC | 17 | (24) | ||
| IHC | 25 | (35) | ||
| PHC (Klatskin tumor) | 22 | (31) | ||
| DC | 7 | (10) | ||
| GBC vs. CC | 0.46 (55) | 0.014* (55) | ||
| Gender | ||||
| Male | 42 | (59) | ||
| Female | 29 | (41) | ||
| Male vs. female | 0.10 (55) | 0.14 (55) | ||
| Age at diagnosis | ||||
| ≤60 years | 31 | (44) | ||
| >60 years | 40 | (56) | ||
| Median age [years] | 63 | NA | ||
| Mean age [years] | 62 | NA | ||
| Range [years] | 36–83 | NA | ||
| ≤60 vs. >60 years | 0.57 (55) | 0.031* (55) | ||
| Tumor grade | ||||
| G1 | 5 | (7) | ||
| G2 | 33 | (46.5) | ||
| G3 | 33 | (46.5) | ||
| G1, G2, vs. G3 | 0.22 (55) | 0.68 (55) | ||
| Tumor stage | ||||
| T1 | 10 | (14) | ||
| T2 | 17 | (24) | ||
| T3 | 38 | (53.5) | ||
| T4 | 6 | (8.5) | ||
| T1, T2 vs. T3, T4 | 0.51 (55) | 0.013* (55) | ||
| Lymph node status | ||||
| N0 | 32 | (45.1) | ||
| N1 | 15 | (21.1) | ||
| N2 | 1 | (1.4) | ||
| Unknown | 23 | (32.4) | ||
| N0 vs. N1, N2 | 0.19 (39) | 0.30 (39) | ||
| Venous invasion | ||||
| V0 | 42 | (59) | ||
| V1 | 19 | (27) | ||
| Unknown | 10 | (14) | ||
| V0 vs. V1 | 0.97 (48) | 0.76 (48) | ||
| Lymphatic invasion | ||||
| L0 | 34 | (48) | ||
| L1 | 20 | (28) | ||
| Unknown | 17 | (24) | ||
| L0 vs. L1 | 0.67 (42) | 0.70 (42) | ||
| Perineural invasion | ||||
| Pn0 | 17 | (24) | ||
| Pn1 | 35 | (49) | ||
| Unknown | 19 | (27) | ||
| Pn0 vs. Pn1 | 0.37 (41) | 0.82 (41) | ||
| Distant metastases | ||||
| M0 | 65 | (91.5) | ||
| M1 | 6 | (8.5) | ||
| M0 vs. M1 | 0.32 (55) | 0.36 (55) | ||
| UICC stage | ||||
| UICC I | 4 | (6) | ||
| UICC II | 9 | (13) | ||
| UICC III | 28 | (39) | ||
| UICC IV | 10 | (14) | ||
| Unknown | 20 | (28) | ||
| UICC I, II vs. UICC III, IV | 0.08 (41) | 0.06 (41) | ||
| Surgical margin | ||||
| R0 | 52 | (73) | ||
| R1 | 19 | (27) | ||
| R0 vs. R1 | 0.24 (55) | 0.66 (55) | ||
| Follow-up | ||||
| Follow-up available | 71 | (100) | ||
| Median follow-up [months] | 15 | NA | ||
| Mean follow-up [months] | 23 | NA | ||
| Range [months] | 0–104 | NA | ||
| Deceased | 50 | (70) | ||
| Censored | 21 | (30) | ||
p values refer to the Mann-Whitney U test
*p < 0.05
DNA methylation levels of SHOX2 and SEPT9
Percent methylated reference (PMR) values of the SHOX2 and SEPT9 gene obtained by quantitative methylation-specific real-time PCR are illustrated in Fig. 1. Valid measurements were obtained from 55 BTC and 41 NAT specimens. The performance of both methylation markers in differentiating tumor tissue from NAT was evaluated in all 55 BTC specimens collectively, as well as in CC (n = 43) and GBC (n = 12) individually. Background methylation with a maximum of 2.52% (SHOX2) and 2.32% (SEPT9) was present in most NAT samples, thus necessitating cut-offs for both gene loci to classify samples as either methylated or unmethylated.
Fig. 1.

SHOX2 and SEPT9 DNA methylation levels in tissue samples. Relative DNA methylation values (PMR in %) of SHOX2 are depicted as black rhombuses for a NAT and BTC specimens, as well as for b NAT, CC, and GBC specimens. PMR values of SEPT9 are also depicted for c NAT and BTC specimens, as well as for d NAT, CC, and GBC specimens. Horizontal lines indicate median PMR values of sample series. p values refer to Mann-Whitney U test. *Bonferroni corrected p values for multiple pairwise compare
SHOX2 showed significantly higher DNA methylation values in BTC specimens and both subpopulations compared to NAT (Fig. 1a, b). Among 55 BTC specimens, 39 were correctly rated as SHOX2 positive, resulting in a sensitivity of 71% with 100% specificity. SHOX2 methylation frequencies in CC and GBC were 72 and 67%, respectively. No significant difference in SHOX2 methylation levels was observed between CC and GBC (Fig. 1b).
Elevated SEPT9 DNA methylation levels were also found in BTC specimens compared to NAT. However, the results were not statistically significant (Fig. 1c). Sixteen BTC samples showed SEPT9 positivity, providing a sensitivity and specificity of 29 and 100%, respectively. If analyzed separately, however, carcinomas of the gallbladder presented significantly higher SEPT9 methylation levels than both NAT and CC (Fig. 1d). While the methylation frequency of SEPT9 reached only 19% in CC, it was as high as 67% in GBC.
In a further step, the combined performance of SHOX2 and SEPT9 as a biomarker panel was evaluated. This panel was considered positive if at least one of both genes displayed DNA hypermethylation. Since high methylation values of SHOX2 did not correlate significantly with SEPT9 hypermethylation (Pearson’s correlation coefficient r = 0.24, p value = 0.08), the combined panel was expected to detect more cancer specimens than SHOX2 or SEPT9 alone. As anticipated, the panel correctly identified 75, 74, and 75% of BTC, CC, and GBC specimens, respectively. All 41 NAT samples were rated as SHOX2 and SEPT9 negative, resulting in a specificity of 100%. The ROC curves and AUC values for SHOX2, SEPT9, and the combined panel in BTC as well as in CC and GBC specimens are illustrated in Fig. 2.
Fig. 2.
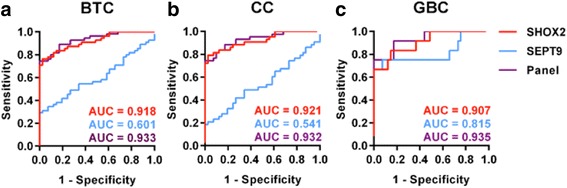
Receiver operating characteristic (ROC) curves for SHOX2, SEPT9, and the combined biomarker panel (based on the sum of PMR values) in tissue specimens. The ROC curves and resulting area under the curve (AUC) values are depicted for a all 55 BTC specimens: AUC—SHOX2 = 0.918 [95% CI 0.865–0.971]; AUC—SEPT9 = 0.601 [95% CI 0.488–0.713]; AUC—Panel 0.933 [95% CI 0.886–0.979] b 43 CC specimens: AUC—SHOX2 = 09.21 [0.863–0.979]; AUC—SEPT9 = 0,.541 [95% CI 0.416–0.666]; AUC—Panel = 0.932 [95% CI 0.880–0.984] and c 12 GBC specimens: AUC—SHOX2 = 0.907 [95% CI 0.808–1.005]; AUC—SEPT9 = 0.815 [95% CI 0.637–0.993]; AUC—Panel = 0.935 [95% CI 0.855–1.015], respectively
Three BTC samples showed relative SHOX2 DNA methylation values above the theoretically expected maximum of 100% (109.2, 127.6, and 140.8%). Similarly high SEPT9 methylation levels were also found in two other cases (129.7 and 183.8%). Relative SHOX2 and SEPT9 DNA methylation values were determined by a triplex assay, which included a reference PCR of the ACTB gene to quantify the total amount of DNA. PMR values >100% have been previously reported for SHOX2 in lung cancer, when ACTB was used as a reference, and were attributed to amplification of the SHOX2 locus or deletion of the ACTB locus [34, 36, 56]. Therefore, we presume that deletion of the ACTB locus or amplification of the SHOX2 and SEPT9 locus, respectively, may have also led to PMR values >100% in these five BTC samples.
Association of DNA methylation with clinicopathological parameters
Elevated SEPT9 methylation levels correlated significantly with advanced size of the primary tumor (T1/T2 vs. T3/T4, p = 0.013) as well as patient age (≤60 years vs. > 60 years, p = 0.031) (Table 1). However, no significant association was found between SHOX2 or SEPT9 methylation and the remaining clinicopathological parameters.
Survival analyses
Data on overall survival were available for all 71 patients. Median follow-up was 15 months (range 0–104 months), and 50 patients (70%) deceased during the follow-up time. Results from uni- and multivariate Cox proportional hazards analyses are shown in Table 2. Univariate survival analysis revealed that lymph node involvement (p = 0.031, HR = 2.2), lymphatic invasion (p = 0.007, HR = 2.4), and surgical margin status (p < 0.001, HR = 3.5) were of prognostic significance. On multivariate analysis merely microscopic residual carcinoma at the final surgical margin (R1) proved to be an independent prognostic factor associated with a dismal outcome (p = 0.026, HR = 3.8). DNA methylation status of SHOX2 and SEPT9 in tumor tissue showed no correlation with overall survival.
Table 2.
Results from univariate and multivariate survival analyses (Cox proportional hazard models)
| Number of patients | Hazard ratio [95% CI] | p value (Wald test) | ||
|---|---|---|---|---|
| Univariate analysis | ||||
| Tumor location | (GBC vs. CC) | 71 | 0.9 [0.5–1.8] | 0.83 |
| Gender | (Male vs. female) | 71 | 1.4 [0.8–2.5] | 0.22 |
| Age at diagnosis | (≤60 vs. >60 years) | 71 | 1.1 [0.6–2.0] | 0.72 |
| Tumor grade | (G1, G2 vs. G3) | 71 | 1.6 [0.9–2.7] | 0.13 |
| Tumor stage | (T1, T2 vs. T3, T4) | 71 | 1.0 [0.6–1.8] | 0.94 |
| Lymph node status | (N1, N2 vs. N0) | 48 | 2.2 [1.1–4.5] | 0.031* |
| Venous invasion | (V0 vs. V1) | 61 | 1.3 [0.7–2.5] | 0.42 |
| Lymphatic invasion | (L1 vs. L0) | 54 | 2.4 [1.3–4.6] | 0.007* |
| Perineural invasion | (Pn0 vs. Pn1) | 52 | 1.3 [0.6–2.5] | 0.50 |
| Distant metastases | (M0 vs. M1) | 71 | 2.4 [0.9–6.1] | 0.08 |
| UICC stage | (UICC I, II vs. UICC III, IV) | 51 | 1.0 [0.5–2.0] | 0.91 |
| Surgical margin | (R1 vs. R0) | 71 | 3.5 [1.8–6.7] | <0.001* |
| SHOX2 methylationa | 55 | 1.0 [1.0–1.0] | 0.52 | |
| SEPT9 methylationa | 55 | 1.0 [1.0–1.0] | 0.78 | |
| SHOX2 methylation | (SHOX2− vs. SHOX2+) | 55 | 1.3 [0.6–2.6] | 0.51 |
| SEPT9 methylation | (SEPT9− vs. SEPT9+) | 55 | 1.1 [0.6–2.4] | 0.72 |
| Multivariate analysis | ||||
| Lymph node status | (N0 vs. N1, N2) | 1.5 [0.6–3.5] | 0.36 | |
| Lymphatic invasion | (L0 vs. L1) | 34 | 2.1 [0.9–5.3] | 0.11 |
| Surgical margin | (R0 vs. R1) | 3.8 [1.2–12.2] | 0.026* | |
SHOX2 and SEPT9 DNA methylation levels were analyzed as continuous and dichotomized variables. p values refer to the Wald test
*p < 0.05
aContinuous variable
Results from plasma analyses
DNA from plasma samples taken preoperatively from 20 cholangiocarcinoma patients, and 100 matched controls was analyzed for relative SHOX2 and SEPT9 DNA methylation (see Additional file 2: Table S1 for clinicopathological characteristics of both cohorts). Valid measurements were obtained from all 120 plasma samples, and respective PMR values are shown in Fig. 3. As in tissue analyses, detectable background methylation in samples from benign controls required a cut-off for dichotomization.
Fig. 3.

Relative DNA methylation values (PMR in %) of a SHOX2 and b SEPT9 in plasma samples from 100 controls and 20 cholangiocarcinoma patients. Each black rhombus represents a patient sample. Horizontal lines indicate median PMR values of sample series. p values refer to Mann-Whitney U test
SHOX2 DNA methylation was significantly higher in plasma specimens from CC patients than from controls and correctly identified 35% (7/20) of carcinoma cases (Fig. 3a). One control sample was falsely classified as SHOX2 positive, resulting in 99% specificity. The area under the curve (AUC) was 0.691 (95% CI 0.554–0.828).
Similarly, plasma samples from patients with CC showed increased PMR values of the SEPT9 gene locus (Fig. 3b). Five plasma samples showed SEPT9 methylation values above the cut-off, resulting in a sensitivity of 25% with 99% specificity and an AUC of 0.699 (95% CI 0.561–0.837).
As in tumor tissue, no correlation between SHOX2 and SEPT9 DNA methylation was observed (Pearson’s correlation coefficient r = 0.072, p = 0.76). Therefore, SHOX2 and SEPT9 as a panel displayed a higher sensitivity than both gene loci. Nine out of 20 cholangiocarcinoma cases (45%) were detected, while 99% of control samples were accurately classified as SHOX2 and SEPT9 negative. The combined biomarker panel achieved an area under the curve (AUC) of 0.752 (95% CI 0.631–0.873). The ROC curves and AUC values of SHOX2, SEPT9, and the combined panel in plasma samples from cholangiocarcinoma patients are shown in Fig. 4
Fig. 4.
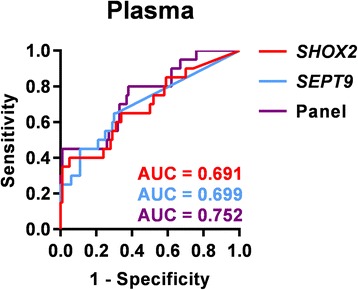
Receiver operating characteristic (ROC) curves and area under the curve (AUC) values for SHOX2, SEPT9, and the combined biomarker panel, based on the sum of PMR values, in plasma samples. AUC—SHOX2 = 0.691 [95% CI 0.554–0.828]; AUC-SEPT9 = 0.699 [95% CI 0.561–0.837]; AUC-Panel = 0.752 [95% CI 0.631–0.873]
Discussion
Epigenetic alterations such as aberrant DNA methylation patterns have been extensively studied across multiple human cancer types, with BTC being no exception. Genome-wide methylation analyses in CC revealed frequent promoter hypermethylation of homeobox genes, a gene class known to be involved in various developmental processes [17, 18]. Recently, Goeppert et al. published global methylation data for intrahepatic and extrahepatic CC and identified numerous aberrantly methylated genes involved in cholangiocarcinogenesis, including components of the Wnt signaling pathway [19]. Unlike the above referenced, the majority of studies has focused on the methylation status of a few specific gene loci and their capability to distinguish between malignant and benign tissues, biliary brush cytologies, and bile juice specimens [16, 20–28]. In the present study, a similar approach was followed by selecting two candidate genes for methylation analysis in BTC, namely SHOX2 and SEPT9, which have been reported to be aberrantly methylated in other tumor entities. Due to afore evidenced clinical implications, we sought to evaluate the methylation status of SHOX2 and SEPT9 and their potential as biomarkers in tissue and plasma specimens from BTC patients.
To our knowledge, this is the first report of recurrent SHOX2 hypermethylation in BTC. SHOX2 methylation status has been previously investigated in other malignant tissue specimens with varying results. Ninety-six percent of lung cancer samples showed more pronounced methylation than matched NAT samples from the same patients [34]. Merely 36% of papillary thyroid carcinomas were found to be methylated at the SHOX2 locus [57]. We observed a SHOX2 hypermethylation in 71% of BTC specimens while no normal tissue sample harbored this alteration emphasizing the malignant association.
SEPT9, as part of a four genes panel, has been recently evaluated as a biomarker for CC in tumor tissue as well as in biliary brush samples [16]. In tissue specimens, SEPT9 was described to differentiate between CC and non-malignant controls with a sensitivity of 26% and a specificity of 100% (AUC = 0.63) [16]. Our study supported these results, although we observed SEPT9 to be slightly less sensitive in CC. This is possibly due to selection bias in relatively small cohorts of rare entities. While our samples solely comprised formalin-fixed and paraffin-embedded tissue, Andresen et al. included fresh frozen tissue samples, too [16]. In a previous study using the same CC series, both sample materials were compared and methylation frequencies of CDO1, ZSCAN18, SFRP1, and DCLK1 were found to be generally lower in FFPE specimens than in the fresh frozen sample set while modern sensitive techniques may overcome this observation [29].
In our study, we demonstrated that the DNA methylation of SHOX2 and SEPT9 can accurately differentiate between tumor tissue from BTC patients and normal adjacent tissue with high specificity. However, the performance of both markers varied markedly in terms of sensitivity, and SHOX2 outperformed SEPT9 in detecting BTC by more than double. The results confirm our hypothesis that epigenetic alterations of SHOX2 and SEPT9 may serve as biomarkers to support the detection of BTC in tissue specimens due to its high specificity, and a use as biomarkers for response prediction can be discussed. However, the application of this methylation panel test in the clinical setting as a diagnostic tool may be limited because of its limited sensitivity. In order to overcome the low sensitivity, other biomarkers could be added to the panel. A recent study identified DNA methylation level of OPCML and SFRP1 as a potential diagnostic biomarker in BTC [58]. The AUC value of OPCML was 0.932 and of SFRP1 was 0.951. The sensitivity and specificity of OPCML were 89 and 100%, respectively, and of SFRP1 were 83.6 and 85.5%. The analysis of a combined methylation panel including SFPR1, OPCML, SHOX2, and SEPT9 in plasma could increase the diagnostic value of the methylation panel.
The fact that SHOX2 and SEPT9 show different promoter methylation in BTC plasma specimens also suggests a clinical application for minimally invasive diagnosis in various clinical samples. Half a century after Mendel and Metais discovered the presence of cell-free nucleic acids in human blood, it has become generally acknowledged that extracellular DNA from cancer patients also harbors tumor-related epigenetic alterations [59]. Accordingly, aberrant methylation of circulating cell-free DNA has been extensively studied as a biomarker in human cancer [60]. To our knowledge, larger studies on quantification of methylated circulating DNA in BTC are thus far not published. Herein, we report for the first time that altered promoter methylation can also be detected in plasma from patients with cholangiocarcinoma. SHOX2 and SEPT9 DNA methylation levels showed a high specificity in differentiating between plasma from CC patients and controls. However, methylation analysis of SHOX2 and SEPT9 in plasma, naturally containing circulating DNA from any site of the body, bears some disadvantages for diagnostic purposes in BTC. In fact, the amount of methylated DNA derived from the biliary tract is presumably low in proportion to total sample DNA. This could explain the limited sensitivity observed in our study. The addition of other established biomarker like CA19-9 to this panel could increase the test sensitivity. This issue should be investigated in future prospective studies in order to asses the value of this methylation panel as a part of a more complex diagnostic tool.
Interestingly, aberrant DNA methylation patterns have been observed in bile fluid as well as in biliary brush cytology specimens and were described to be capable of differentiating between BTC and benign controls [16, 25–28]. SEPT9 has recently been reported to achieve a sensitivity of 57% with 100% specificity in biliary brush samples from CC patients, while respective tissue samples were methylated in less than 30% [16]. In our tissue study, SHOX2 methylation proved to be superior to SEPT9 in terms of sensitivity to correctly identify BTC specimens. Therefore, it seems reasonable to suggest that SHOX2 DNA methylation could also serve as biomarker for BTC in biliary brush samples or bile fluids. Andresen et al. investigated a four-gene methylation biomarker panel (CDO1, CNRIP1, SEPT9, and VIM) in biliary brush sample of patients with CC. This test achieved a 85% sensitivity and a 98% specificity. Thus, future investigations should analyze the role of a five-gene panel including CDO1, CNRIP1, VIM, SEPT9, and SHOX2 as a diagnostic tool in plasma from patients with BTC.
In our cohort, residual carcinoma at the final histological margin proved to be the only independent predictor of survival. This finding is in line with previous studies linking R1-resection to an unfavorable outcome in BTC [61–63]. Methylation status of SHOX2 and SEPT9 showed no relation to overall survival in our cohort. Nevertheless, a prognostic value of methylated SHOX2 and SEPT9 has been reported in the literature, although the findings were partially ambiguous. For instance, low SHOX2 DNA methylation values predicted a shorter progression-free survival following resection in non-small cell lung cancer, whereas hypermethylation of SHOX2 in pleural effusions was associated with an adverse outcome [39, 56]. Schmidt et al. recently proposed quantitative measurement of methylated SHOX2 DNA in plasma as a useful tool to monitor therapy response in advanced stage lung cancer patients [36]. Furthermore, an increased methylation level of SEPT9 was a predictor of poor prognosis in esophageal squamous cell carcinoma, as well as in prostate cancer on androgen deprivation [52, 53]. Likewise, colorectal cancer patients with high SEPT9 serum methylation levels at 1-year follow-up were found to be at high risk of disease recurrence [64]. Although these promising findings in other cancer entities suggested that aberrant SHOX2 and SEPT9 methylation may also correlate with prognosis in BTC, our results did not confirm this hypothesis. This could be attributed to our relatively limited sample number. Therefore, we recommend further research on the prognostic value of SHOX2 and SEPT9 DNA methylation in a larger cohort of BTC patients.
Our study has some limitations: Due to the rareness of BTC, we only included a limited number of patients, and CCs were not subgrouped into IHC, PHC, and DC though distinct differences in methylation profiles have been observed between intrahepatic and extrahepatic CC [22, 23]. Moreover, a subgroup analysis of clinicopathological data and methylation status separately for CC and GBC was not performed. The subgroups were not large enough in order to obtain a sufficient statistical power. Therefore, we did not perform an association analysis for CC and GBC. Thus, a larger study cohort, possibly based on a multicenter approach in a prospective fashion, is needed to more precisely assess the potential of SHOX2 and SEPT9 methylation as a biomarker for all four subgroups of biliary tract cancer. Another limitation of our study is the lack of a targeted sequencing of SHOX2 and SEPT9. This could add some important information concerning the promoter methylation status of both genes. Thus, we cannot provide information about the methylated CpG sites in tumor and normal tissue. Wasserkort et al. already demonstrated that hypermethylation of SEPT9 in adenoma and CRC specimens is limited to one of the several CpG islands [47]. Moreover, investigation of intragenic CpGs and alternative promoters could add valuable information about these two genes. Therefore, a targeted sequencing should be performed in further studies in order to clarify the methylation status of SHOX2 and SEPT9 in cancer.
Conclusions
Biliary tract cancer remains a fatal disease; thus, novel molecular markers enabling early diagnosis, treatment implementation, and therapy monitoring are urgently needed. Epigenetic alterations are promising for the development of biomarkers in human cancer. In this study, we showed that the SHOX2 gene locus is frequently methylated in BTC. In addition, our results confirmed the presence of aberrant methylation of the SEPT9 locus in BTC. Together, both markers identified 75% of BTC specimens with 100% specificity. Furthermore, we demonstrated that methylated SHOX2 and SEPT9 DNA can be detected in plasma from BTC patients highlighting a use in clinical samples for minimally invasive diagnostic applications and therapy monitoring. Future studies are needed to validate the biomarker capabilities of SHOX2 and SEPT9 in BTC before a possible establishment in clinical practice.
Acknowledgements
None.
Funding
This study was supported by a BONFOR grant of the Bonn University Medical Faculty provided to Hanno Matthaei (O-112005).
Availability of data and materials
The datasets analysed during the current study available from the corresponding author on reasonable request.
Authors’ contributions
VB and PS made substantial contributions to conception and coordination of the study, acquisition and analysis of data. HM and DD made substantial contributions to the study design, data interpretation, and study supervision. All authors critically revised the manuscript and gave final approval of the version to be submitted.
Competing interests
Dimo Dietrich has been an employee and is a stockholder of Epigenomics AG, a company that aims to commercialize the DNA methylation biomarkers SEPT9 and SHOX2. Dimo Dietrich is co-inventor and owns patents on methylation biomarkers and related technologies. These patents are commercially exploited by Epigenomics AG. Dimo Dietrich receives inventor’s compensation from Epigenomics AG. Dimo Dietrich is a consultant for AJ Innuscreen GmbH (Berlin, Germany), a 100% daughter company of Analytik Jena AG (Jena, Germany), and receives royalties from product sales. All other authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was conducted with approval of the Institutional Review Board (IRB) at University of Bonn, Germany.
Abbreviations
- AJCC
American Joint Committee on Cancer
- AUC
Area under the ROC curve
- BTC
Biliary tract carcinoma
- CC
Cholangiocarcinoma
- CEA
Carcinoembryonic antigen
- CRC
Colorectal cancer
- DC
Distal cholangiocarcinoma
- EBUS-TBNA
Endobronchial ultrasound with transbronchial needle aspiration
- ERC
Endoscopic retrograde cholangiography
- EUS-FNA
Endoscopic ultrasonography fine needle aspiration
- FFPE
Formalin-fixed paraffin-embedded
- GBC
Gallbladder carcinoma
- IHC
Intrahepatic cholangiocarcinoma
- NAT
Normal adjacent tissue
- PCR
Polymerase chain reaction
- PHC
Perihilar cholangiocarcinoma
- PMR
Percent methylated reference
- ROC
Receiver operating characteristic
Additional files
Primers locations and sequences. (DOCX 181 kb)
Clinicopathological data of the 20 biliary tract cancer cases and 100 gender- and age-matched controls included in plasma study. (XLSX 116 kb)
Footnotes
Vittorio Branchi and Pauline Schaefer have joint first authorship.
Dimo Dietrich and Hanna Matthaei are joint senior authors.
Contributor Information
V. Branchi, Email: vittorio.branchi@ukb.uni-bonn.de
P. Schaefer, Email: pauline.schaefer@gmx.de
A. Semaan, Email: alexander.semaan@ukb.uni-bonn.de
A. Kania, Email: alexander.kania@ukb.uni-bonn.de
P. Lingohr, Email: philipp.lingohr@ukb.uni-bonn.de
J. C. Kalff, Email: kalff@uni-bonn.de
N. Schäfer, Email: nico.schaefer@ukb.uni-bonn.de
G. Kristiansen, Email: kristiansen@uni-bonn.de
D. Dietrich, Email: dimo.dietrich@ukb.uni-bonn.de
H. Matthaei, Phone: +49-(0)-228-287-15151, Email: hanno.matthaei@ukb.uni-bonn.de
References
- 1.Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8(4):189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertuccio P, Bosetti C, Levi F, Decarli A, Negri E, La Vecchia C. A comparison of trends in mortality from primary liver cancer and intrahepatic cholangiocarcinoma in Europe. Ann Oncol. 2013;24(6):1667–74. doi: 10.1093/annonc/mds652. [DOI] [PubMed] [Google Scholar]
- 3.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33(6):1353–7. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 4.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, Sant M, Trama A, Faivre J; EUROCARE-5 Working Grouph. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999-2007: Results of EUROCARE-5. Eur J Cancer. 2015. doi:10.1016/j.ejca.2015.07.034. [Epub ahead of print]. [DOI] [PubMed]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 7.Skipworth JRA, Olde Damink SWM, Imber C, Bridgewater J, Pereira SP, Malagó M. Review article: surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment Pharmacol Ther. 2011;34(9):1063–78. doi: 10.1111/j.1365-2036.2011.04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–79. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(9):512–22. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnett AS, Calvert TJ, Chokshi RJ. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J Surg Res. 2013;184(1):304–11. doi: 10.1016/j.jss.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 11.Skipworth JR, Timms JF, Pereira SP. Novel diagnostic and prognostic biomarkers in biliary tract cancer. Expert Opin Med Diagn. 2013;7(5):487–99. doi: 10.1517/17530059.2013.826646. [DOI] [PubMed] [Google Scholar]
- 12.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10(8):789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 13.Shen H, Laird PW. Interplay between the cancer genome and epigenome. Cell. 2013;153(1):38–55. doi: 10.1016/j.cell.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6(8):597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 15.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 16.Andresen K, Boberg KM, Vedeld HM, Honne H, Jebsen P, Hektoen M, et al. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61(5):1651–9. doi: 10.1002/hep.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sriraksa R, Zeller C, Dai W, Siddiq A, Walley AJ, Limpaiboon T, et al. Aberrant DNA methylation at genes associated with a stem cell-like phenotype in cholangiocarcinoma tumors. Cancer Prev Res (Phila) 2013;6(12):1348–55. doi: 10.1158/1940-6207.CAPR-13-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu Y, Wang B, Wang J, Wang J, Zou S. Identification of methylation profile of HOX genes in extrahepatic cholangiocarcinoma. World J Gastroenterol. 2011;17(29):3407–19. doi: 10.3748/wjg.v17.i29.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goeppert B, Konermann C, Schmidt CR, Bogatyrova O, Geiselhart L, Ernst C, et al. Global alterations of DNA methylation in cholangiocarcinoma target the Wnt signaling pathway. Hepatology. 2014;59(2):544–54. doi: 10.1002/hep.26721. [DOI] [PubMed] [Google Scholar]
- 20.Andresen K, Boberg KM, Vedeld HM, Honne H, Hektoen M, Wadsworth CA, et al. Novel target genes and a valid biomarker panel identified for cholangiocarcinoma. Epigenetics. 2012;7(11):1249–57. doi: 10.4161/epi.22191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim B, Cho N, Shin SH, Kwon H, Jang JJ, Kang GH. CpG island hypermethylation and repetitive DNA hypomethylation in premalignant lesion of extrahepatic cholangiocarcinoma. Virchows Arch. 2009;455(4):343–51. doi: 10.1007/s00428-009-0829-4. [DOI] [PubMed] [Google Scholar]
- 22.Yang B, House MG. Guo M, Herman JG, Clark DP. Promoter methylation profiles of tumor suppressor genes in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol. 2005;18(3):412–20. doi: 10.1038/modpathol.3800287. [DOI] [PubMed] [Google Scholar]
- 23.Kim B, Cho N, Choi M, Lee S, Jang JJ, Kang GH. Methylation profiles of multiple CpG island loci in extrahepatic cholangiocarcinoma versus those of intrahepatic cholangiocarcinomas. Arch Pathol Lab Med. 2007;131(6):923–30. doi: 10.5858/2007-131-923-MPOMCI. [DOI] [PubMed] [Google Scholar]
- 24.Kagohara LT, Schussel JL, Subbannayya T, Sahasrabuddhe N, Lebron C, Brait M, et al. Global and gene-specific DNA methylation pattern discriminates cholecystitis from gallbladder cancer patients in Chile. Future Oncol. 2014. [DOI] [PMC free article] [PubMed]
- 25.Shin S, Lee K, Kim B, Cho N, Jang J, Kim Y, et al. Bile-based detection of extrahepatic cholangiocarcinoma with quantitative DNA methylation markers and its high sensitivity. J Mol Diagn. 2012;14(3):256–63. doi: 10.1016/j.jmoldx.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Klump B, Hsieh C, Dette S, Holzmann K, Kiebetalich R, Jung M, et al. Promoter methylation of INK4a/ARF as detected in bile-significance for the differential diagnosis in biliary disease. Clin Cancer Res. 2003;9(5):1773–8. [PubMed] [Google Scholar]
- 27.Kato N, Yamamoto H, Adachi Y, Ohashi H, Taniguchi H, Suzuki H, et al. Cancer detection by ubiquitin carboxyl-terminal esterase L1 methylation in pancreatobiliary fluids. World J Gastroenterol. 2013;19(11):1718–27. doi: 10.3748/wjg.v19.i11.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsi MA, Li A, Li C, Goggins M. DNA methylation alterations in endoscopic retrograde cholangiopancreatography brush samples of patients with suspected pancreaticobiliary disease. Clin Gastroenterol Hepatol. 2008;6(11):1270–8. doi: 10.1016/j.cgh.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letelier P, Brebi P, Tapia O, Roa JC. DNA promoter methylation as a diagnostic and therapeutic biomarker in gallbladder cancer. Clin Epigenetics. 2012;4(1):11. doi: 10.1186/1868-7083-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Baere E, Speleman F, van Roy N, de Paepe A, Messiaen L. Assignment of SHOX2 (alias OG12X and SHOT) to human chromosome bands 3q25--q26.1 by in situ hybridization. Cytogenet Cell Genet. 1998;82(3–4):228–9. doi: 10.1159/000015108. [DOI] [PubMed] [Google Scholar]
- 31.Blaschke RJ, Monaghan AP, Schiller S, Schechinger B, Rao E, Padilla-Nash H, et al. SHOT, a SHOX-related homeobox gene, is implicated in craniofacial, brain, heart, and limb development. Proc Natl Acad Sci U S A. 1998;95(5):2406–11. doi: 10.1073/pnas.95.5.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu L, Liu H, Yan M, Yang J, Long F, Muneoka K, et al. Shox2 is required for chondrocyte proliferation and maturation in proximal limb skeleton. Dev Biol. 2007;306(2):549–59. doi: 10.1016/j.ydbio.2007.03.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Espinoza-Lewis RA, Chen C, Hu X, Zhang Y, Chen Y. The role of Shox2 in SAN development and function. Pediatr Cardiol. 2012;33(6):882–9. doi: 10.1007/s00246-012-0179-x. [DOI] [PubMed] [Google Scholar]
- 34.Schneider KU, Dietrich D, Fleischhacker M, Leschber G, Merk J, Schaper F, et al. Correlation of SHOX2 gene amplification and DNA methylation in lung cancer tumors. BMC Cancer. 2011;11:102. doi: 10.1186/1471-2407-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kneip C, Schmidt B, Seegebarth A, Weickmann S, Fleischhacker M, Liebenberg V, et al. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer in plasma. J Thorac Oncol. 2011;6(10):1632–8. doi: 10.1097/JTO.0b013e318220ef9a. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt B, Beyer J, Dietrich D, Bork I, Liebenberg V, Fleischhacker M, et al. Quantification of cell-free mSHOX2 plasma DNA for therapy monitoring in advanced stage non-small cell (NSCLC) and small-cell lung cancer (SCLC) patients. PLoS One. 2015;10(2):e0118195. doi: 10.1371/journal.pone.0118195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt B, Liebenberg V, Dietrich D, Schlegel T, Kneip C, Seegebarth A, et al. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer based on bronchial aspirates. BMC Cancer. 2010;10:600. doi: 10.1186/1471-2407-10-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietrich D, Kneip C, Raji O, Liloglou T, Seegebarth A, Schlegel T, et al. Performance evaluation of the DNA methylation biomarker SHOX2 for the aid in diagnosis of lung cancer based on the analysis of bronchial aspirates. Int J Oncol. 2012;40(3):825–32. doi: 10.3892/ijo.2011.1264. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich D, Jung M, Puetzer S, Leisse A, Holmes EE, Meller S, et al. Diagnostic and prognostic value of SHOX2 and SEPT9 DNA methylation and cytology in benign, paramalignant and malignant pleural effusions. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilse P, Biesterfeld S, Pomjanski N, Fink C, Schramm M. SHOX2 DNA methylation is a tumour marker in pleural effusions. Cancer Genomics Proteomics. 2013;10(5):217–23. [PubMed] [Google Scholar]
- 41.Jung M, Pützer S, Gevensleben H, Meller S, Kristiansen G, Dietrich D. Diagnostic and prognostic value of SHOX2 and SEPT9 DNA methylation and cytology in benign, paramalignant, and malignant ascites. Clin Epigenetics. 2016;8:24. doi: 10.1186/s13148-016-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Darwiche K, Zarogoulidis P, Baehner K, Welter S, Tetzner R, Wohlschlaeger J, et al. Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Ann Oncol. 2013;24(11):2866–70. doi: 10.1093/annonc/mdt365. [DOI] [PubMed] [Google Scholar]
- 43.Hall PA, Russell SE. The pathobiology of the septin gene family. J Pathol. 2004;204(4):489–505. doi: 10.1002/path.1654. [DOI] [PubMed] [Google Scholar]
- 44.Estey MP, Di Ciano-Oliveira C, Froese CD, Fung YY, Steels JD, Litchfield DW, et al. Mitotic regulation of SEPT9 protein by cyclin-dependent kinase 1 (Cdk1) and Pin1 protein is important for the completion of cytokinesis. J Biol Chem. 2013;288(42):30075–86. doi: 10.1074/jbc.M113.474932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connolly D, Abdesselam I, Verdier-Pinard P, Montagna C. Septin roles in tumorigenesis. Biol Chem. 2011;392(8–9):725–38. doi: 10.1515/BC.2011.073. [DOI] [PubMed] [Google Scholar]
- 46.Lofton-Day C, Model F, Devos T, Tetzner R, Distler J, Schuster M, et al. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54(2):414–23. doi: 10.1373/clinchem.2007.095992. [DOI] [PubMed] [Google Scholar]
- 47.Wasserkort R, Kalmar A, Valcz G, Spisak S, Krispin M, Toth K, et al. Aberrant septin 9 DNA methylation in colorectal cancer is restricted to a single CpG island. BMC Cancer. 2013;13:398. doi: 10.1186/1471-2407-13-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.deVos T, Tetzner R, Model F, Weiss G, Schuster M, Distler J, et al. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem. 2009;55(7):1337–46. doi: 10.1373/clinchem.2008.115808. [DOI] [PubMed] [Google Scholar]
- 49.Church TR, Wandell M, Lofton-Day C, Mongin SJ, Burger M, Payne SR, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63(2):317–25. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, et al. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011;9:133. doi: 10.1186/1741-7015-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bennett KL, Karpenko M, Lin M, Claus R, Arab K, Dyckhoff G, et al. Frequently methylated tumor suppressor genes in head and neck squamous cell carcinoma. Cancer Res. 2008;68(12):4494–9. doi: 10.1158/0008-5472.CAN-07-6509. [DOI] [PubMed] [Google Scholar]
- 52.Kuo I, Chang J, Jiang S, Chen C, Chang I, Sheu B, et al. Prognostic CpG methylation biomarkers identified by methylation array in esophageal squamous cell carcinoma patients. Int J Med Sci. 2014;11(8):779–87. doi: 10.7150/ijms.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angulo JC, Andrés G, Ashour N, Sánchez-Chapado M, López JI, Ropero S. Development of castration resistant prostate cancer can be predicted by a DNA hypermethylation profile. J Urol. 2016;195(3):619-26. doi:10.1016/j.juro.2015.10.172. Epub 2015 Nov 6. [DOI] [PubMed]
- 54.Edge SB. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 55.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 56.Dietrich D, Hasinger O, Liebenberg V, Field JK, Kristiansen G, Soltermann A. DNA methylation of the homeobox genes PITX2 and SHOX2 predicts outcome in non-small-cell lung cancer patients. Diagn Mol Pathol. 2012;21(2):93–104. doi: 10.1097/PDM.0b013e318240503b. [DOI] [PubMed] [Google Scholar]
- 57.Kikuchi Y, Tsuji E, Yagi K, Matsusaka K, Tsuji S, Kurebayashi J, et al. Aberrantly methylated genes in human papillary thyroid cancer and their association with BRAF/RAS mutation. Front Genet. 2013;4:271. doi: 10.3389/fgene.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amornpisutt R, Proungvitaya S, Jearanaikoon P, Limpaiboon T. DNA methylation level of OPCML and SFRP1: a potential diagnostic biomarker of cholangiocarcinoma. Tumour Biol. 2015;36(7):4973–8. doi: 10.1007/s13277-015-3147-2. [DOI] [PubMed] [Google Scholar]
- 59.Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11(6):426–37. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 60.Warton K, Samimi G. Methylation of cell-free circulating DNA in the diagnosis of cancer. Front Mol Biosci. 2015;2:13. doi: 10.3389/fmolb.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg. 2014;18(3):562–72. doi: 10.1007/s11605-013-2447-3. [DOI] [PubMed] [Google Scholar]
- 62.Sasaki R, Takeda Y, Funato O, Nitta H, Kawamura H, Uesugi N, et al. Significance of ductal margin status in patients undergoing surgical resection for extrahepatic cholangiocarcinoma. World J Surg. 2007;31(9):1788–96. doi: 10.1007/s00268-007-9102-7. [DOI] [PubMed] [Google Scholar]
- 63.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Prognostic factors of patients with advanced gallbladder carcinoma following aggressive surgical resection. J Gastrointest Surg. 2011;15(6):1007–16. doi: 10.1007/s11605-011-1479-9. [DOI] [PubMed] [Google Scholar]
- 64.Tham C, Chew M, Soong R, Lim J, Ang M, Tang C, et al. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer. 2014;120(20):3131–41. doi: 10.1002/cncr.28802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study available from the corresponding author on reasonable request.


