Abstract
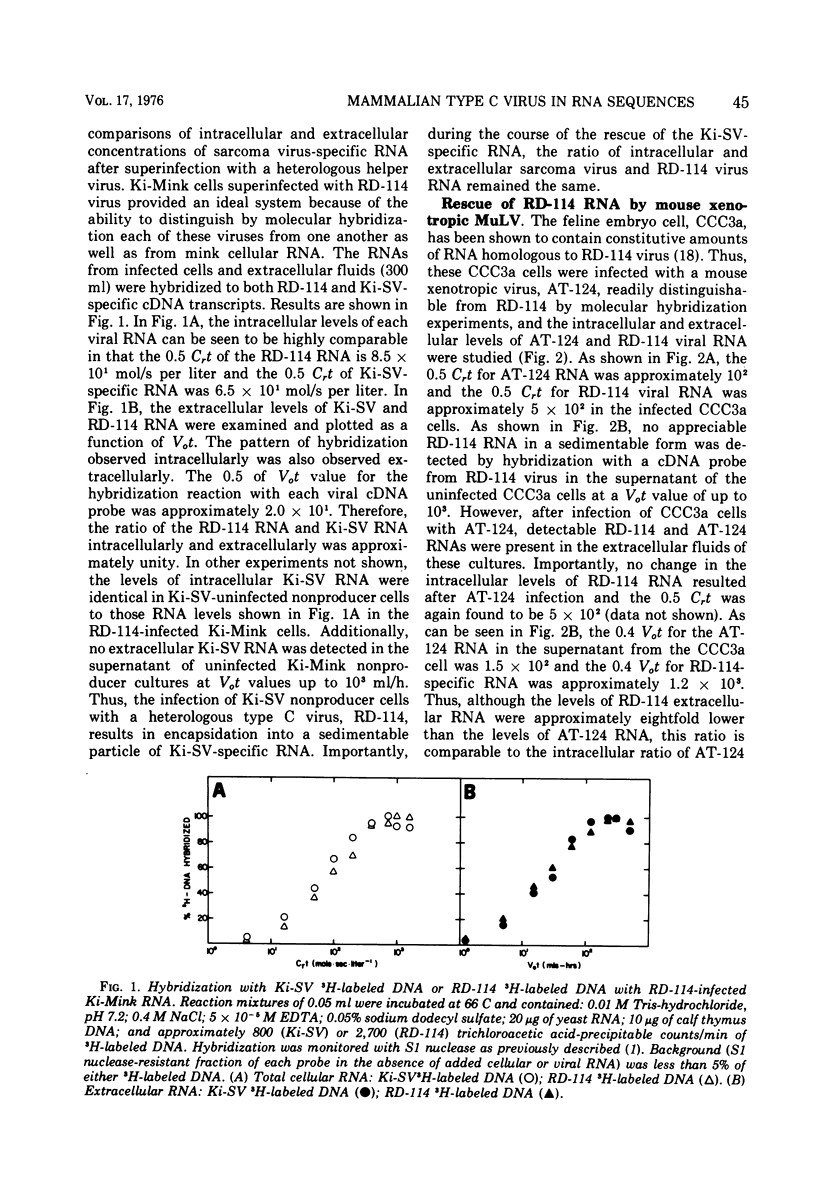
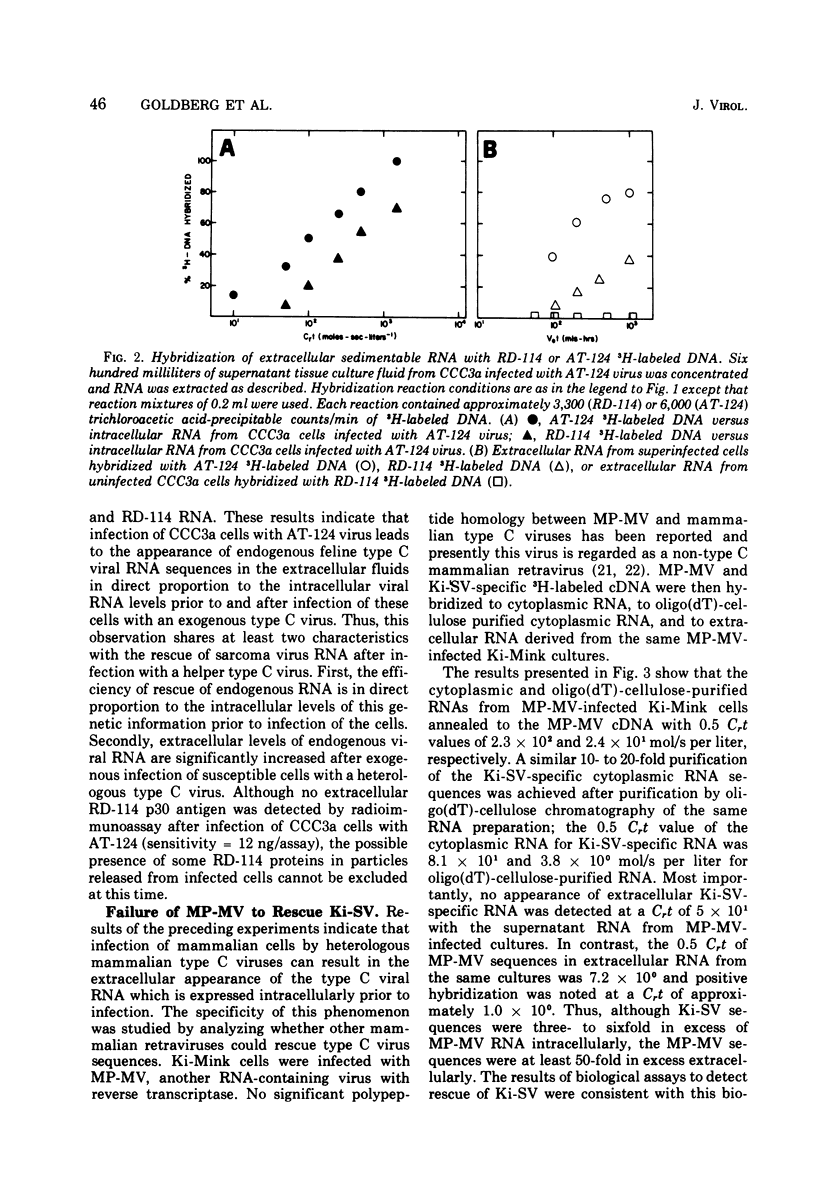
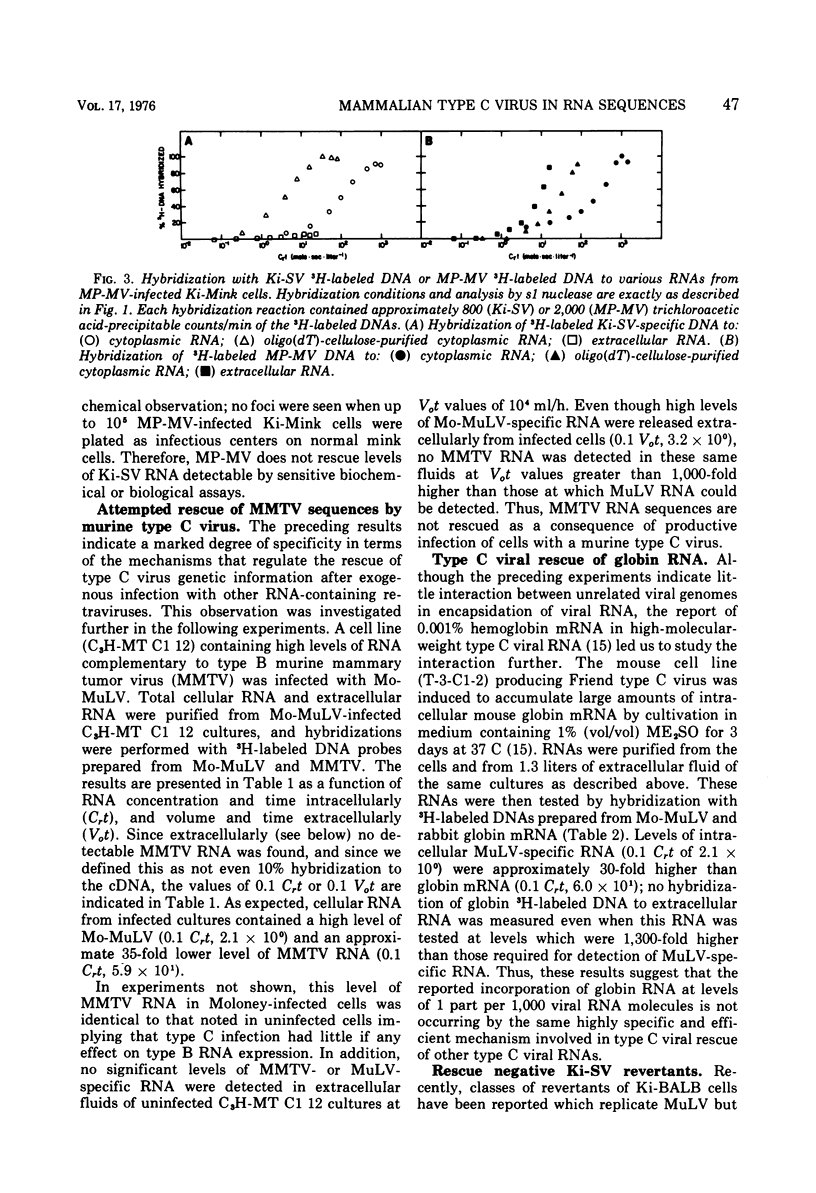
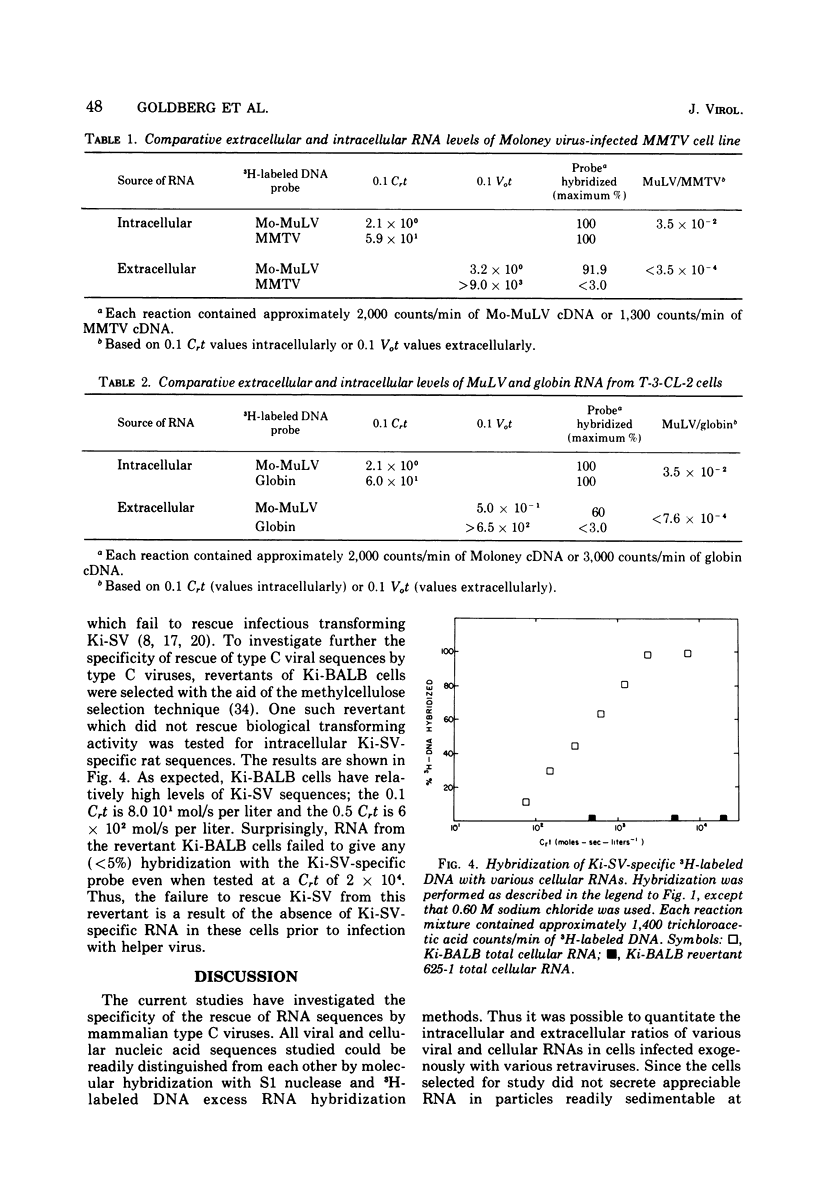
The specificity and quantitation of the rescue of RNA sequences by mammalian type C viruses has been investigated. Type C viruses can package with specificity only type C viral RNA. Type C viruses do not encapsidate with comparable efficiency either type B viral or cellular globin mRNA. Conversely, a non-type C mammalian retravirus, MP-MV, cannot encapsidate type C RNA. A revertant of Kirsten sarcoma virus (Ki-SV)-transformed nonproducer cells which fails to rescue biologically active Ki-SV after superinfection with helper virus had no detectable intracellular Ki-SV -specific RNA. The results suggest specific mechanisms by which type C viral proteins can package type C viral RNA and provide an approach to classifying RNA of potentially defective endogenous retraviruses as type C in origin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D. Reversion and induction of Rous sarcoma virus expression in virus-transformed baby hamster kidney cells. Virology. 1974 Dec;62(2):522–529. doi: 10.1016/0042-6822(74)90412-7. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Boettiger D., Macpherson I., Varmus H. E. The persistence and expression of virus-specific DNA in revertants of Rous sarcoma virus-transformed BHK-21 cells. Virology. 1974 Dec;62(2):512–521. doi: 10.1016/0042-6822(74)90411-5. [DOI] [PubMed] [Google Scholar]
- Fidanián H. M., Drohán W. N., Baluda M. A. RNA of simian sarcoma-associated virus type 1 produced in human tumor cells. J Virol. 1975 Mar;15(3):449–457. doi: 10.1128/jvi.15.3.449-457.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Peebles P. T., Nomura S., Haapala D. K. Isolation of RD-114-like oncornavirus from a cat cell line. J Virol. 1973 Jun;11(6):978–985. doi: 10.1128/jvi.11.6.978-985.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering G., Aoki T., Old L. J. Shared viral antigen of mammalian leukaemia viruses. Nature. 1970 Apr 18;226(5242):265–266. doi: 10.1038/226265a0. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Anderson G. R., Aaronson S. A. Transformation-defective virus mutants in a class of morphologic revertants of sarcoma virus transformed nonproducer cells. Cell. 1974 Aug;2(4):279–286. doi: 10.1016/0092-8674(74)90022-1. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Miyamoto T. Recovery of a new virus from apparently normal chick cells by infection with avian tumor viruses. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1797–1803. doi: 10.1073/pnas.67.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Recombination between endogenous and exogenous RNA tumor virus genes as analyzed by nucleic acid hybridization. J Virol. 1975 Jun;15(6):1367–1377. doi: 10.1128/jvi.15.6.1367-1377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Ikawa Y., Ross J., Leder P. An association between globin messenger RNA and 60S RNA derived from Friend leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1154–1158. doi: 10.1073/pnas.71.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Ross J., Gielen J., Packman S., Ikawa Y., Aviv H., Swan D. Regulated expression of mammalian genes: globin and immunoglobulin as model systems. Cold Spring Harb Symp Quant Biol. 1974;38:753–761. doi: 10.1101/sqb.1974.038.01.080. [DOI] [PubMed] [Google Scholar]
- Nomura S., Dunn K. J., Mattern C. F., Hartley J. W., Fischinger P. J. Revertants of mouse cells transformed by murine sarcoma virus: flat variants without a rescuable sarcoma virus from a clone of BALB/3T3 transformed by Kirsten MSV. J Gen Virol. 1974 Nov;25(2):207–218. doi: 10.1099/0022-1317-25-2-207. [DOI] [PubMed] [Google Scholar]
- Okabe H., Gilden R. V., Hatanaka M. RD 114 virus-specific sequences in feline cellular RNA: detection and characterization. J Virol. 1973 Nov;12(5):984–994. doi: 10.1128/jvi.12.5.984-994.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Ozanne B., Vogel A. Selection of revertants of Kirsten sarcoma virus transformed nonproducer BALB-3T3 cells. J Virol. 1974 Aug;14(2):239–248. doi: 10.1128/jvi.14.2.239-248.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Radioimmunoassay of mammalian type-C viral proteins: interspecies antigenic reactivities of the major internal polypeptide. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1766–1770. doi: 10.1073/pnas.69.7.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Ross J., Todaro G. J., Aaronson S. A. Immunological relationships of reverse transcriptases from ribonucleic acid tumor viruses. J Virol. 1972 Jan;9(1):110–115. doi: 10.1128/jvi.9.1.110-115.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Huebner R. J. Trans-species rescue of defective genomes of murine sarcoma virus from hamster tumor cells with helper feline leukemia virus. Proc Natl Acad Sci U S A. 1970 Jan;65(1):81–87. doi: 10.1073/pnas.65.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Maryak J. M., Parks W. P. Levels of rat cellular RNA homologous to either Kirsten sarcoma virus or rat type-C virus in cell lines derived from Osborne-Mendel rats. J Virol. 1974 Dec;14(6):1435–1444. doi: 10.1128/jvi.14.6.1435-1444.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P. Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. J Virol. 1974 Jun;13(6):1211–1219. doi: 10.1128/jvi.13.6.1211-1219.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Rands E., Williams D., Parks W. P. Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. J Virol. 1973 Sep;12(3):458–463. doi: 10.1128/jvi.12.3.458-463.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Anderson G. R., Tronick S. R., Aaronson S. A. Evidence for genetic recombination between endogenous and exogenous mouse RNA type C viruses. Cell. 1974 Jun;2(2):87–94. doi: 10.1016/0092-8674(74)90096-8. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Todaro G. J., Arnstein P., Parks W. P., Lennette E. H., Huebner R. J. A type-C virus in human rhabdomyosarcoma cells after inoculation into NIH Swiss mice treated with antithymocyte serum. Proc Natl Acad Sci U S A. 1973 Mar;70(3):859–862. doi: 10.1073/pnas.70.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Gilden R. V., Hatanaka M. Sarcoma-virus-related RNA sequences in normal rat cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4503–4507. doi: 10.1073/pnas.71.11.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K. Genetically stable reassortment of markers during mixed infection with avian tumor viruses. Virology. 1971 Dec;46(3):947–952. doi: 10.1016/0042-6822(71)90093-6. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Wyke J. A. The selective isolation of temperature-sensitive mutants of Rous sarcoma virus. Virology. 1973 Apr;52(2):587–590. doi: 10.1016/0042-6822(73)90357-7. [DOI] [PubMed] [Google Scholar]



