Abstract
Objective
To investigate contrast enhanced ultrasound (CEUS) assessment of tumor response to anti-angiogenic therapy in children and adolescents with solid malignancies.
Methods & Materials
After IRB approval, 4 girls and 9 boys, mean age 13 years (range, 1.8 to 19.8 years) with recurrent solid tumors enrolled on an institutional phase I study of anti-angiogenic therapy, underwent CEUS of target lesions before therapy, on days 3 and 7 and end of course 1. Acoustic data from target lesion regions of interest were used to measure peak enhancement (PE), time to peak (TTP), rate of enhancement (RE), total area under the curve (AUC), AUC during wash-in (AUC1) and AUC during wash-out (AUC2). Using Cox regression analysis we assessed the association between changes in parameters from baseline to follow-up time points and time to tumor progression. P-values ≤ 0.050 were considered significant.
Results
Target lesion sites included: liver (n=3), pleura (n=2), and supraclavicular mass, soft tissue component of a bone metastasis, lung, retroperitoneum, peritoneum, lymph node, muscle mass and perineum (n=1 each). Hazard ratios for changes from baseline to end course 1 in PE (1.17, p=0.034), RE (3.3, p=0.029) and AUC1 (1.023, p=0.040) were significantly associated with time to progression. Greater decreases in these parameters correlated with longer time to progression.
Conclusions
CEUS measurements of tumor PE, RE and AUC1 were early predictors of time to progression in our cohort of children and adolescents with recurrent solid tumors treated with anti-angiogenic therapy. Further investigation of these findings in a larger population is warranted.
Introduction
Angiogenesis plays a critical role in tumor growth, metastasis and survival and targeted anti-angiogenic therapies are being increasingly used in cancer clinical trials. Unlike conventional cytotoxic chemotherapy, these agents are cytostatic and may be effective without causing tumors to shrink. Therefore, traditional methods that depend on changes in tumor size are not suitable for assessing response to anti-angiogenic agents and techniques that provide quantitative measurements of tumor perfusion are needed. Dynamic contrast enhanced ultrasonography (CEUS) is emerging as a reliable indicator of tumor response to anti-angiogenic therapy in adults with hepatocellular carcinoma, renal cell carcinoma, malignant melanoma and gastrointestinal stromal tumor (1-11). However, there are no reports of the value of this modality in children and adolescents receiving anti-angiogenic cancer therapy. Children are ideally suited for ultrasound because their small size facilitates placement of the US transducer near the structure of interest, thus reducing artifact. Furthermore, ultrasound does not require sedation and, most importantly, does not use ionizing radiation, an issue of considerable concern in children.
We previously reported that tumor enhancement measured by CEUS as early as 24 hours after initiation of anti-angiogenic therapy, is significantly associated with the degree of tumor vascularity in murine models of pediatric malignancies (12-15). We have also shown that US contrast agents are safe and well tolerated in children with underlying solid malignancies and can improve visualization of tumor margins (16-17). The purpose of this pilot study was to build on our preclinical and clinical experience and to investigate the value of quantitative dynamic CEUS in the assessment of tumor response in children and adolescents with recurrent or refractory solid tumors treated on an institutional phase 1 trial of anti-angiogenic therapy.
Materials and Methods
Patient population and outcome measures
Our cohort comprised a subset of patients, age ≤ 21 years at the time of diagnosis of a solid malignancy, with recurrent or refractory disease, enrolled on an institutional phase 1 study of bevacizumab, sorafenib and low-dose cyclophosphamide (NCT00665990) between December, 2008 and April, 2013. The study was approved by our Institutional Review Board, was HIPAA compliant and performed under FDA IND 62,852. Treatment study design, eligibility and exclusion criteria, preliminary study results and initial CEUS findings in 4 subjects have been reported (18). In that report only 2 CEUS parameters (peak enhancement and rate of enhancement) were investigated and here we report 6 paramenters (see below) from a larger cohort. To be eligible for CEUS patients had to have a target lesion (residual primary tumor or metastasis) that was visible on grey-scale sonography. Because of a theoretical risk of microemboli caused by microsphere contrast agent bypassing the pulmonary circulation, all subjects were required to undergo complete history and physical, 12-lead electrocardiography and echocardiography. Those with a history of ultrasound contrast allergy, a right to left or intra-cardiac shunt, pulmonary hypertension or oxygen saturation < 92% on room air were not eligible for CEUS. Eligible patients/guardians signed informed assent/consent for CEUS at the time of enrollment on the phase 1 study. Subjects were monitored with continuous pulse oximetry and 3 lead ECG (Welch-Allen Propaq monitor, Beaverton, OR) immediately before, during and for 30 minutes after contrast agent injection.
Conventional imaging was obtained at baseline, end of courses 1 and 2, then after every other course until disease progression. Each course was 21 days in duration. Tumor response was assessed using the original Response Evaluation Criteria in Solid Tumors (RECIST) with progression defined as ≥ 20% increase in sum of diameters of target lesions compared to the smallest sum of diameters achieved or the presence of new disease (19). Time to progression was defined as the time in days, between the on-study date and the date of tumor progression.
CEUS technique, image analysis and timing
Target lesions were chosen based on ease of visibility on grey-scale sonography and identification of landmarks to ensure similar transducer placement on follow-up (Figs. 1A, 2A). In general, the largest tumor area was identified in the transverse or longitudinal plane and the transducer was held in that position throughout a 60 second recording (rate, 10 Hz), that began just before contrast administration. All subjects received perflutren protein-type A microsphere contrast material (GE Healthcare, Princeton, NJ). One subject was removed from protocol but continued the same treatment off study and received perflutren lipid microsphere contrast agent (Lantheus, N. Billerica, MA) during follow-up because the protein type-A agent was available only for the phase 1 study participants. Subjects < 20 kg received 0.3 mL and those ≥ 20 kg received 0.5-0.6 mL through in-dwelling central venous lines, except one who preferred peripheral IV access. Bolus hand injections were administered by the principal investigator or radiologist co-investigator (PI, MBM or JLC) followed by a 5 mL sterile saline flush. All examinations were performed on an Acuson Sequoia ultrasound machine (Siemens Medical Solutions, Mountain View, CA) using a 6C2 or 15L8 MHz transducer and the vendor’s Contrast Pulse Sequencing (CPS) software. Scanning parameters were preset by the CPS system including a low mechanical index of 0.2 to avoid contrast agent destruction. The focal zone, depth and gain were optimized for tumor visualization. Tumor region-of-interest (ROI) analysis was performed on-line using the CPS Auto-Tracking Contrast Quantification system which corrects for respiratory motion. Because video compressed data are known to be less reliable for quantitation of tumor perfusion, the CPS system utilizes non-compressed, acoustic data (20). The PI, blinded to RECIST response, drew an ROI on the target lesion keeping inside tumor margins to avoid including adjacent, normal tissue (Figs. 1B, C, 2B, C). If the tumor did not enhance at least 5 dB at baseline, the contrast dose was doubled and the injection repeated after waiting 10 minutes for the first dose to clear. If the tumor did not enhance at least 5 dB after the second, higher dose, the subject was not eligible for follow-up.
Fig. 1.
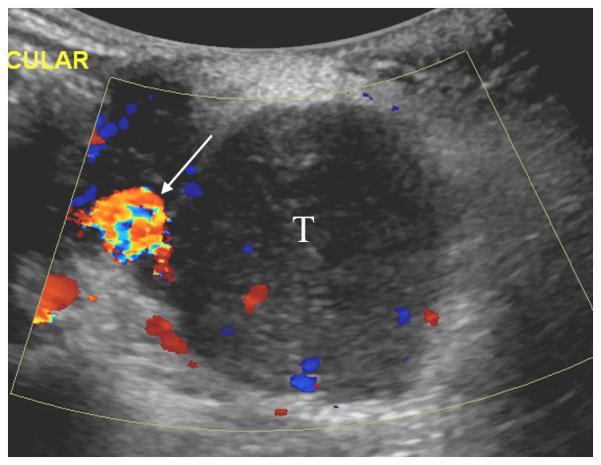
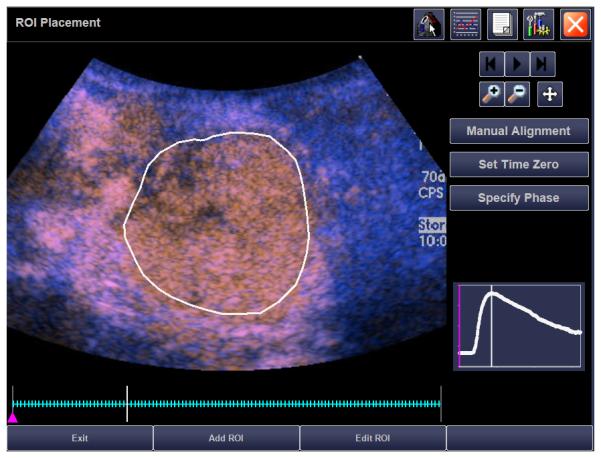
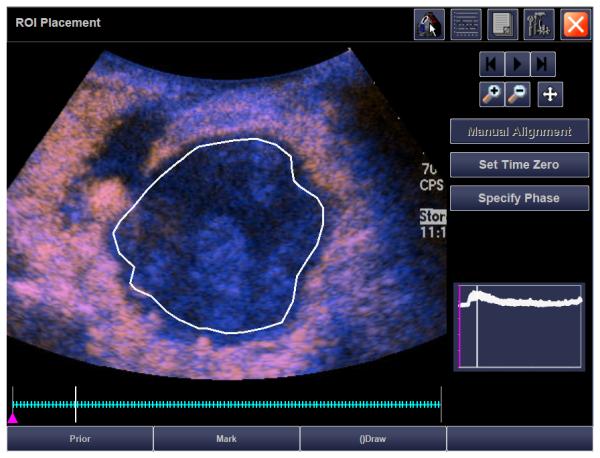
15 year old girl with recurrent synovial sarcoma. A) Transverse color Doppler grey-scale sonogram of the largest transverse area of a left supraclavicular tumor (T). Common carotid artery (arrow) was used as a landmark to insure similar transducer placement on follow-up contrast enhanced ultrasound (CEUS) studies. B) Baseline transverse CEUS with region-of-interest (ROI, solid line) drawn just inside tumor margins. Vertical line in the inset time intensity curve (TIC) indicates that this image was obtained at peak enhancement (PE) of 28.9 dB. C) Day 7 after initiation of therapy, transverse CEUS image obtained at PE of 2.0 dB giving a 93% reduction compared to baseline. This subject’s time to progression was 242 days.
Figure2.
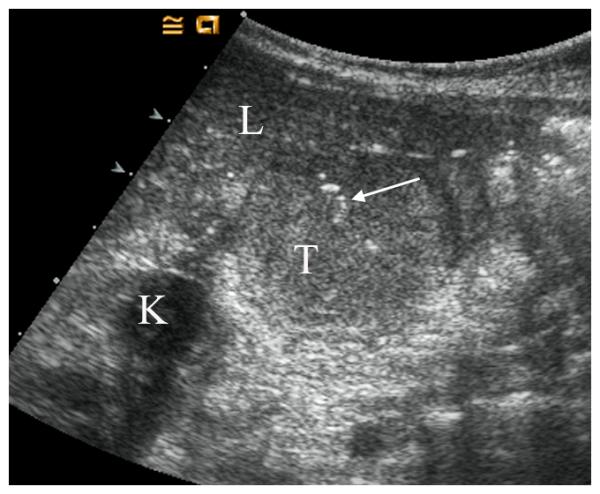
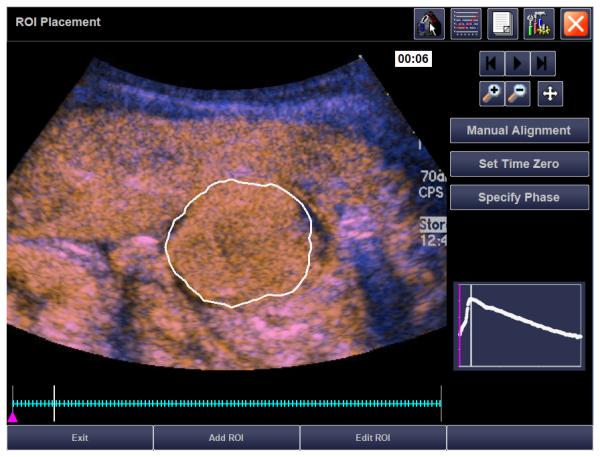
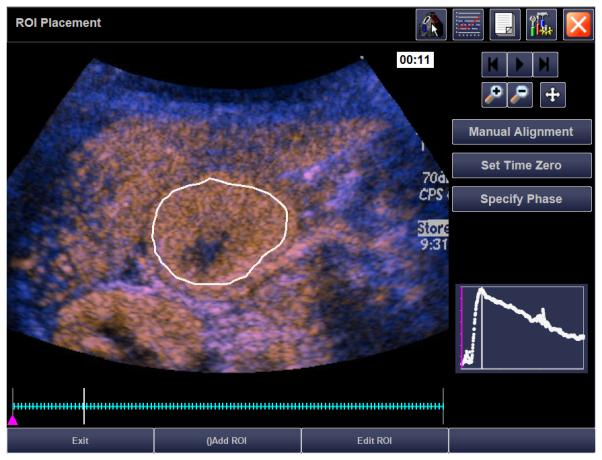
21 month old girl with recurrent rhabdoid tumor. A) Transverse grey-scale sonogram shows a peritoneal tumor (T) located posterior to the liver (L) and medial to the right kidney (K). Tumoral calcification (arrow) and adjacent organs were used as landmarks for transducer placement. B) Baseline transverse CEUS image with ROI inside tumor margins, obtained at PE of 33.5 dB. C) Day 7 after initiation of therapy, transverse CEUS image obtained at PE of 30.6 dB giving a 8.7% reduction compared to baseline. This subject progressed before the end of course 1, at 22 days.
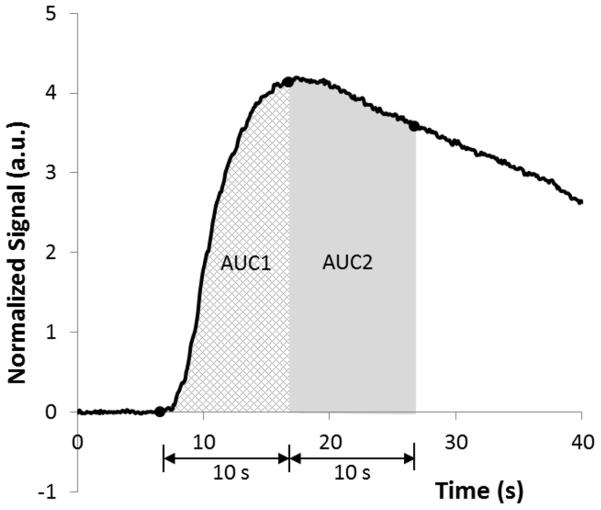
The ROI time intensity curve (TIC) data was used to measure peak enhancement (PE), time to peak enhancement (TTP) and rate of enhancement (RE) as shown in Fig. 3A. Raw data were exported to excel files and TICs normalized to their baseline values were created for measurement of the area under the curve (AUC) in arbitrary units (a.u.). The AUC was divided into wash-in (AUC1) and wash-out (AUC2). Because maximal enhancement was seen at about 10 seconds after arrival of the contrast agent, we defined wash-in as the first 10 seconds and wash-out as the subsequent 10 seconds of the AUC (Fig. 3B). All subjects underwent initial CEUS within an average of 1 day (range, 0 – 6 days) prior to treatment initiation. Based on our pre-clinical data we performed follow-up on days 3 and 7 (± 2 days) after starting study therapy, then at conventional imaging response assessment time points until disease progression (12-15). To assess inter-observer variability, 3 reviewers (2 radiologists, with 11 and 5 years CEUS experience, and 1 sonographer with 7 years CEUS experience; MBM, JC, PH) drew ROIs on each tumor at the baseline, days 3 and 7 and end of course 1 time points. To assess intra-observer variability, each reviewer repeated the ROI measurements for each tumor at each time point.
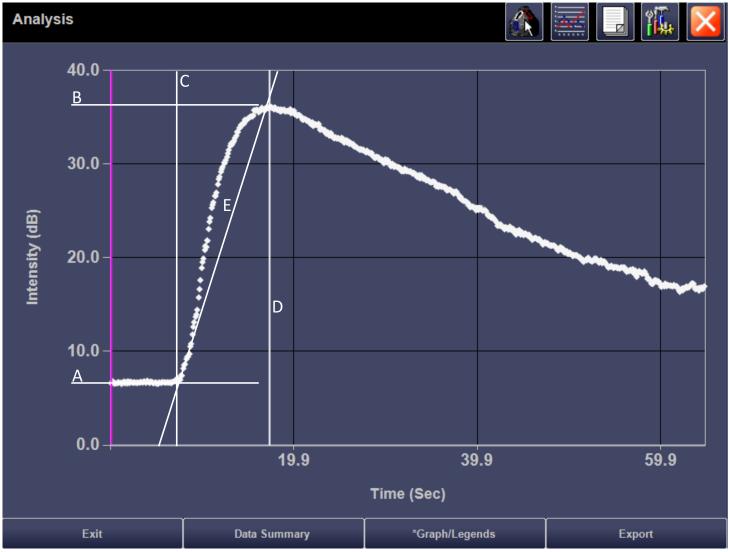
Fig. 3.
A = baseline, pre-contrast signal (dB)
B = maximal enhancement: Peak enhancement (PE) = B – A in dB
C = time of arrival of contrast agent into region of interest (sec)
D = time of maximal enhancement (sec): Time to peak (TTP) = D – C (sec)
E = rate of change in enhancement (RE) calculated as PE/TTP in dB/sec
AUC1 = wash-in
AUC2 = wash-out
AUC = AUC1 + AUC2
A) Time intensity curve and parameters obtained from a region of interest analysis within the tumor shown in Figure 1A. B) Normalized time intensity curve for measurement of the area under the curve (AUC), created off-line from exported raw data for the same patient.
Statistical analysis
Statistical analyses were performed using SAS software 9.3. Using reviewer 1’s first ROI measurements the Cox regression analysis was performed to assess the association between each of the CEUS parameters (PE, TTP, RE, AUC (AUC1 + AUC2), AUC1 and AUC2) at baseline and changes in these parameters from baseline to early follow-up time points (days 3, 7, end course 1) and time to progression separately. We also investigated the association between the time (in days) between the maximal and minimal values of each parameter, caliber of change between baseline and maximal change, and caliber of change between the maximal and minimal values for each parameter at any time point with time to progression separately. Due to the study’s small sample size, multiple regression models were not explored. Subjects who progressed at ≤ 42 days (end course 2) were considered non-responders and those who progressed at > 42 days were responders. We compared the baseline values and percentage change of each parameter at the early follow-up time points for responders vs. non-responders. P-values ≤ 0.050 were considered significant. The variability of ROI measurements was investigated using an analysis of variance model to obtain inter- and intra-observer reliability coefficients that characterize the consistency and reproducibility of measurements made by different observers and by the same observer at review 1 or 2. Reliability coefficients vary between 0 and 1, with higher values indicating greater consistency and reproducibility in repeated measurements.
Results
Twenty-five patients underwent CEUS screening; 4 had lesions that were not adequately visualized by grey-scale ultrasound (skull metastasis, middle mediastinal mass, pleural nodule and orbital apex nodule), 2 had visible lesions but declined participation, 1 had a cystic mass that was not suitable for CEUS and 1 signed consent for CEUS but clinically deteriorated and became ineligible before starting therapy. Of the remaining 17 consenting subjects, 4 were excluded; 2 because they had no baseline study (1 missed appointment, 1 equipment failure), 1 due to oxygen saturation < 92% on room air and 1 because the target lesion enhanced < 5 dB even after doubling the contrast dose. Of the 13 evaluable subjects there were 4 girls and nine boys, mean age 13 years (range, 1.8 to 19.8 years). Primary diagnoses were rhabdomyosarcoma (n=2), rhabdoid tumor (n=2), Wilms tumor (n=2), renal cell carcinoma, hepatocellular carcinoma, osteosarcoma, synovial sarcoma, epithelioid sarcoma and Ewing sarcoma (n=1 each). Target lesion sites included; liver (n=3), pleura (n=2) and supraclavicular mass, soft-tissue component of a bone metastasis, lung, retroperitoneum, peritoneum, lymph node, muscle mass and perineum (n=1 each). A total of 74 CEUS examinations were performed at the following time points: baseline (n=13), day 3 (n=11), day 7 (n=12), end course 1 (n=12), end course 2 (n=8), end course 4 (n=8), end course 6 (n=5), end course 8 (n=3), and end courses 10 and 12 (n=1 each). End of course 1 AUC and AUC2 data were excluded for 1 non-responder and 1 responder due to inadequate AUC2 data. One subject reported brief taste alteration after contrast injection during 3 of 5 examinations. There were no other adverse events. There were 9 responders and 4 non-responders. The median time to progression for all 13 was 95 days (range, 22 to 242 days), for responders it was 142 days (range, 61 to 242 days) and for non-responders, 23 days (range, 22 to 27 days).
Table 1 summarizes descriptive data for CEUS parameters at baseline and changes from baseline to each early follow-up time point. Table 2 summarizes results of the Cox regression analysis showing hazard ratios for the association between time to progression and changes in parameters from baseline to early follow-up time points. Notably, changes in PE, RE, and AUC1 at the end of course 1 were significantly associated with time to progression; greater decreases predicted longer time to progression (Figs. 1, 2). Changes in PE at days 3 and 7, change in AUC1 at day 7 and changes in AUC and AUC2 at the end of course 1 approached significance. The baseline values of parameters, time between the maximal and minimal values of each parameter, caliber of change between baseline and maximal change, and caliber of change between the maximal and minimal values at any time point did not predict time to progression with the exception of the caliber change between the maximal and minimal PE (p = 0.018) such that subjects with larger differences had longer time to progression. There were too few subjects to reach statistical power for a comparison of responders to non-responders. However, median baseline values for each group were similar (with the exception of AUC1 and AUC2) while responders had substantially greater percentage reductions in all parameters by day 3, day 7 and/or end of course 1 compared to non-responders (Table 3). The PE, AUC, AUC1 and AUC2 showed consistently greater reductions in responders than non-responders at all early time points. Inter and intra-observer reliability was generally high for all parameters at all time points with a few exceptions. The parameter showing the best reliability was the PE; inter-observer reliability ranged from 0.87 to 0.99 and intra-observer reliability from 0.91 to 1.0.
Table 1.
Values of contrast enhanced ultrasound parameters at baseline and changes in parameters at various time points during and at the end of course 1.
| Parameter | |||||||
|---|---|---|---|---|---|---|---|
| Time Point | n | PE (dB) | RE (dB/sec) | TTP (sec) | AUC (a.u.) | AUC1 (a.u.) | AUC2 (a.u.) |
| Baseline | 13 | 17.30(4.47 - 33.4) | 1.54(0.20 - 10.26) | 11.21(3.21 - 33.59) | 175.99(55.13 - 447.60) | 90.15(18.69 - 290.12) | 80.89(30.71 - 275.13) |
| Day 3 | 11 | −2.08(−12.37 - 5.88) | −0.23(−4.14 - 1.54) | −1.03(−16.9 - 2.97) | −6.62(−184.9 – 86.31) | −5.58(−97.96 - 26.93) | −11.14(−147.83 – 59.38) |
| Day 7 | 12 | −4.59(−26.83 - 3.84) | −0.48(−6.64 - 1.49) | −2.11(−7.37 - 12.73) | −49.25(−427.42 - 52.86) | −32.69(−161.72 - 15.46) | −30.45(−265.70 – 37.41) |
| #End Course 1 | 12 | −3.24(−24.15 - 10.64) | −0.85(−2.73 - 2.31) | 0.65(−16.08 - 19.38) | −31.81(−385.21 - 124.7) | −5.26(−147.10 - 54.30) | −13.62(−238.11 – 70.41) |
Data are median values and range
Table 2.
Hazard ratios and 95% confidence intervals for risk of progression based on changes in CEUS parameters at baseline and early follow-up time points.
| Parameter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Point | PE | p-value | RE |
p-
value |
TTP |
p-
value |
AUC |
p-
value |
AUC1 | p-value | AUC2 |
p-
value |
| Baseline | 0.96(0.87-1.05) | 0.38 | 1.18(0.76-1.83) | 0.47 | 1.03(0.94-1.13) | 0.53 | 0.995(0.99-1.0) | 0.18 | 1.00(0.99-1.01) | 0.96 | 0.99(0.98-1.0) | 0.17 |
| Day 3 | 1.26 (0.99-1.59) | 0.051 | 0.97 (0.49-1.89) | 0.92 | 0.99 (0.83-1.16) | 0.87 | 1.01(1.0-1.03) | 0.12 | 1.03(0.99-1.07) | 0.15 | 1.02(0.99-1.05) | 0.15 |
| Day 7 | 1.24 (0.98-1.57) | 0.072 | 1.23 (0.61-2.46) | 0.56 | 0.99(0.90-1.09) | 0.83 | 1.01(1.0-1.03) | 0.14 | 1.03(1.0-1.06) | 0.065 | 1.02(0.99-1.04) | 0.17 |
| End Course 1 | 1.17 (1.01-1.35) | 0.034 | 3.25 (1.13-9.35) | 0.029 | 0.95(0.86-1.04) | 0.28 | 1.00(0.99-1.02) | 0.06 | 1.02(1.0-1.04) | 0.04 | 1.02(1.0-1.03) | 0.059 |
Table 3.
Median baseline CEUS parameter values and percent changes in median values at early follow-up time points in responders and non-responders
| Non-Responders (n = 4) | Responders (n = 9) | |
|---|---|---|
| Parameters | Baseline | |
| PE (dB) | 16.78 | 17.30 |
| RE (dB/sec) | 2.82 | 1.54 |
| TTP (sec) | 8.50 | 11.21 |
| AUC (a.u.) | 175.99 | 188.92 |
| AUC1 (a.u.) | 102.96 | 53.81 |
| AUC2 (a.u.) | 80.89 | 116.94 |
| Day 3 | ||
| PE | −9.63% | −40.17% |
| RE | −53.86% | −29.93% |
| TTP | 16.95% | −25.42% |
| AUC | 3.51% | −41.78% |
| AUC1 | −15.89% | −27.58% |
| AUC2 | 60.93% | −37.28% |
| Day 7 | ||
| PE | −10.34% | −59.97% |
| RE | −24.25% | −54.37% |
| TTP | −10.30% | 35.33% |
| AUC | −36.54% | −65.42% |
| AUC1 | −20.68% | −39.15% |
| AUC2 | 7.19% | −66.04% |
| End Course 1 | ||
| PE | −8.49% | −49.31% |
| RE | −1.37% | −55.16% |
| TTP | −1.47% | −8.03% |
| AUC | 15.99% | −35.49% |
| AUC1 | 5.60% | −28.28% |
| AUC2 | 21.58% | −35.08% |
Discussion
With the increasing number of molecularly targeted agents being evaluated in clinical trials there is a growing need for imaging methods that go beyond an assessment of anatomic change yet provide quantitative measurements of tumor response. Numerous functional and metabolic imaging modalities are being incorporated into clinical trials and each has unique indications, merits and limitations. In addition to dynamic CEUS, promising methods of assessing tumor vascularity include dynamic contrast enhanced CT and MRI and 15O-labeled water positron emission tomography (PET) (21-24). Dynamic CT and PET expose patients to ionizing radiation and, therefore, are less attractive for the pediatric population. Dynamic contrast enhanced MRI avoids exposure to radiation but is technically challenging. Because MR contrast agents diffuse freely across the vascular membrane, complex pharmacokinetic models are needed to analyze gadolinium concentration TICs (23). Furthermore, sedation is often required for young patients who cannot lie still for lengthy MRI scans. In contrast, dynamic CEUS is an ideal modality for children because it is well tolerated, does not require sedation and has the important attribute of not exposing this vulnerable population to radiation. Ultrasound contrast agents are composed of microspheres that remain within the vascular space and, because they contain a gas, are highly reflective on ultrasound imaging allowing detection at the capillary level (25). These contrast agents, therefore, are ideal surrogate markers of tumor blood flow and can be quantitated with modern CEUS software. Dynamic, quantitative CEUS is becoming an important method of monitoring the effects of anti-angiogenic therapies in adults. The current challenge is to define which CEUS parameters predict tumor response or patient outcome, the ideal timing for assessment and, with regard to children, which malignancies are most suitable for this technique.
Our findings show that CEUS detects changes in blood flow in a variety of solid malignancies in children very early in the course of anti-angiogenic therapy and that these changes can predict time to progression. Importantly, this imaging modality may identify poor responders before conventional RECIST criteria, thus affording the opportunity for early intervention and tailored management. This approach could avoid maintaining patients on toxic and ineffective therapy for weeks or months before conventional imaging identifies morphologic changes that indicate tumor progression. In our study, subjects with greater decreases in PE, RE and AUC 1 at the end of course 1 had significantly longer time to progression. Additionally, reductions in PE at day 3, PE and AUC1 at day 7 and the AUC and AUC2 at the end of course 1 were marginally significant. Although our cohort was too small to attain statistical power, there were substantial differences in percent changes in all parameters at early time points in responders compared to non-responders. Among the 6 parameters that we investigated, the PE appears to be the most robust indicator of tumor response. We found that the early percentage decreases of PE, relative to baseline, were 5 to 6 times greater in responders than non-responders. This is perhaps not surprising since the PE depends on tumor perfusion and tumors that respond well to anti-angiogenic therapies will have substantially reduced blood flow and contrast enhancement. Our findings agree with those of Lassau et al who investigated the value of CEUS in 24 adults with gastrointestinal stromal tumors treated with imatinib. Those investigators found a strong correlation (p < 10-4) between changes in percent of contrast uptake within tumors from baseline to day 7, day 14 and at 2 months in patients with a good response but no significant change in poor responders (4). However, our findings differ slightly from a subsequent study by Lassau and colleagues that assessed 42 adults with hepatocellular carcinoma treated with bevacizumab (2). In that study changes in AUC, AUC1, AUC2, TTP and mean transit time at day 3 trended toward a significant correlation with RECIST response, but there was no correlation between PE and RECIST response or patient survival. The reason for these differences is not clear but could be related to differences in the perfusion pattern of liver lesions compared to other tumor sites and differences in biological behavior of the tumor types. We found a high degree of inter- and intra-observer reliability for measurement of all parameters at most time points. Importantly, this was especially true of the PE which showed excellent reproducibility both within a single reviewer and between reviewers. These findings further support the suitability of the PE as a meaningful parameter to assess tumor response to anti-angiogenic therapy.
Our study has several limitations. Our sample size was small precluding a statistical comparison of responders vs. non-responders. Although our findings suggest that CEUS can predict time to progression very early in the course of anti-angiogenic treatment, they should be interpreted with caution. Our cohort was comprised of patients with a wide variety of solid malignancies and CEUS may be more valuable in some than others. In our phase 1 study, all patients had recurrent or refractory disease and many had numerous metastatic sites. Since ultrasound contrast agents are not FDA approved for pediatric use we limited our analysis to a single target lesion in order to minimize the cumulative contrast agent dose. It is possible that performing multiple contrast injections to assess several target lesions would better represent total tumor burden and overall response to therapy. Also, due to limitations in current software, we imaged only a single slice of tumor. Because tumor composition is often heterogenous, it may be preferable to assess the entire tumor volume or numerous slices throughout the tumor mass (26-27).
In conclusion, our pilot study suggests that several quantitative CEUS parameters obtained very early in the course of anti-angiogenic therapy are significant predictors of time to progression in children and adolescents with solid malignancies. Although our cohort was too small to achieve statistical power, we found substantial differences in percent reductions in all parameters as early as days 3 and 7 in responders vs. non-responders. In this patient population the PE appears to be the most robust and reproducible parameter. Our findings require validation in larger clinical trials with more homogenous patient populations. We are currently investigating the value of CEUS in children with Ewing sarcoma who are being treated at our institution with an mTOR inhibitor in combination with cytotoxic chemotherapy. Because tumor microenvironments involve complex interactions between angiogenesis, tissue hypoxia, altered metabolism, cell proliferation and apoptosis, the best approach to assessing tumor response will likely require a multimodality and multiparametric approach utilizing imaging technologies that can quantify these various processes (24). Dynamic CEUS appears to be an ideal modality for assessing solid tumor vascularity in pediatric patients.
Acknowledgements
We thank Stacey Glass for excellent technical assistance in performing CEUS, Kim Johnson for data management, our sedation nursing team for vigilant patient monitoring, Erika Thompson and Adrianne Matthews for administrative assistance and General Electric Healthcare for product support.
Source Funding: Supported in part by The American Lebanese Syrian Associated Charities and Cancer Center Core Grant 027165. Beth McCarville receives product support from General Electric Healthcare.
This study was approved by the St. Jude Children’s Research Hospital IRB and informed consent was obtained from all participants.
Footnotes
Conflicts of Interest: None of the other authors have a conflict of interest or financial disclosure.
Contributor Information
M. Beth McCarville, Department of Diagnostic Imaging (MS 220), St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
Jamie L. Coleman, Department of Diagnostic Imaging (MS 220), St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, Phone:(901) 595-6650, Fax: (901) 595-3962, jamie.coleman@stjude.org.
Junyu Guo, Department of Diagnostic Imaging (MS 220), St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, Phone:(901) 595-2085, Fax: (901) 595-3962, junyu.guo@stjude.org.
Yimei Li, Department of Biostatistics (MS 768), St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, Phone:(901) 595-6735, Fax: (901) 595-8843, yimei.li@stjude.org.
Xingyu Li, Department of Biostatistics (MS 723), St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, Phone:(901) 595-7191, Fax: (901) 595-4585, xingyu.li@stjude.org.
Patricia Honnoll, Department of Diagnostic Imaging (MS 220), St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, Phone:(901) 595-3319, Fax: (901) 595-4585, patti.honnoll@stjude.org.
Andrew M. Davidoff, Department of Surgery (MS 133), St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, Phone:(901) 595-3728, Fax: (901) 595-6621, andrew.davidoff@stjude.org.
Fariba Navid, Department of Oncology, St. Jude Children's Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105.
References
- 1.Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5(1):53–58. doi: 10.1007/s11523-010-0136-7. [DOI] [PubMed] [Google Scholar]
- 2.Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification--preliminary results. Radiology. 2011;258(1):291–300. doi: 10.1148/radiol.10091870. [DOI] [PubMed] [Google Scholar]
- 3.Lamuraglia M, Escudier B, Chami L, et al. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer. 2006;42(15):2472–2479. doi: 10.1016/j.ejca.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Lassau N, Lamuraglia M, Chami L, et al. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006;187(5):1267–1273. doi: 10.2214/AJR.05.1192. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Lassau N, Angevin E, et al. Phase I trial of sorafenib in combination with IFN alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13(6):1801–1809. doi: 10.1158/1078-0432.CCR-06-1432. [DOI] [PubMed] [Google Scholar]
- 6.De Giorgi U, Aliberti C, Benea G, Conti M, Marangolo M. Effect of angiosonography to monitor response during imatinib treatment in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2005;11(17):6171–6176. doi: 10.1158/1078-0432.CCR-04-2046. [DOI] [PubMed] [Google Scholar]
- 7.Lassau N, Lamuraglia M, Vanel D, et al. Doppler US with perfusion software and contrast medium injection in the early evaluation of isolated limb perfusion of limb sarcomas: prospective study of 49 cases. Ann Oncol. 2005;16(7):1054–1060. doi: 10.1093/annonc/mdi214. [DOI] [PubMed] [Google Scholar]
- 8.Goetti R, Reiner CS, Knuth A, et al. Quantitative perfusion analysis of malignant liver tumors: dynamic computed tomography and contrast-enhanced ultrasound. Invest Radiol. 2012;47(1):18–24. doi: 10.1097/RLI.0b013e318229ff0d. [DOI] [PubMed] [Google Scholar]
- 9.Averkiou M, Lampaskis M, Kyriakopoulou K, et al. Quantification of tumor microvascularity with respiratory gated contrast enhanced ultrasound for monitoring therapy. Ultrasound Med Biol. 2010;36(1):68–77. doi: 10.1016/j.ultrasmedbio.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Frohlich E, Muller R, Cui XW, Schreiber-Dietrich D, Dietrich CF. Dynamic contrast-enhanced ultrasound for quantification of tissue perfusion. J Ultrasound Med. 2015;34(2):179–196. doi: 10.7863/ultra.34.2.179. [DOI] [PubMed] [Google Scholar]
- 11.Lassau N, Chami L, Benatsou B, Peronneau P, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US) with quantification of tumor perfusion: a new diagnostic tool to evaluate the early effects of antiangiogenic treatment. Eur Radiol. 2007;17(Suppl 6):F89–98. doi: 10.1007/s10406-007-0233-6. [DOI] [PubMed] [Google Scholar]
- 12.Sims TL, McGee M, Williams RF, et al. IFN-beta restricts tumor growth and sensitizes alveolar rhabdomyosarcoma to ionizing radiation. Mol Cancer Ther. 2010;9(3):761–771. doi: 10.1158/1535-7163.MCT-09-0800. [DOI] [PubMed] [Google Scholar]
- 13.Dickson PV, Hamner JB, Sims T, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. 2007;13(13):3942–3950. doi: 10.1158/1078-0432.CCR-07-0278. [DOI] [PubMed] [Google Scholar]
- 14.Dickson PV, Hamner JB, Streck CJ, et al. Continuous delivery of IFN-beta promotes sustained maturation of intratumoral vasculature. Molecular cancer research : MCR. 2007;5(6):531–542. doi: 10.1158/1541-7786.MCR-06-0259. [DOI] [PubMed] [Google Scholar]
- 15.McCarville MB, Streck CJ, Dickson PV, Li CS, Nathwani AC, Davidoff AM. Angiogenesis inhibitors in a murine neuroblastoma model: quantitative assessment of intratumoral blood flow with contrast-enhanced gray-scale US. Radiology. 2006;240(1):73–81. doi: 10.1148/radiol.2401050709. [DOI] [PubMed] [Google Scholar]
- 16.McCarville MB, Kaste SC, Hoffer FA, et al. Contrast-enhanced sonography of malignant pediatric abdominal and pelvic solid tumors: preliminary safety and feasibility data. Pediatr Radiol. 2012;42(7):824–833. doi: 10.1007/s00247-011-2338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman JL, Navid F, Furman WL, McCarville MB. Safety of ultrasound contrast agents in the pediatric oncologic population: a single-institution experience. AJR Am J Roentgenol. 2014;202(5):966–970. doi: 10.2214/AJR.13.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navid F, Baker SD, McCarville MB, et al. Phase I and clinical pharmacology study of bevacizumab, sorafenib, and low-dose cyclophosphamide in children and young adults with refractory/recurrent solid tumors. Clin Cancer Res. 2013;19(1):236–246. doi: 10.1158/1078-0432.CCR-12-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Nat Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Peronneau P, Lassau N, Leguerney I, Roche A, Cosgrove D. Contrast ultrasonography: necessity of linear data processing for the quantification of tumor vascularization. Ultraschall Med. 2010;31(4):370–378. doi: 10.1055/s-0029-1245450. [DOI] [PubMed] [Google Scholar]
- 21.Specht JM, Kurland BF, Montgomery SK, et al. Tumor metabolism and blood flow as assessed by positron emission tomography varies by tumor subtype in locally advanced breast cancer. Clin Cancer Res. 2010;16(10):2803–2810. doi: 10.1158/1078-0432.CCR-10-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eary JF, Link JM, Muzi M, et al. Multiagent PET for risk characterization in sarcoma. J Nucl Med. 2011;52(4):541–546. doi: 10.2967/jnumed.110.083717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winfield JM, Payne GS, deSouza NM. Functional MRI and CT biomarkers in oncology. Eur J Nuc Med Mol Imaging. 2015;42(4):562–578. doi: 10.1007/s00259-014-2979-0. [DOI] [PubMed] [Google Scholar]
- 24.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256(2):348–364. doi: 10.1148/radiol.10091760. [DOI] [PubMed] [Google Scholar]
- 25.Greis C. Quantitative evaluation of microvascular blood flow by contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2011;49(1-4):137–149. doi: 10.3233/CH-2011-1464. [DOI] [PubMed] [Google Scholar]
- 26.Miles KA, Ganeshan B, Hayball MP. CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging. 2013;13(3):400–406. doi: 10.1102/1470-7330.2013.9045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoyt K, Sorace A, Saini R. Quantitative mapping of tumor vascularity using volumetric contrast-enhanced ultrasound. Invest Radiol. 2012;47(3):167–174. doi: 10.1097/RLI.0b013e318234e6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]