ABSTRACT
RNA interference (RNAi) technology is considered as an alternative for control of pests. However, RNAi has not been used in field conditions yet, since delivering exogenous ds/siRNA to target pests is very difficult. The laboratory methods of introducing the ds/siRNA into insects through feeding, micro feeding / dripping and injecting cannot be used in fields. Transgenic crop is perhaps the most effective application of RNAi for pest control, but it needs long-time basic researches in order to reduce the cost and evaluate the safety. Therefore, transgenic microbe is maybe a better choice. Entomopathogenic fungi generally invade the host insects through cuticle like chemical insecticides contact insect to control sucking sap pests. Isaria fumosorosea is a common fungal entomopathogen in whitefly, Bemisia tabaci. We constructed a recombinant strain of I. fumosorosea expressing specific dsRNA of whitefly's TLR7 gene. It could silence the TLR7 gene and improve the virulence against whitefly. Transgenic fungal entomopathogen has shown great potential to attain the application of RNAi technology for pests control in fields. In the future, the research interests should be focused on the selection of susceptible target pests and their vital genes, and optimizing the methods for screening genes and recombinants as well.
KEYWORDS: Isaria fumosorosea, RNAi, recombinant fungal entomopathogen, whitefly
The numerous pests seriously damage agricultural production. Chemical insecticides play a very important role in control of pests. However, the side effect of “3R” (resistance, residue and resurgence) to non-target species and pest-resistance development have limited long-time usage of any one of chemical pesticides. New methods for pest's integrated management are still essential. RNA interference (RNAi) is considered to be a new technology for control of pests.1 However, RNAi technology has not been used in fields yet. The reasons mainly include the high cost for the practical application, the unstable effectiveness and the difficulties for delivering exogenous ds/siRNA to target pests. Actually, there are many direct and indirect utilizations of RNAi technologies for insects (Fig. 1). For direct methods, the specific ds/siRNA of target genes are obtained from biological or artificial synthesis, and then the ds/siRNA could be delivered through feeding, micro feeding / dripping, and injecting. These direct methods can be used for genes' function study and for screening susceptible genes, but cannot be expected for control of pests in field conditions.2-4
Figure 1.
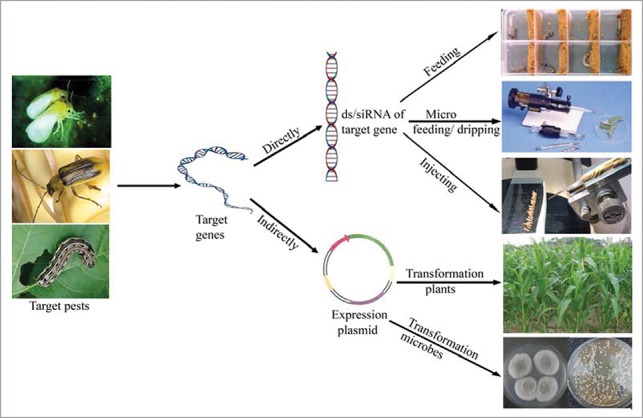
The methods of RNAi insect's gene.
The indirect way is transgenic crops or microbes including fungi and bacteria (Fig. 1). Transgenic crop expressing specific dsRNA targeted for insect genes is perhaps the most effective application of RNAi for pest control. In fact, RNAi-based transgenic bait of corn was validated as an alternative of Bt-based transgenic bait for management of corn rootworm, Diabrotica virgifera virgifera LeConte.5 Recently, transgenic tobacco lines were developed for the expression of long dsRNA precursor to make siRNA and down-regulate the v-ATPaseA mRNA in whitefly, Bemisia tabaci. The transcript level of v-ATPaseA was reduced up to 62% after feeding on the transgenic plants.6 Some researchers reported the expression of dsRNA in bacteria to target insect genes. The Escherichia coli expressing dsRNA of chitin synthase gene A was fed to Spodoptera exigua, resulting in the inhibition of the growth and development of S. exigua larvae.7 However, transgenic crops need long-time research on food and environmental safety issue, while transgenic bacteria are only effective for feeding insects that means it cannot be used to control sucking sap pests.
Entomopathogenic fungi are often used as myco-insecticides to control insect pests. The fungi generally invade the hosts through cuticle, which gives them the characteristic of contact-like insecticide that can be applied for sucking sap pests. Meanwhile, after entering the host's hemocoel the fungi can change morphologically to blastospores to adapt the hemolymph immunity.8,9 Isaria fumosorosea is a common fungal entomopathogen with wide geographical distribution and extensive range of host insects.10,11 I. fumosorosea is also an important pathogen of whitefly and mainly infects on the nymphs.
Whitefly, Bemisia tabaci, is a very important insect pest with more than 20 cryptic species or biotypes. Its B-biotype is a very important invading insect, which was firstly named as superbug in the world.12,13 Whitefly is a representative of piercing-sucking mouthparts and not only damages directly the growth and development of host plants by sucking sap, but also injures seriously crops production by spreading begomoviruses.12,13 Because of the sucking sap, it means that whitefly cannot be RNAi effective by feeding ds/siRNA. It also means that recombinant bacteria or virus expressing specific genes that cannot be used to whitefly's RNAi, since the ds/siRNA are unable to enter the whitefly's gut and penetrate the cuticle. So, in order to use RNAi for control of whitefly, constructing recombinant plants and fungi are the only choice. However, genetic modified plant is probably difficult to use in a short time because they need long-time to figure out the food safety issues and problems of the environmental compatibility. On the contrary, entomogenous fungus is easy to be transformed and used in fields. Therefore, I. fumosorosea as a commonly used fungal pathogen should be given priority as a host to produce ds/siRNA for RNAi in whitefly.
In our previous research, we constructed a recombination strain of I. fumosorosea that expresses specific dsRNA of whitefly's TLR7 gene (Fig. 2). The gene modified strain, IfB01-TLR7, could silence the TLR7 gene and improve its virulence against whitefly.14
Figure 2.
Constructing the recombinant fungal strain of I. fumosorosea to RNAi whitefly's TLR7 gene.
Selecting a proper target gene is very important for application of RNAi technology. However, screening the susceptible gene suitable for RNAi guided pest control is very difficult. Insects have a large amount genes, such as a model insect Drosophila melanogaster with 10,000–15,000 genes, what is the best choice? Because entomogenous fungus directly penetrates insect cuticle and inters to the hemocoel, the fungal cells should firstly confront the hemolymph immunity response of the host, so we believe that the insect immunity-related genes should be primarily concerned as the target gene of RNAi. Before the current research, we had firstly tested the RNAi susceptibility of 7 immunity-related genes of whitefly, by means of feeding the adults with specific dsRNA. However, there were some problems. The first one was that the nymph whiteflies could not be used for oral feeding assay because they always stayed the same place to eat and didn't move to any other place, but the entomogenous fungus generally infects at the nymph stage, so we had to use adults to screen susceptible genes. In fact, even the some genes are maybe differently expressed between adults and larvae. The second one was that the oral feeding of dsRNA differed from fungal delivering dsRNA, as oral feeding led to the dsRNA entering insect from gut, while the dsRNA entered to the hemoceol after transgenic fungus infecting insect. Therefore, the genes selected from adults might not be suitable for RNAi in larvae. In addition, the screening methods of susceptible genes used for RNAi of transgenic fungal entomopathogen should be carefully concerned in the future researches.
The RNAi effectiveness of recombinant fungal strain is influenced by numerous factors mainly including target gene length and expression, fungal species, plasmid, transformation method, and dsRNA degradation in the host, etc. when the fungus is transformed, the plasmid for gene silences perhaps randomly inserted in the fungal chromosome. The loci and copies number of the inserted plasmid will seriously affect the target gene's expression. In fact, we cannot control the loci and copies number, but we can improve the RNAi effectiveness and virulence of the recombinant strain, by means of screening recombinant fungal strain highly expressing the dsRNA fragment of target genes. In that research, we surveyed hundreds of recombinants for the best biological characteristics and bioactivity, and finally the IfB01-TRL7 strain was picked out. The selecting methods of recombinants should be improved in the future.
Strain degeneration is a common problem of entomopathogenic fungi. The degraded strains usually show decreased sporulation and virulence. Similarly, the recombinant fungal strain, IfB01-TRL7, has the degeneration problem as well. In laboratory, the strain cannot produce conidia after inoculation in succession for 4 generations, and if inoculated from the infected insects, it showed not the same phenotype as the parent strain. To prevent or reduce the strain degeneration, the original recombinant strain should be carefully reserved, on the other hand, rejuvenation and re-selection of strain must be carried on.
In conclusion, recombinant fungal entomopathogen expressing dsRNA targeted to insect vital genes, which was confirmed with great promising for delivering exogenous dsRNA and control of the host pest. In the future, researches of transgenic entomopathogenic fungi for RNAi should be focused on selecting susceptible pests and suitable target genes, and improving the methods used for screening genes and recombinants as well.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Funding
This research was supported by the National High Technology Research and Development Program (‘863’ Program) of China (2012AA101505).
References
- [1].Murphy M. Agrochemicals - RNAi for pest control. Chemistry & Industry 2008; 11:10-10. [Google Scholar]
- [2].Nandety RS, Kuo YW, Nouri S, Falk BW. Emerging strategies for RNA interference (RNAi) applications in insects. Bioengineered 2015; 6:8-19; PMID:25424593; http://dx.doi.org/ 10.4161/21655979.2014.979701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim YH, Issa MS, Cooper AMW, Zhu KY. RNA interference: Applications and advances in insect toxicology and insect pest management. Pestic Biochem Phys 2015; 120:109-17; PMID:25987228; http://dx.doi.org/ 10.1016/j.pestbp.2015.01.002 [DOI] [PubMed] [Google Scholar]
- [4].Rangasamy M, Siegfried BD. Validation of RNA interference in western corn rootworm Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) adults. Pest Manag Sci 2012; 68:587-91; PMID:22500293; http://dx.doi.org/ 10.1002/ps.2301 [DOI] [PubMed] [Google Scholar]
- [5].Narva KE, Siegfried BD, Storer NP. Transgenic Approaches to Western Corn Rootworm Control. Adv Biochem Eng Biot 2013; 136:135-62; PMID:23604211 [DOI] [PubMed] [Google Scholar]
- [6].Thakur N, Upadhyay SK, Verma PC, Chandrashekar K, Tuli R, Singh PK. Enhanced Whitefly Resistance in Transgenic Tobacco Plants Expressing Double Stranded RNA of v-ATPase A Gene. Plos One 2014; 9:e87235; PMID:24595215; http://dx.doi.org/ 10.1371/journal.pone.0087235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tian HG, Peng H, Yao Q, Chen HX, Xie Q, Tang B, et al.. Developmental Control of a Lepidopteran Pest Spodoptera exigua by Ingestion of Bacteria Expressing dsRNA of a Non-Midgut Gene. Plos One 2009; 4:e6225; PMID:19593438; http://dx.doi.org/ 10.1371/journal.pone.000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hajek AE, St Leger RJ. Interaction between fungal pathogens and insect hosts. Annu Rev Entomol 1994; 39:293-322; http://dx.doi.org/ 10.1146/annurev.en.39.010194.001453 [DOI] [Google Scholar]
- [9].Boomsma JJ, Jensen AB, Meyling NV, Eilenberg J. Evolutionary Interaction Networks of Insect Pathogenic Fungi. Annual Review of Entomology 2014; 59:467-85; PMID:24160418; http://dx.doi.org/ 10.1146/annurev-ento-011613-162054 [DOI] [PubMed] [Google Scholar]
- [10].Zimmermann G. The entomopathogenic fungi Isaria farinosa (formerly Paecilomyces farinosus) and the Isaria fumosorosea species complex (formerly Paecilomyces fumosoroseus): biology, ecology and use in biological control. Biocontrol Science and Technology 2008; 18:865-901; http://dx.doi.org/ 10.1080/09583150802471812 [DOI] [Google Scholar]
- [11].Meyling NV, Schmidt NM, Eilenberg J. Occurrence and diversity of fungal entomopathogens in soils of low and high Arctic Greenland. Polar Biology 2012; 35:1439-45; http://dx.doi.org/ 10.1007/s00300-012-1183-6 [DOI] [Google Scholar]
- [12].Culotta E. Biological Immigrants Under Fire. Science 1991; 254:444-1447; PMID:17773285 [DOI] [PubMed] [Google Scholar]
- [13].De Barro PJ, Liu SS, Boykin LM, Dinsdale AB. Bemisia tabaci: A Statement of Species Status. Annu Rev Entomol 2011; 56:1-19; PMID:20690829; http://dx.doi.org/ 10.1146/annurev-ento-112408-085504 [DOI] [PubMed] [Google Scholar]
- [14].Chen XR, Li L, Hu QB, Zhang BW, Wu W, Jin FL, et al.. Expression of dsRNA in recombinant Isaria fumosorosea strain targets the TLR7 gene in Bemisia tabaci. Bmc Biotechnol 2015; 15:64; PMID:26198409 [DOI] [PMC free article] [PubMed] [Google Scholar]


