Abstract
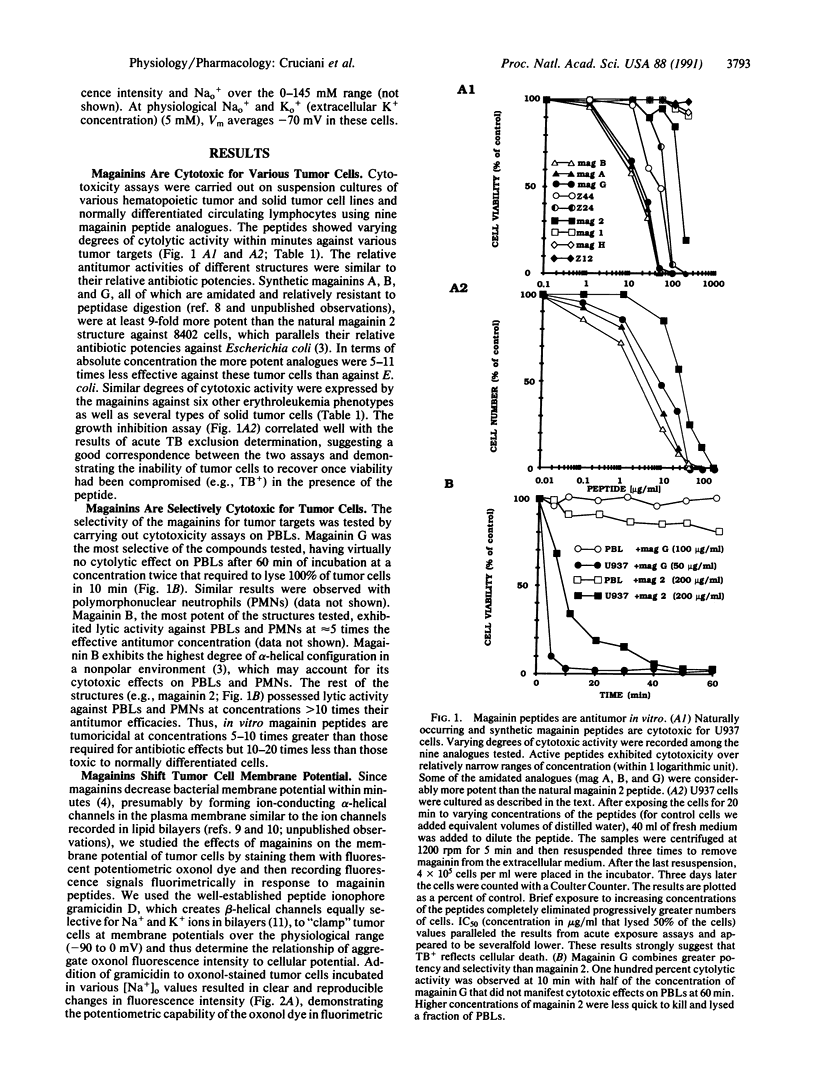
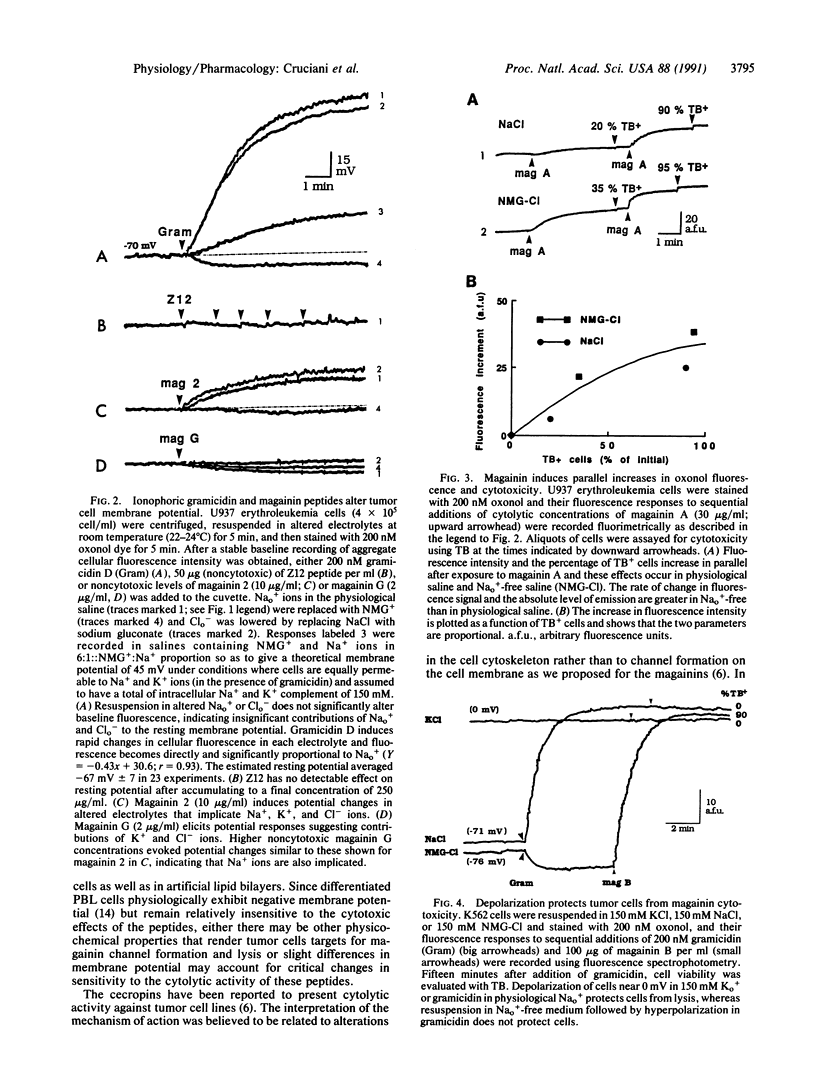
Magainins are an ionophoric class of vertebrate peptides with antibiotic activity against various microorganisms. Here we show that magainin 2 and synthetic analogues can rapidly and irreversibly lyse hematopoietic tumor and solid tumor target cells with a relative cytotoxic potency that parallels their antibacterial efficacy and at concentrations that are relatively nontoxic to well-differentiated cells. The cytotoxicity is prevented by cell depolarization. Magainins represent a natural cytolytic agent in vertebrates and may provide another therapeutic strategy for certain tumors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen H. C., Brown J. H., Morell J. L., Huang C. M. Synthetic magainin analogues with improved antimicrobial activity. FEBS Lett. 1988 Aug 29;236(2):462–466. doi: 10.1016/0014-5793(88)80077-2. [DOI] [PubMed] [Google Scholar]
- Christensen B., Fink J., Merrifield R. B., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclohier H., Molle G., Spach G. Antimicrobial peptide magainin I from Xenopus skin forms anion-permeable channels in planar lipid bilayers. Biophys J. 1989 Nov;56(5):1017–1021. doi: 10.1016/S0006-3495(89)82746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. M., Julian G. R., Jeffers G. W., White K. L., Enright F. M. In vitro cytocidal effect of lytic peptides on several transformed mammalian cell lines. Pept Res. 1989 Mar-Apr;2(2):157–160. [PubMed] [Google Scholar]
- Juretić D., Chen H. C., Brown J. H., Morell J. L., Hendler R. W., Westerhoff H. V. Magainin 2 amide and analogues. Antimicrobial activity, membrane depolarization and susceptibility to proteolysis. FEBS Lett. 1989 Jun 5;249(2):219–223. doi: 10.1016/0014-5793(89)80627-1. [DOI] [PubMed] [Google Scholar]
- MacDougall S. L., Grinstein S., Gelfand E. W. Activation of Ca2+-dependent K+ channels in human B lymphocytes by anti-immunoglobulin. J Clin Invest. 1988 Feb;81(2):449–454. doi: 10.1172/JCI113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D., Zasloff M., Bax A. A two-dimensional NMR study of the antimicrobial peptide magainin 2. FEBS Lett. 1988 Jan 18;227(1):21–26. doi: 10.1016/0014-5793(88)81405-4. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Harada M., Handa T., Funakoshi S., Fujii N., Yajima H., Miyajima K. Magainin 1-induced leakage of entrapped calcein out of negatively-charged lipid vesicles. Biochim Biophys Acta. 1989 May 19;981(1):130–134. doi: 10.1016/0005-2736(89)90090-4. [DOI] [PubMed] [Google Scholar]
- Urrutia R., Cruciani R. A., Barker J. L., Kachar B. Spontaneous polymerization of the antibiotic peptide magainin 2. FEBS Lett. 1989 Apr 10;247(1):17–21. doi: 10.1016/0014-5793(89)81230-x. [DOI] [PubMed] [Google Scholar]
- Westerhoff H. V., Juretić D., Hendler R. W., Zasloff M. Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6597–6601. doi: 10.1073/pnas.86.17.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H. A., Chused T. M. Lymphocyte membrane potential and Ca2+-sensitive potassium channels described by oxonol dye fluorescence measurements. J Cell Physiol. 1985 Oct;125(1):72–81. doi: 10.1002/jcp.1041250110. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]



