Abstract
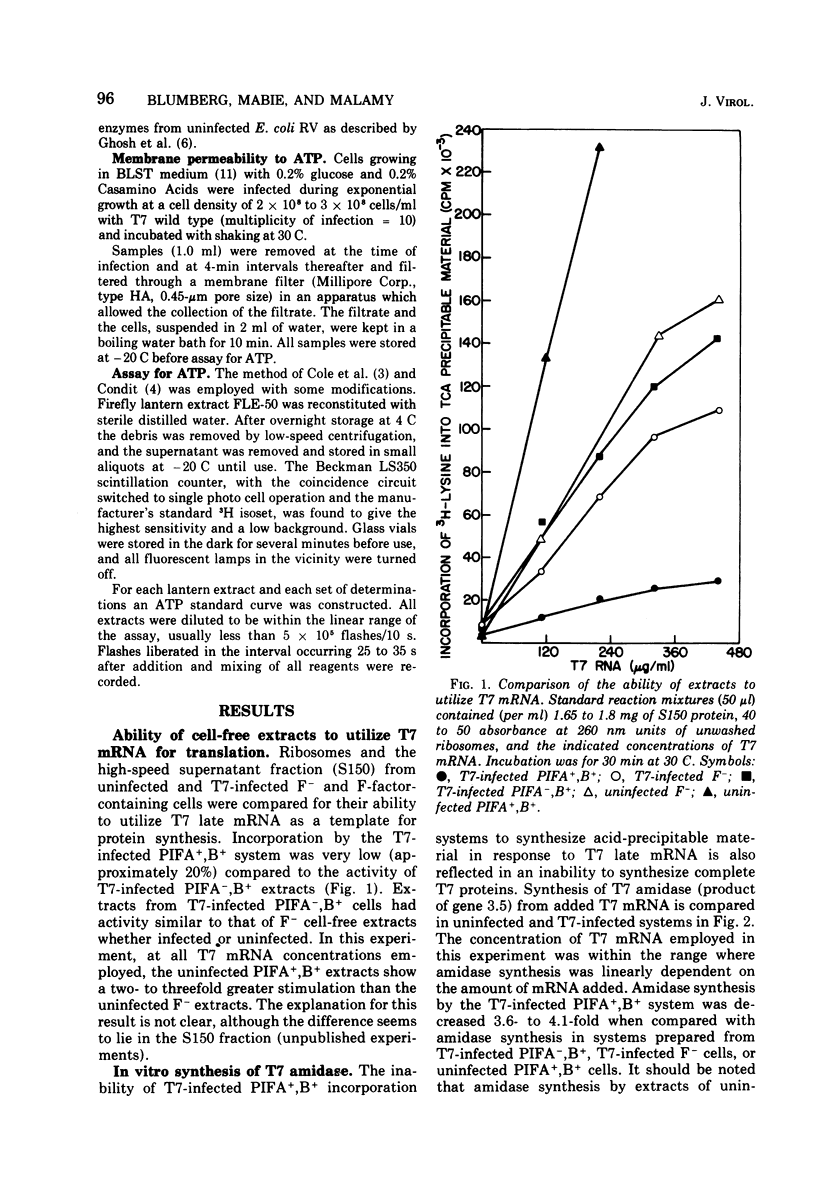
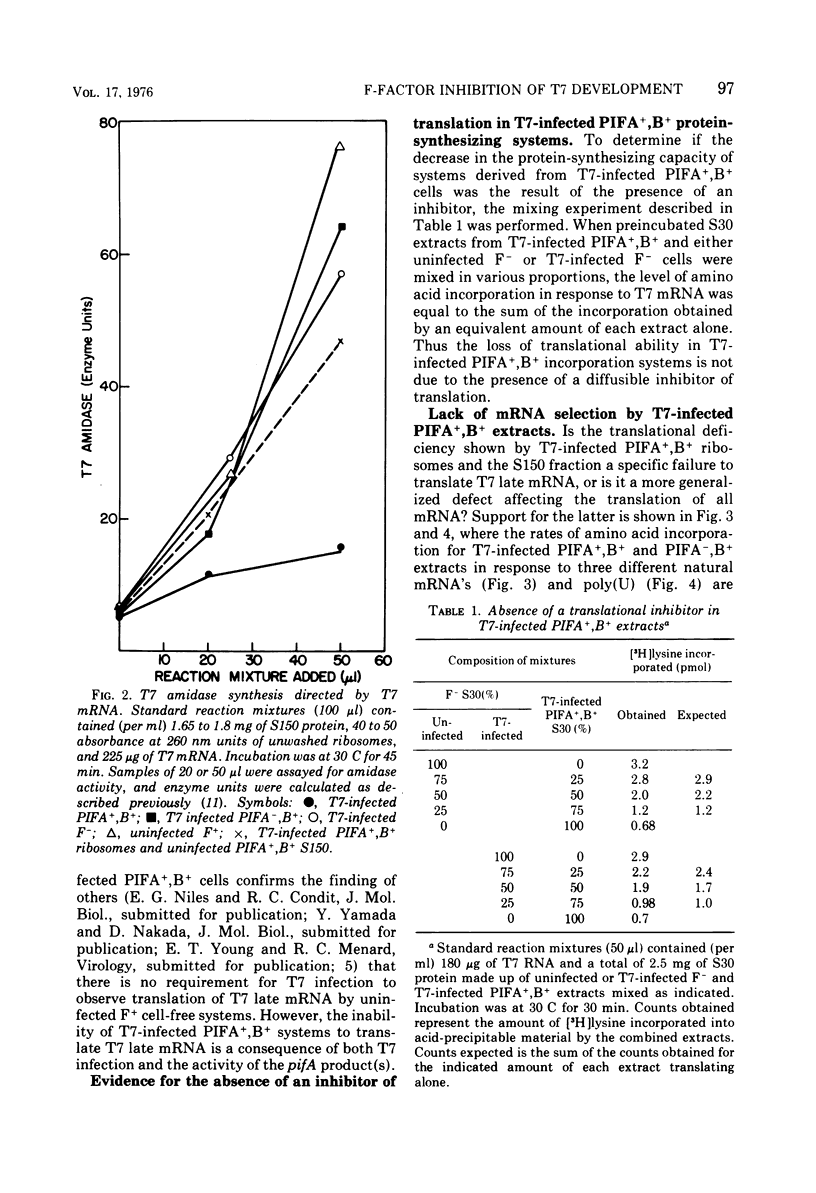
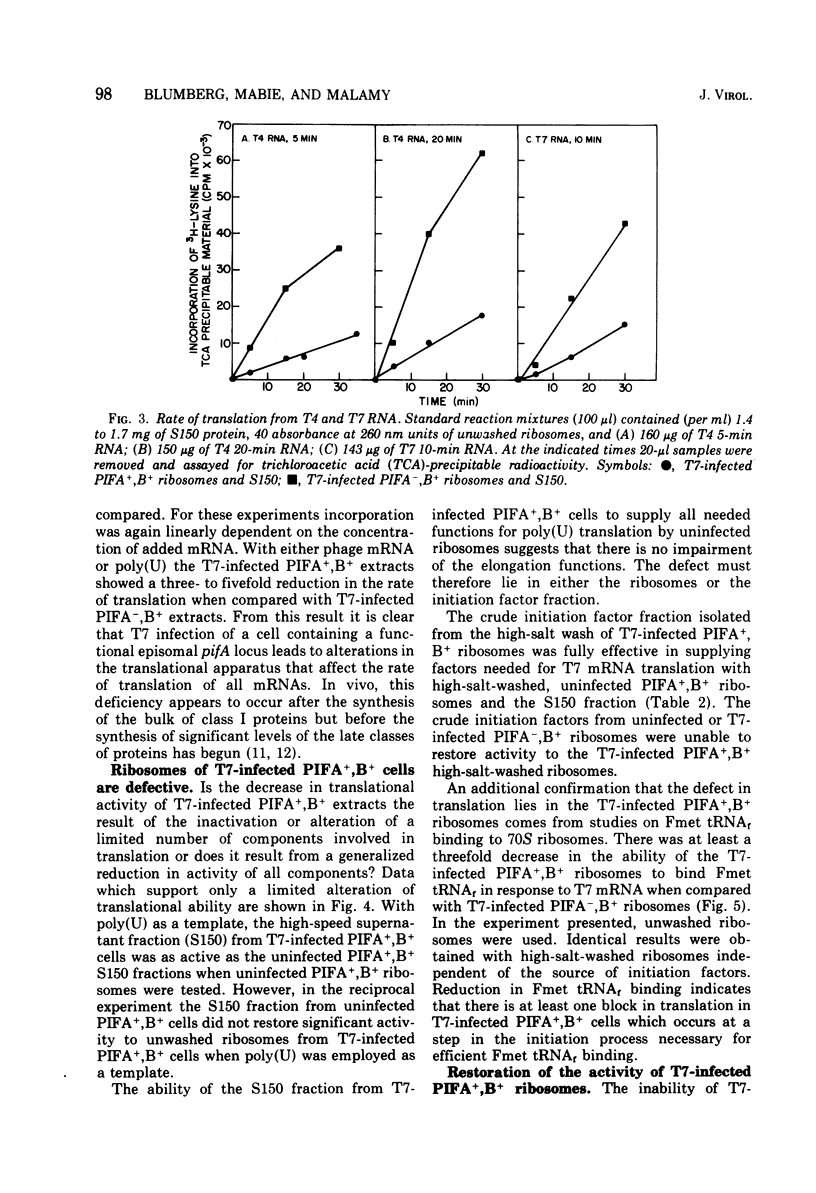
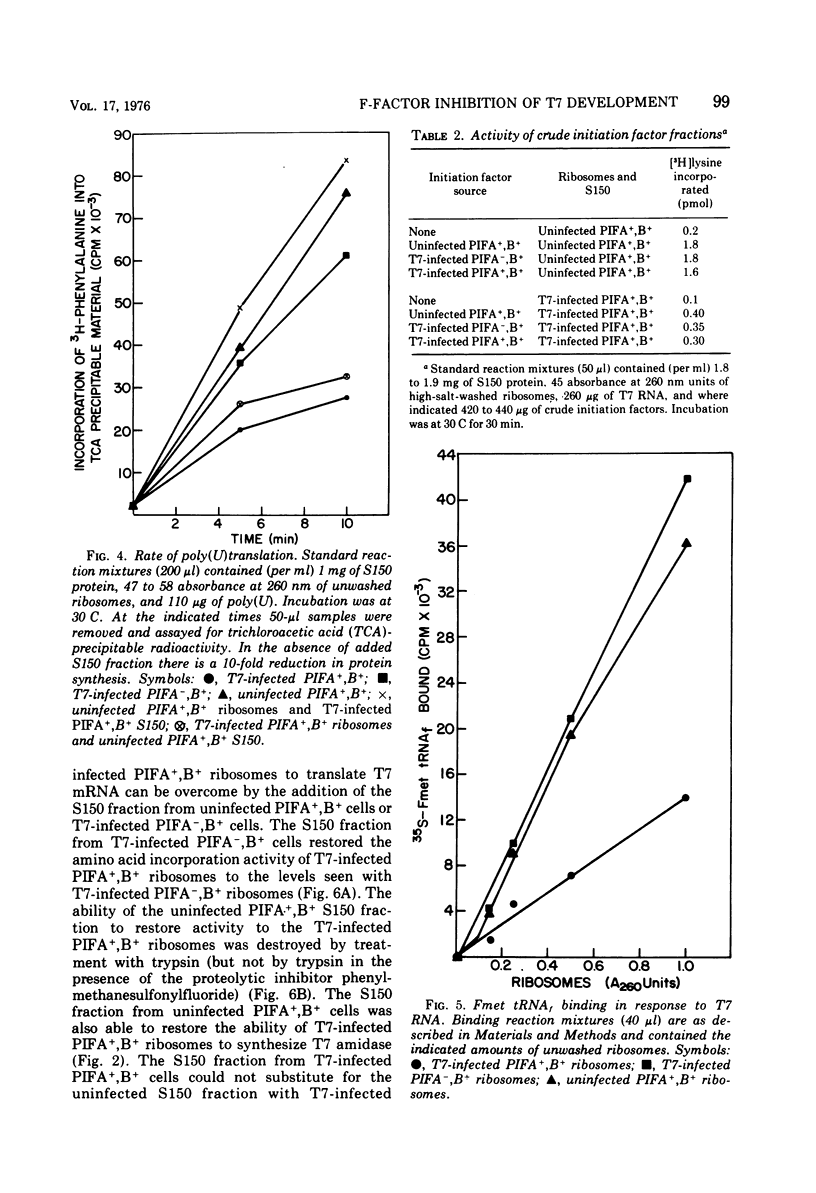
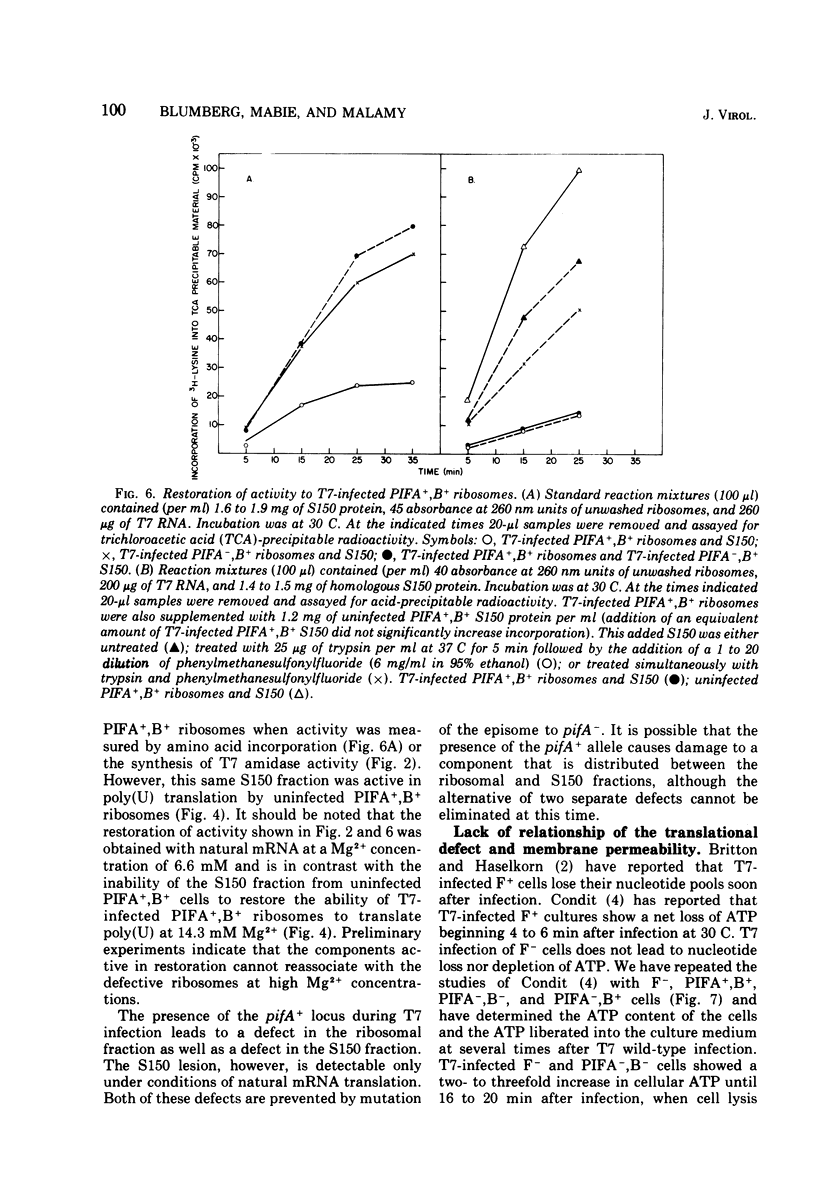
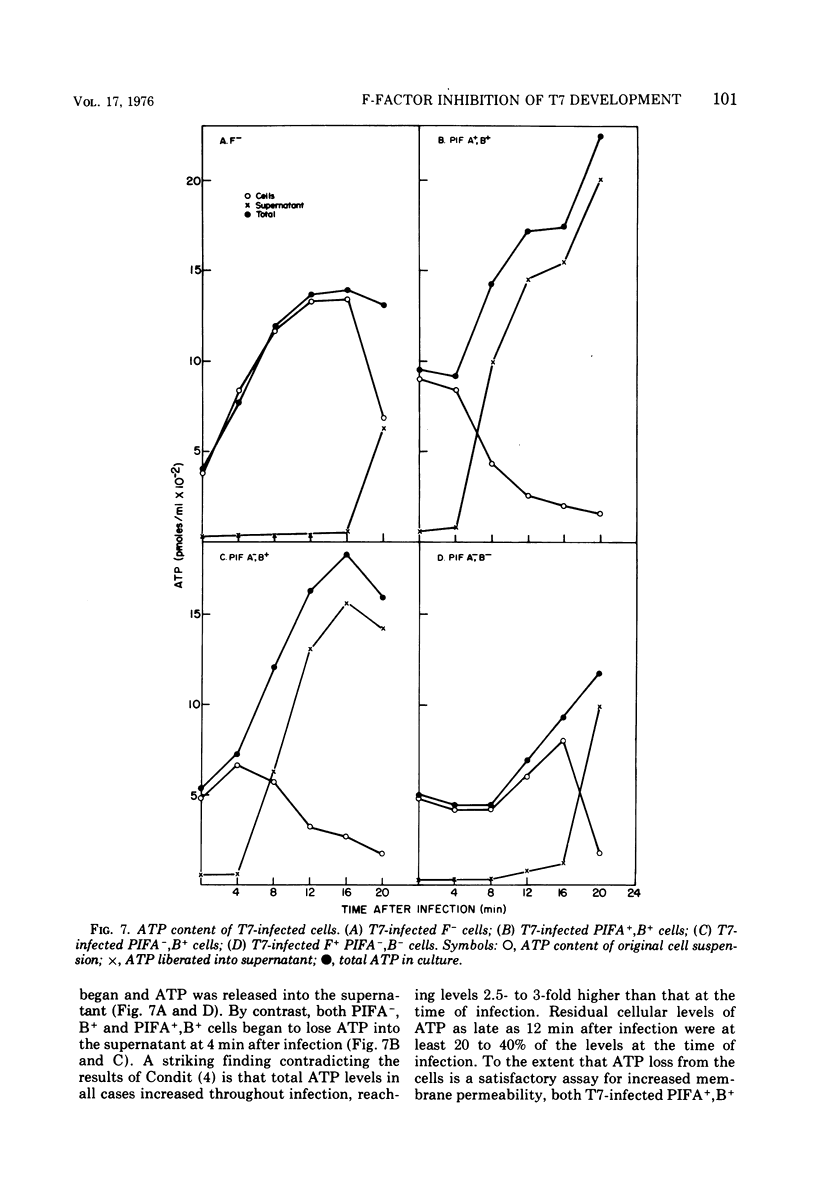
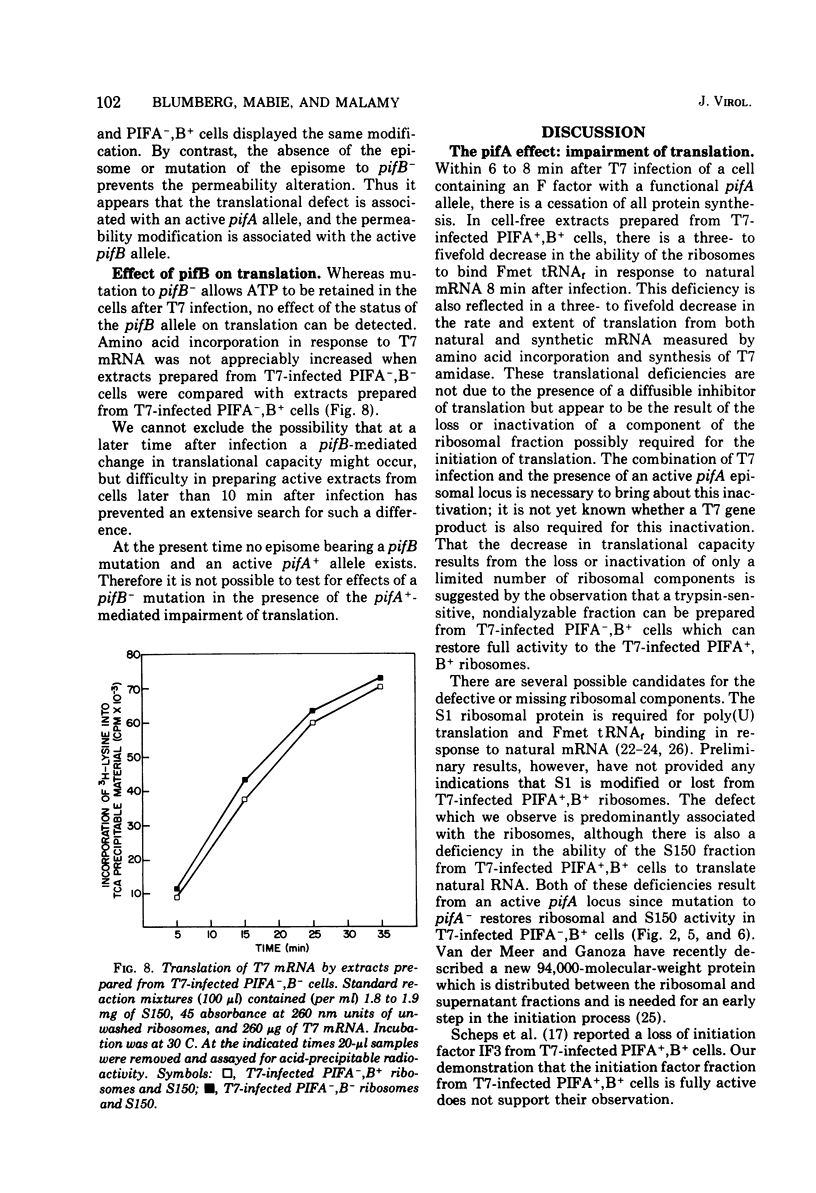
T7 infection of F-factor-containing PIFA+,B+ cells is abortive. In spite of the presence of mRNA for all three classes of T7 proteins, only the earliest of the T7 proteins are synthesized. A crucial question is whether the failure of T7 to develop in PIFA+,B+ cells is the result of an inability to translate the late classes of T7 mRNA or, as has been recently suggested (Britton, and Haselkorn, 1975; Condit, 1975), whether it is the result of a more generalized alteration in membrane permeability. We have examined the effects of the wild-type PIFA+,B+ episome and two episomal mutations (pifA− and pifB−) on in vitro translation and membrane permeability. In vivo the episomal mutations allow partial or complete T7 development to occur. We demonstrate that cell-free protein-synthesizing systems from T7-infected PIFA+,B+ cells show a three- to fivefold decrease in the rate of translation of both natural and synthetic mRNA. In addition, ribosomes from T7-infected PIFA+,B+ cells are defective in their ability to bind Fmet tRNAf in response to natural mRNA. By contrast, cell-free extracts from T7-infected pifA− (PIFA−,B+) cells retain the ability to bind Fmet tRNAf and to translate natural and synthetic mRNA at normal rates. The defective T7-infected PIFA+,B+ ribosomes can be restored to full activity by a trypsin-sensitive fraction from uninfected PIFA+,B+ or T7-infected PIFA−,B+ cells. Despite the differences in translational capacity of these extracts, both T7-infected PIFA+,B+ and PIFA−,B+ cells display the same permeability lesions as measured by the loss of ATP from the cells into the supernatant. Mutation of the episome to pfiB− prevents the loss of ATP from the cells after T7 infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg D. D., Malamy M. H. Evidence for the presence of nontranslated T7 late mRNA in infected F'(PIF+) episome-containing cells. J Virol. 1974 Feb;13(2):378–385. doi: 10.1128/jvi.13.2.378-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Söll D., Khorana H. G. Studies on polynucleotides. LXVII. Initiation of protein synthesis in vitro as studied by using ribopolynucleotides with repeating nucleotide sequences as messengers. J Mol Biol. 1967 Apr 28;25(2):275–298. doi: 10.1016/0022-2836(67)90142-8. [DOI] [PubMed] [Google Scholar]
- Goldman E., Lodish H. F. Specificity of protein synthesis by bacterial ribosomes and initiation factors: absence of change after phage T4 infection. J Mol Biol. 1972 Jun 14;67(1):35–47. doi: 10.1016/0022-2836(72)90384-1. [DOI] [PubMed] [Google Scholar]
- Leder P., Skogerson L. S., Callahan R., 3rd Translational initiation: defects arising in Escherichia coli infected with phage T7, , and Q . Arch Biochem Biophys. 1972 Dec;153(2):814–822. doi: 10.1016/0003-9861(72)90403-1. [DOI] [PubMed] [Google Scholar]
- Leffler S., Szer W. Purification and properties of initiation factor IF-3 from Caulobacter crescentus. J Biol Chem. 1974 Mar 10;249(5):1458–1464. [PubMed] [Google Scholar]
- Morrison T. G., Blumberg D. D., Malamy M. H. T7 protein synthesis in F' episome-containing cells: assignment of specific proteins to three translational groups. J Virol. 1974 Feb;13(2):386–393. doi: 10.1128/jvi.13.2.386-393.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Nugent K., Kennell D. Polypeptide synthesis by extracts from Escherichia coli treated with T2 ghosts. J Virol. 1972 Dec;10(6):1199–1204. doi: 10.1128/jvi.10.6.1199-1204.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. I. An increase in host cell permeability induced by bacteriophage infection. J Exp Med. 1954 May 1;99(5):481–494. doi: 10.1084/jem.99.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. II. Demonstration of cyclic permeability change accompanying virus infection of Escherichia coli B cells. J Exp Med. 1955 Feb 1;101(2):151–175. doi: 10.1084/jem.101.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salser W., Gesteland R. F., Bolle A. In vitro synthesis of bacteriophage lysozyme. Nature. 1967 Aug 5;215(5101):588–591. doi: 10.1038/215588a0. [DOI] [PubMed] [Google Scholar]
- Scheps R., Zeller H., Revel M. Deficiency in initiation factors of protein synthesis induced by phage T7 in E. coli F(+) strains. FEBS Lett. 1972 Oct 15;27(1):1–4. doi: 10.1016/0014-5793(72)80394-6. [DOI] [PubMed] [Google Scholar]
- Simon M., Kennell D. Defective 30S ribosomal subunits after infection of Escherichia coli by T2 ghosts. J Virol. 1974 Nov;14(5):1310–1313. doi: 10.1128/jvi.14.5.1310-1313.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Summers W. C., Jakes K. Phage T7 lysozyme mRNA transcription and translation in vivo and in vitro. Biochem Biophys Res Commun. 1971 Oct 15;45(2):315–320. doi: 10.1016/0006-291x(71)90820-5. [DOI] [PubMed] [Google Scholar]
- Szer W., Hermoso J. M., Leffler S. Ribosomal protein S1 and polypeptide chain initiation in bacteria. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2325–2329. doi: 10.1073/pnas.72.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szer W., Leffler S. Interaction of Escherichia coli 30S ribosomal subunits with MS2 phage RNA in the absence of initiation factors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3611–3615. doi: 10.1073/pnas.71.9.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakada D. F-Factor-mediated restriction of bacteriophage T7: protein synthesis in cell-free systems from T7-infected Escherichia coli F- and F+ cells. J Virol. 1975 Dec;16(6):1483–1491. doi: 10.1128/jvi.16.6.1483-1491.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin J., van Knippenberg P. H. Functional heterogeneity of the 30 S ribosomal subunit of Escherichia coli. 3. Requirement of protein S1 for translation. J Mol Biol. 1974 Mar 25;84(1):185–195. doi: 10.1016/0022-2836(74)90221-6. [DOI] [PubMed] [Google Scholar]
- van der Meer J. P., Ganoza M. C. Purification and characterization of a new factor which restores protein synthesis in a conditionally lethal mutant of Escherichia coli. Eur J Biochem. 1975 May;54(1):229–237. doi: 10.1111/j.1432-1033.1975.tb04132.x. [DOI] [PubMed] [Google Scholar]



