Abstract
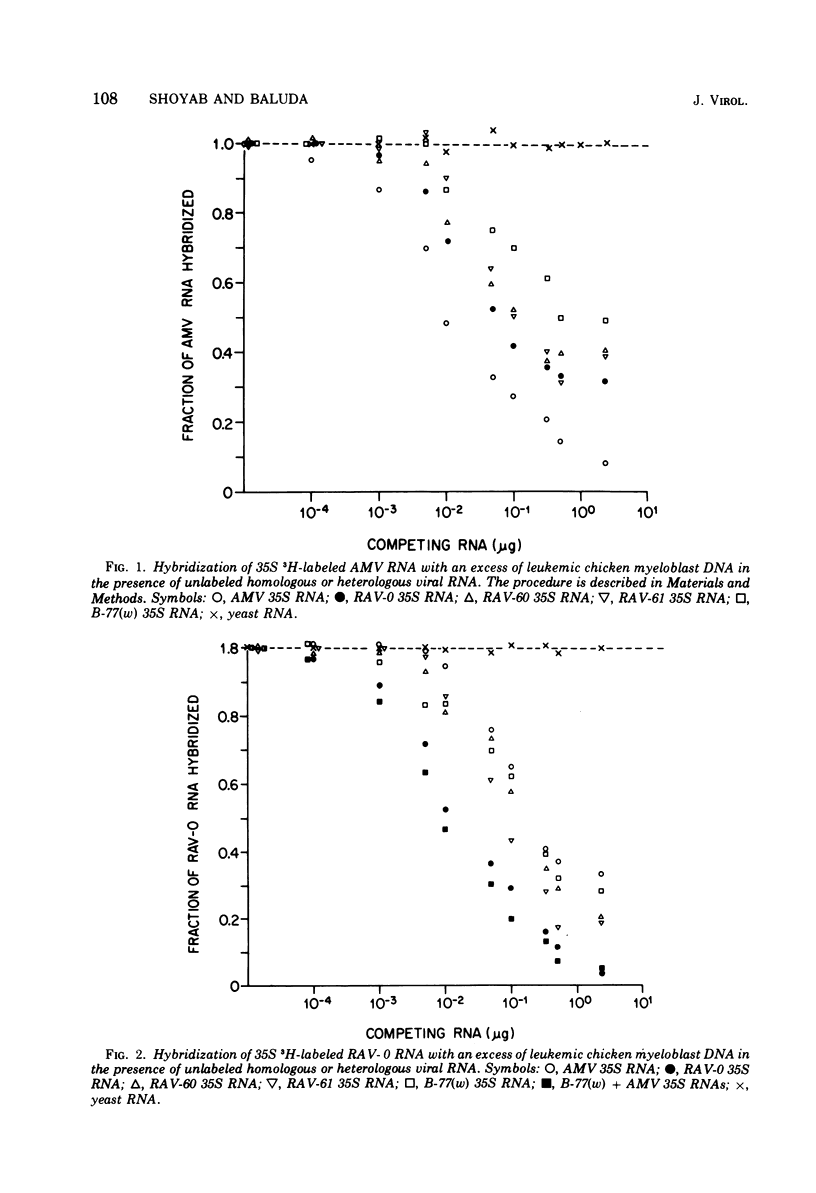
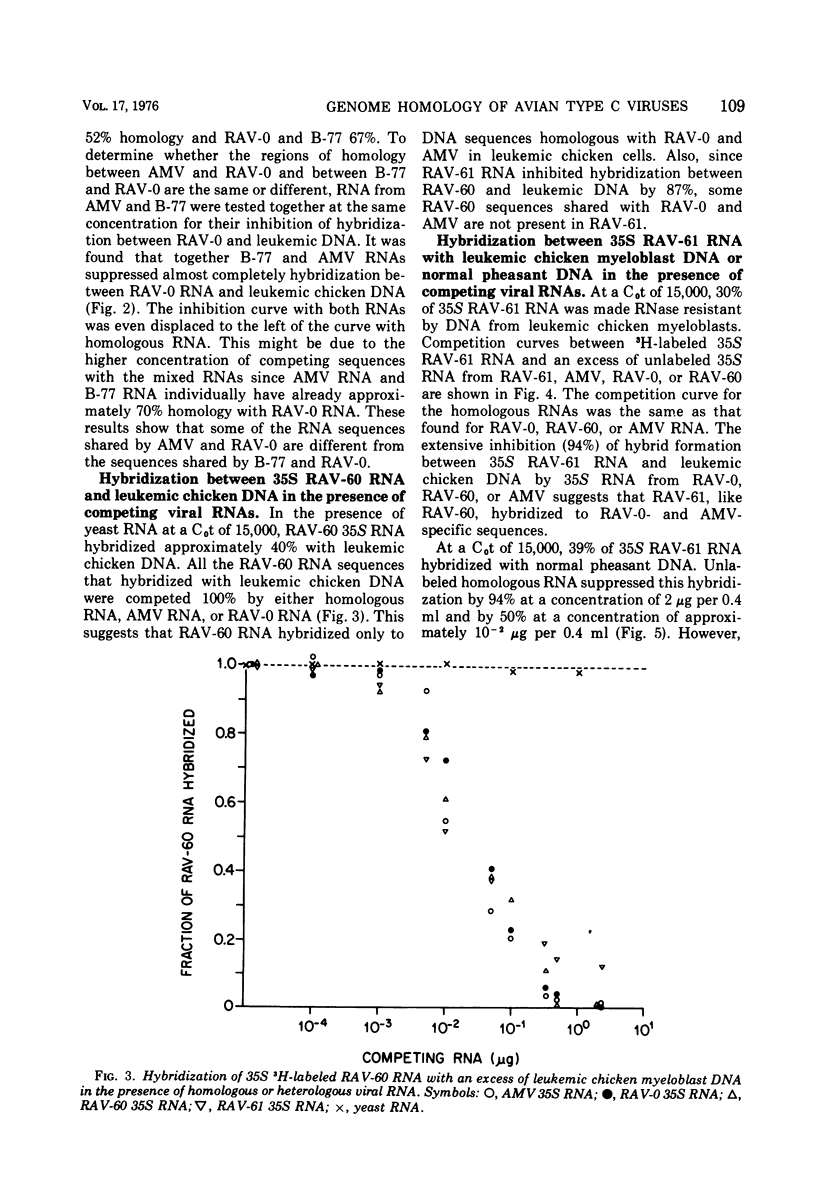
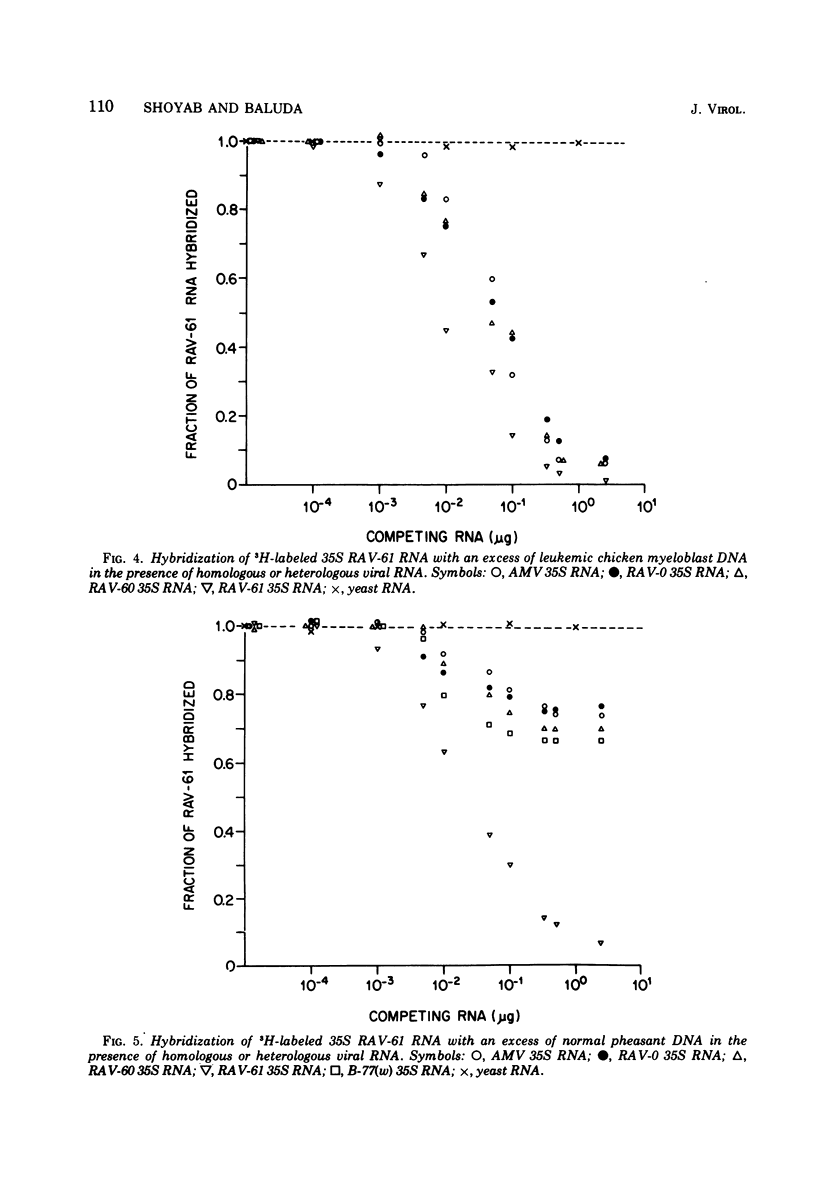
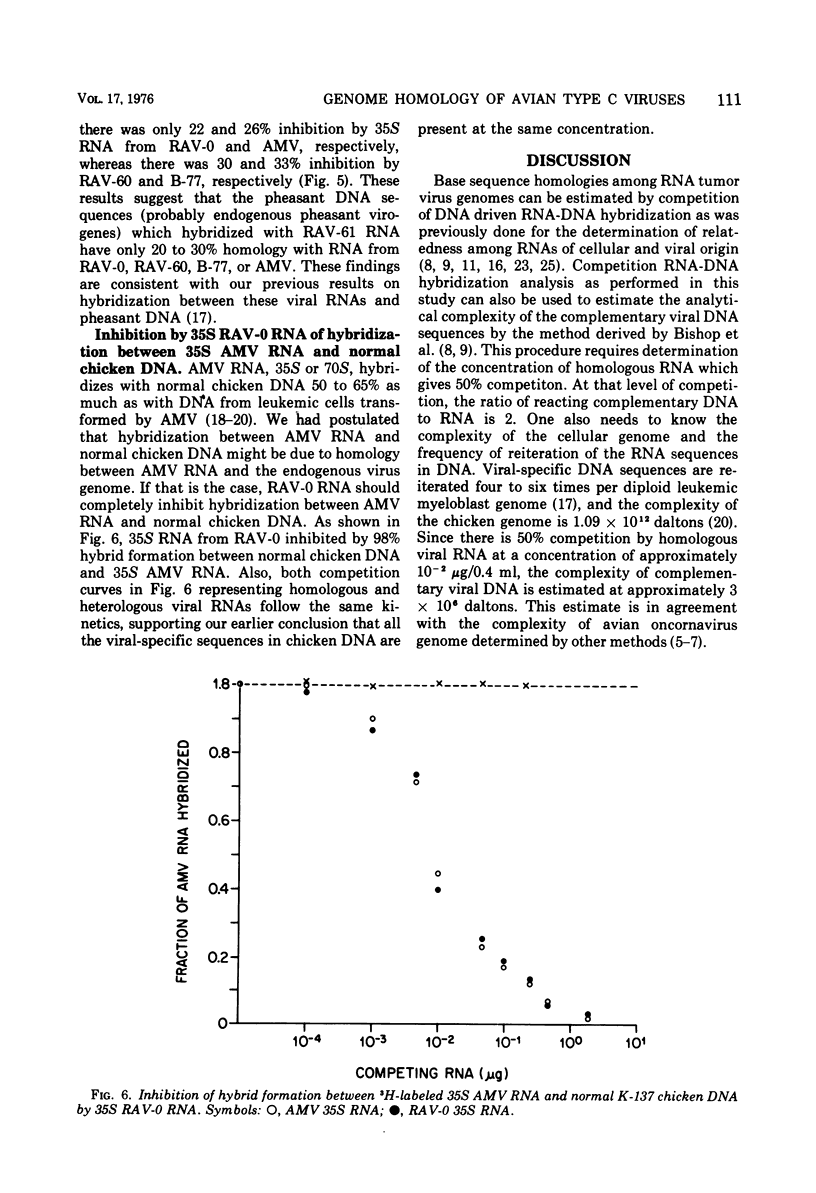
RNA sequence relatedness among avian RNA tumor virus genomes was analyzed by inhibition of DNA-RNA hybrid formation between 3H-labeled 35S viral RNA and an excess of leukemic or normal chicken cell DNA with increasing concentrations of unlabeled 35S viral RNA. The avian viruses tested were Rous associated virus (RAV)-0, avian myeloblastosis virus (AMV), RAV-60, RAV-61, and B-77 sarcoma virus. Hybridization of 3H-labeled 35S AMV RNA with DNA from normal chicken cells was inhibited by unlabeled 35S RAV-0 RNA as efficiently (100%) as by unlabeled AMV RNA. Hybridization between 3H-labeled 35S AMV RNA and DNA from leukemic chicken myeloblasts induced by AMV was suppressed 100 and 68% by unlabeled 35S RNA from AMV and RAV-0, respectively. Hybridization between 3H-labeled RAV-0 and leukemic chicken myeloblast DNA was inhibited 100 and 67% by unlabeled 35S RNA from RAV-0 and AMV, respectively. It appears therefore that the AMV and RAV-0 genomes are 67 to 70% homologous and that AMV hybridizes to RAV-0 like sequences in normal chicken DNA. Hybridization between AMV RNA and leukemic chicken DNA was inhibited 40% by RNA from RAV-60 or RAV-61 and 50% by B-77 RNA. Hybridization between RAV-0 RNA and leukemic chicken DNA was inhibited 80% by RAV-60 or RAV-61 and 70% by B-77 RNA. Hybridization between 3H-labeled 35S RNA from RAV-60 or RAV-61 and leukemic chicken myeloblast DNA was reduced equally by RNA from RAV-60, RAV-61, AMV or RAV-0; this suggests that RNA from RAV-60 and RAV-61 hybridizes with virus-specific sequences in leukemic DNA which are shared by AMV, RAV-0, RAV-60, and RAV-61 RNAs. Hybridization between 3H-labeled 35S RNA from RAV-61 and normal pheasant DNA was inhibited 100% by homologous viral RNA, 22 to 26% by RNA from AMV or RAV-0, and 30 to 33% by RNA from RAV-60 or B-77. Nearly complete inhibition of hybridization between RAV-0 RNA and leukemic chicken DNA by a mixture of AMV and B-77 35S RNAs indicates that the RNA sequences shared by B-77 virus and RAV-0 are different from the sequences shared by AMV and RAV-0. It appears that different avian RNA tumor virus genomes have from 50 to 80% homology in nucleotide sequences and that the degree of hybridization between normal chicken cell DNA and a given viral RNA can be predicted from the homology that exists between the viral RNA tested and RAV-0 RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A., JAMIESON P. P. In vivo infectivity studies with avian mveloblastosis virus. Virology. 1961 May;14:33–45. doi: 10.1016/0042-6822(61)90129-5. [DOI] [PubMed] [Google Scholar]
- BALUDA M. A. Properties of cells infected with avian myeloblastosis virus. Cold Spring Harb Symp Quant Biol. 1962;27:415–425. doi: 10.1101/sqb.1962.027.001.039. [DOI] [PubMed] [Google Scholar]
- BURMESTER B. R., WALTER W. G., GROSS M. A., FONTES A. K. The oncogenic spectrum of two pure strains of avian leukosis. J Natl Cancer Inst. 1959 Aug;23:277–291. [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. DNA complementary to viral RNA in leukemic cells induced by avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):329–336. doi: 10.1073/pnas.66.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluda M. A., Shoyab M., Markham P. D., Evans R. M., Droham W. N. Base sequence complexity of 35S avian myeloblastosis virus RNA determined by molecular hybridization kinetics. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):869–874. doi: 10.1101/sqb.1974.039.01.101. [DOI] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. O. DNA-RNA hybridization. Acta Endocrinol Suppl (Copenh) 1972;168:247–276. doi: 10.1530/acta.0.071s247. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Grouse L., Chilton M. D., McCarthy B. J. Hybridization of ribonucleic acid with unique sequences of mouse deoxyribonucleic acid. Biochemistry. 1972 Feb 29;11(5):798–805. doi: 10.1021/bi00755a019. [DOI] [PubMed] [Google Scholar]
- Hanafusa H., Hayward W. S., Chen J. H., Hanafusa T. Control expression of tumor virus genes in uninfected chicken cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1139–1144. doi: 10.1101/sqb.1974.039.01.130. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Detection of avian tumor virus RNA in uninfected chicken embryo cells. J Virol. 1973 Feb;11(2):157–167. doi: 10.1128/jvi.11.2.157-167.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., McMillin C., MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. doi: 10.1128/jvi.13.4.837-846.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A., Evans R. Acquisition of new DNA sequences after infection of chicken cells with avian myeloblastosis virus. J Virol. 1974 Feb;13(2):331–339. doi: 10.1128/jvi.13.2.331-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Homology between avian oncornavirus RNAs and DNA from several avian species. J Virol. 1975 Dec;16(6):1492–1502. doi: 10.1128/jvi.16.6.1492-1502.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Evans R. M., Baluda M. A. Presence in leukemic cells of avian myeloblastosis virus-specific DNA sequences absent in normal chicken cells. J Virol. 1974 Jul;14(1):47–49. doi: 10.1128/jvi.14.1.47-49.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Markham P. D., Baluda M. A. Host induced alteration of avian sarcoma virus B-77 genome. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1031–1035. doi: 10.1073/pnas.72.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Markham P. D., Baluda M. A. Reliability of the RNA-DNA filter hybridization for the detection of oncornavirus-specific DNA sequences. J Virol. 1974 Aug;14(2):225–230. doi: 10.1128/jvi.14.2.225-230.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkovic D. Characteristics of tumors induced in mammals, especially rodents, by viruses of the avian leukosis sarcoma group. Adv Virus Res. 1972;17:95–127. [PubMed] [Google Scholar]
- Tereba A., McCarthy B. J. Hybridization of 125I-labeled ribonucleic acid. Biochemistry. 1973 Nov 6;12(23):4675–4679. doi: 10.1021/bi00747a020. [DOI] [PubMed] [Google Scholar]
- Wright S. E., Neiman P. E. Base-sequence relationships between avian ribonucleic acid endogenous and sarcoma viruses assayed by competitive ribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 26;13(7):1549–1554. doi: 10.1021/bi00704a035. [DOI] [PubMed] [Google Scholar]


