Abstract
Background
Neutralizing antibodies against nerve growth factor (NGF) are analgesic in rodent models, naturally occurring degenerative joint disease (DJD) pain in dogs, and chronic pain in humans.
Objectives
To evaluate the efficacy of a fully felinized anti‐NGF antibody (NV‐02) for the treatment of DJD pain and mobility impairment in cats.
Animals
Thirty‐four client‐owned cats with DJD‐associated pain and mobility impairment.
Methods
In a placebo‐controlled, pilot, masked clinical study, cats were randomized to a single treatment with NV‐02 (0.4 mg/kg SC [n = 11] or 0.8 mg/kg SC [n = 12]) or placebo (saline, SC [n = 11]). Activity was measured objectively. Additionally, owners completed clinical metrology instruments (client‐specific outcome measures [CSOM] and feline musculoskeletal pain index [FMPI]) on days 0 (screening), 14 (baseline), 35, 56, and 77. A repeated‐measures model was used to evaluate the objective activity data.
Results
NV‐02 significantly increased objectively measured activity overall (P = .017) and at 2 (P = .035), 3 (P = .007), 4 (P = .006), 5 (P = .007), and 6 (P = .017) weeks after treatment. CSOM scores (P = .035) and pain (P = .024) showed a significant effect of treatment 3 weeks after administration. In the treatment group, 83% of the owners correctly identified the treatment administered compared with 45% of owners in the placebo group (P = .013). No treatment‐related adverse effects were identified.
Conclusions
These pilot data demonstrate a 6‐week duration positive analgesic effect of this fully felinized anti‐NGF antibody in cats suffering from DJD‐associated pain.
Keywords: Client‐specific outcome measures, Feline musculoskeletal pain index, Osteoarthritis
Abbreviations
- AC
activity counts
- AM
activity monitors
- CMI
clinical metrology instrument
- CSOM
client‐specific outcome measures
- DJD
degenerative joint disease
- FMPI
feline musculoskeletal pain index
- NCSU‐CVM
North Carolina State University College of Veterinary Medicine
- NGF
nerve growth factor
- OA
osteoarthritis
Many adult and geriatric cats have radiographic evidence of degenerative joint disease (DJD),1, 2 and a large proportion of these have associated chronic pain, manifested as alterations in mobility and activity. In the United States, there is no approved medication for the long‐term treatment of chronic pain in cats, despite the clear need for such a treatment.
Currently, the nonsteroidal anti‐inflammatory drug(NSAID) meloxicam is approved in Europe for use in treating chronic pain in cats, but has not been approved for this use in the United States. There are concerns about the use of NSAIDs for long periods of time in cats, especially because the majority of cats presenting with DJD‐associated pain have evidence of chronic kidney disease.3 Because of these concerns, dosages lower than the European‐approved dosage of meloxicam have been tried, and there are several suggestions from open‐label studies that these lower dosages are effective in the management of DJD‐associated pain in cats.4, 5 Only one blinded, placebo‐controlled study assessing a dosage lower than the approved 0.05 mg/kg daily dosage has been performed, and that study found that a dosage of 0.035 mg/kg daily produced measureable improvement over a 3‐week period of administration.6, 7 Indeed, only 2 placebo‐controlled, masked, clinical studies of the efficacy of meloxicam in cats have been published.6, 7, 8
Neutralizing antibodies against nerve growth factor (NGF) are analgesic in rodent models9 and in humans10 with chronic pain, although none currently are approved for use in humans. Using a proprietary technique for interspecies conversion of antibodies based on expressed cDNA sequence analysis (PETization™) Nexvet Biopharma developed a novel therapeutic fully felinized anti‐NGF mAb (named NV‐02) for the alleviation of pain in cats.11
We hypothesized that a single SC dose of anti‐NGF antibody would alleviate pain and improve mobility for up to 6 weeks. Improvements would be able to be detected by owners and reflected in objectively measured activity. The primary objective of this pilot study was to assess the overall efficacy potential of SC administered NV‐02 in improving owner‐evaluated mobility and activity and increasing objectively measured activity counts in cats with DJD as compared with placebo. Secondary objectives were to (1) assess the overall duration of SC administered NV‐02 in improving owner‐evaluated mobility and activity and increasing objectively measured activity in cats with DJD‐associated pain compared with placebo over an 11‐week period (2 weeks pretreatment baseline, 9 weeks posttreatment); (2) to determine the relative efficacy potential and optimal dosage of NV‐02 using 2 dosage groups; and (3) to assess the adverse effects associated with NV‐02 in cats beyond preliminary investigations in laboratory cats.
Methods and Materials
This study was approved by the Animal Care and Use Committee (Protocol # 14‐043‐O) at North Carolina State University College of Veterinary Medicine (NCSU‐CVM), and written owner consent was granted in each case after verbal discussion of the study. The reporting of data follows the CONSORT guidelines (http://www.consort-statement.org/consort-2010).
Study Design
This study was designed as a double‐blind, placebo‐controlled, randomized pilot study with 12 cats in each of 3 groups. Outcome measures included changes in owner ratings and changes in activity (as measured by accelerometry) after treatment with the active drug or placebo. Additionally, adverse events, including changes in clinical pathology assessments, were evaluated.
Animals
Animals enrolled in the study were all client‐owned cats with naturally occurring DJD‐associated pain and mobility impairment. Subjects were recruited using a combination of advertising directly to owners and to veterinarians. All study‐related activities were performed at zero cost to the owner. We also employed ideas on recruitment and incentives identified recently on owners' attitudes to clinical studies involving cats.12
Prescreening
Owners either emailed or telephoned in response to advertisement and outreach. The study and requirements were explained to them, with a focus on the requirements that the cats be indoor only, able to wear a collar with the activity monitor on it, and be impaired in at least 3 activities. Owners were questioned about whether they had any upcoming life changes, such as moves, new babies, new pets, or extended vacations. Suitable cases were scheduled for visits to the Veterinary Teaching Hospital.
Inclusion and Exclusion Criteria
Eligibility criteria were similar to previous DJD‐associated pain studies in cats performed by our program.6 Cats were eligible to participate in the study if they had a qualifying degree of owner‐noted mobility or activity impairment (described in the next section), evidence of pain during manipulation of at least 2 joints or spinal segments during veterinary orthopedic evaluation (discussed in Orthopedic evaluation section), and radiographic evidence of DJD in at least 2 of the painful joints or spinal segments (discussed in Radiographic evaluation section). Cats were required to be >1 year old and weigh more than 1 kg. Predetermined exclusion criteria for all cats included the presence of suspected or diagnosed infectious diseases, symptomatic cardiac disease, immune‐mediated disease, neoplasia, inflammatory bowel disease, urinary tract infection, hyperthyroidism, and diabetes mellitus. These conditions were ruled out using review of the referring veterinarian's medical records, owner history, physical examination, CBC, serum biochemistry panels, T4 testing, and urinalysis. Cats with chronic kidney disease (CKD) up to and including IRIS stage 2 were eligible to enroll, provided the disease was stable as determined by repeated serum biochemistry panels and urinalyses. Cats with CKD of IRIS stage 3 or 4 were excluded from participating in the study.
Owner Evaluation of Mobility Impairment
During the screening interview, owners were asked to identify 3 activities that their cat showed impairment in performing as described previously.6 These 3 items then were used to construct the client‐specific outcome measures (CSOM) assessment and used for subsequent study assessments. Owners rated their cat's ability to perform each activity on a Likert scale ranging from 4 (no problem) to 0 (impossible) with intermediate values of 3 (mild difficulty), 2 (moderate difficulty), and 1 (severe difficulty). Owner ratings were converted to numerical scores, and the total CSOM score was the sum of these 3 scores with a possible range of 0–12. In order to ensure that cats enrolled in this study were sufficiently impaired by the DJD‐associated pain (as rated by their owners), cats were eligible for inclusion if they received an owner‐rated score of ≤5 on day 0 (lower numbers indicating greater impairment). Owners completed the CSOM without knowledge of the cutoff for inclusion.
Orthopedic Evaluation
Orthopedic evaluations were carried out as previously described6, 13, 14 and were performed by a single veterinarian (MG), with a single assistant (AT). Joints were designated a pain score before radiographs were taken and evaluated. As previously described,6 a total pain score was calculated as the sum of all the individual appendicular joint and axial skeletal segment pain scores.
Radiographic Evaluation
Cats meeting eligibility criteria for owner‐noted mobility or activity impairment and pain on orthopedic evaluation were sedated, and orthogonal radiographs were taken of every joint and spinal segment. Radiographs were reviewed for the presence of DJD by a board‐certified veterinary radiologist who was not aware of the presence or location of pain.
Randomization Method
Cats were randomized to receive drug (low or high dosage, see below) or placebo by the NCSU‐CVM pharmacy once the cat was enrolled. Randomization of treatment occurred in groups of 3, and the randomization key was held by pharmacy staff. The dispensing of treatment or placebo was performed by pharmacy staff, with all personnel involved in data collection being masked to the treatment given. For balance, cats were stratified by higher or lower impairment based on CSOM score for randomization (CSOM 0, 1, 2 = high impairment; CSOM 3, 4, 5 = low impairment), but not analyzed separately. The randomization code and allocation of cats to NV‐02 or placebo was only made known to investigators once the data had all been entered and quality control checked by personnel not directly involved in the study (Clinical Studies Core).
Masking
A preloaded syringe was placed in a brown, light resistant plastic bag labeled with the case number, name, and instructions “Give NV‐02 OR placebo by SC injection once.” The prescribing veterinarian and the quantity (mL) also were listed on the label. The syringe and contents were allowed to reach room temperature for 1 hour, and the injection was given by a veterinary technician not associated with the study (Clinical Studies Core personnel). Each cat was restrained by AT, who was required to turn her head away as the treatment or placebo was being administered. The volume of placebo was balanced, so even observation of volume would not reveal the contents of the syringe.
Study Timeline
Table 1 outlines the study protocol and activities. The owner visited the clinic with the cat on 3 occasions over the 11‐week study (day 0, day 14, and day 77), and on 2 other occasions without the cat (day 35 and day 56). The expected duration of efficacy of NV‐02, if seen, was thought to be 4–6 weeks. The study was extended beyond this time period to ensure the full profile of efficacy was captured.
Table 1.
Outline of study protocol and activities at each time point
| Day of Study | Action |
|---|---|
| Prior to Veterinary Hospital visit |
|
|
Screening—Day 0 Owner and cat visit |
Screening of cat and owner:
|
|
Days 1–14 (weeks 1 and 2) |
|
|
Day 14 (± 2 days) Owner and cat visit |
|
|
Days 15–35 (weeks 3, 4, and 5) |
|
|
Day 35 (± 2 days) Owner to visit |
|
|
Days 36–56 (weeks 6, 7, and 8) |
|
|
Day 56 (± 2 days) Owner visit |
|
|
Days 57–77 (weeks 9, 10, and 11) |
|
|
Day 77 (± 2 days) Owner and cat visit |
|
| Withdrawals | Day 77 schedule is followed (ie, if patient drops out, the “day 77” evaluation was performed) |
CSOM, Client‐Specific Outcome Measures; FMPI, Feline Musculoskeletal Pain Index.
Investigational Compound
A fully felinized anti‐NGF mAb that is in development for the control of pain associated with DJD in cats was used in this study, referred to as NV‐02.11 The NV‐02 was supplied by Nexvet as a sterile solution containing 1.9 mg/mL of the anti‐NGF antibody in phosphate‐buffered saline (pH 7.2). The treatment groups were T1: single SC dose of 0.4 mg/kg NV‐02; T2: single SC dose of 0.8 mg/kg NV‐02; and P: single SC dose of sterile saline, at a volume of either 0.21 mL/kg or 0.42 mL/kg (randomized).
Description of Outcome Measures
Several efficacy outcome measures were chosen to fully characterize the potential response seen with NV‐02.
Primary
Activity measured by accelerometer counts (activity counts; objective)
- Owner assessment using clinical metrology instruments (subjective):
- Feline musculoskeletal pain index (FMPI)
- Client‐specific outcome measure (CSOM)
Secondary
Owner assessment of whether active treatment had been administered (subjective)
Accelerometery (Activity)
The accelerometers1 used to measure activity (hereafter referred to as “Activity Monitors,” AM), and their use in cats, have both been described previously.6, 8, 15 The AMs were mounted on a neck collar for all cats. The AMs were programmed to collect data with an epoch duration of 1 minute.
Owner Assessments
Feline Musculoskeletal Pain Index (FMPI)
The version used in this study (v10) was published recently.6 The FMPI queries owners on their cat's ability to perform each of 17 activities (rated on a Likert scale from “normal” to “not at all”) with an option to select “don't know or not applicable.” Two additional items (pain domain questions) ask owners to rate their cat's level of pain on a standardized 100‐mm visual analog scale. Owner ratings were converted to scores ranging from 0 to 4 for each item with 0 = not at all and 4 = normal. Scores on the visual analog scale were calculated by measuring, in mm, from the start (zero point) to the owner's mark, with 100 indicating “no pain.” The range of possible scores was 0–68 for items 1–17 and 0–100 each of the final 2 questions.
Client‐Specific Outcome Measures (CSOM) Questionnaire
Construction of the CSOM was described above. The CSOM items were each presented in the same order for each cat at each visit. Owner ratings were converted to numerical scores, and the total CSOM score represented the sum of these 3 scores with a possible range of 0–12, with higher numbers indicating less impairment.
Owner Assessment of Whether Active Treatment Had Been Administered
At the end of the study (day 77), owners were asked “Do you think your cat received the anti‐nerve growth factor antibody?” They were required to answer “yes” or “no,” and their response was recorded.
Three approaches were taken in the assessment of safety in this pilot study:
Clinical assessment of cats after the administration of NV‐02 or placebo, for evidence of an allergic response
Evaluation of changes in CBC, serum biochemistries, and urinalyses
Owner‐reported adverse events
After administration of NV‐02 or placebo, cats were observed for 4 hours for signs indicative of an allergic response, including scratching or rubbing, increased respiratory rate, tachycardia, urticaria, and weakness. A bank of cages was positioned in the main Comparative Pain Research room where 2 technicians in the room could closely observe the cats. Respiratory rate was taken every 15 minutes.
Blood samples and urine samples were taken from all of the cats at day 0 (baseline) and day 77 to evaluate any changes in CBC or serum biochemistry results over the course of the study.
Owners were asked to report any events that were unusual for their cat as soon as they occurred, using provided contact information. If adverse events (AE) occurred, owners were instructed to take their cat immediately to the NCSU‐CVM Emergency Service or their local emergency veterinary clinic. Adverse events were defined as any observations in the cat that were unfavorable and unintended and that occurred during the study, whether they were considered to be treatment related. A serious adverse event (SAE) for this study was defined as any AE that was either fatal or life‐threatening, required professional intervention (by a veterinarian), and considered by the investigators to be clinically serious.
Statistical Analysis
Sample Size Estimation
This study was designed as a pilot study, and a formal power analysis was not conducted because anti‐NGF antibodies had not been used in cats previously, and the expected difference between treatment groups, therefore, could not be estimated. One aim of this study was to gather sufficient data to enable a power analysis to be conducted for a larger efficacy study.
Handling of Data
Efficacy was analyzed for all cats completing the study (through day 77) unless another disease or problem was discovered that could not be attributed to the test articles at the end of the study, and safety was analyzed for all cats enrolled in the study using day 0 and day 77 laboratory results and AE reported by owners. Activity data analyses were performed comparing each group (T1 and T2) to placebo. After the analysis of the objective activity data, the treatment groups were combined for the analysis of the subjective data. A P‐value of .05 was considered significant. There was no adjustment of P‐value for multiple comparisons due because of the pilot nature of this study.
Groups (treatment and placebo) were compared for distribution of age and weight using t‐tests, sex of the cats using chi‐square analysis, and body condition score using Wilcoxon rank‐sum test.
Data points (counts) for activity were generated at every minute of every day throughout the study. These counts were averaged for each week to generate a single data point (mean activity per minute) for each cat over each week. Baseline activity was defined as the mean activity count per minute over the first 2 weeks of the study for each cat, before treatment. Values of mean activity count per minute were used to calculate the change from baseline for each week of the study posttreatment (weeks 3–11) for each cat. These data were analyzed using a repeated‐measures ANOVA, including a week × week interaction because the collection of activity data continued for longer than the expected duration of efficacy of NV‐02. Contrasts were used to look at differences between groups at individual weeks.
For the FMPI scores, a percentage possible score was calculated as follows:
Changes from baseline (day 14) were calculated for the %FMPIposs and for CSOM scores for each cat at each assessment time point (day 35, day 56, and day 77) and used for within‐group comparisons (Wilcoxon signed rank) and between‐group comparisons of change (Wilcoxon rank sum). Questions 18 and 19 on the FMPI were analyzed separately because these were visual analog scale questions.
A success‐failure analysis also was performed on the CSOM data, using an increase in CSOM score of ≥2, with no decrease in score for any individual activity, as the criteria for success.6, 7 Success‐failure analysis was performed using a Pearson chi‐square approach. Effect size (Cohen's d for treatment over placebo) was calculated using the change in score from baseline for treatment and placebo groups, and standard deviation for the change in score according to the following equation:
For the owners' ability to identify whether anti‐NGF had been administered, success was defined as correctly choosing the treatment given, and data were analyzed using a Pearson chi‐square approach.
Any allergic responses and AEs were described. Paired t‐tests were used to compare day 0 laboratory results with end of study laboratory results (day 77) for the pooled treatment group and the placebo group and also to compare pooled treatment group and placebo group at day 0 and day 77. The Wilcoxon signed‐rank test was used to compare day 0 and end of study results for laboratory variables lacking a normal distribution (using a Kolmogorov‐Smirnov test for normality).
Results
Recruitment and prescreening occurred between March 31, 2014, and April 23, 2015 (approximately 56 weeks). Successful recruitment to the study occurred at a rate of 0.61 cats/week, with a screening rate of 0.86 cats/week. Inquiries were received and 114 potential candidates contacted either through e‐mail or phone. After the initial telephone contact with owners, 66 cats were deemed ineligible for screening for reasons outlined in Table 2.
Table 2.
Reasons for cats being deemed ineligible for screening following the initial contact with the owner
| Number of Cats | Reason for not Screening |
|---|---|
| 7 | Exclusionary health issues |
| 27 | No response to inquiry |
| 5 | Insufficient impairment |
| 4 | Inappropriate temperament for study |
| 1 | Cat allowed outdoors |
| 11 | Owner schedule/time constraints |
| 2 | Owner health |
| 3 | Cat unable to wear collar |
| 4 | Owner not interested in participating |
| 2 | Cat on exclusionary medications |
A total of 48 cats was screened, and 14 were deemed unsuitable for the study because of the reasons outlined in Table 3.
Table 3.
Reasons for screening failures in the cats screened at day 0
| Number of Cats | Reason for Screening Failure |
|---|---|
| 4 | Insufficient radiographic evidence of DJD |
| 3 | Mass/neoplasia detected on radiographs |
| 2 | Cardiac disease |
| 1 | Lab work abnormalities suggesting systemic disease |
| 4 | Other health issues |
There were no differences between the cats treated with NV‐02 or placebo for age (P = .63), sex (P =.255), body weight (P = .356), or body condition score (P = .228). These results are presented in Table 4. Eleven cats received placebo, and 23 received NV‐02. Of the 23 cats that received NV‐02, 11 cats received 0.4 mg/kg and 12 cats received 0.8 mg/kg.
Table 4.
Demographic summary of the cats enrolled in the study
| NV‐02 (n = 23) | Placebo (n = 11) | P‐Value for Comparison of Treatment Groups | |
|---|---|---|---|
| Age, years, mean (SD) | 12.2 (3.1) | 12.6 (1.94) | P = .63 (t‐test) |
| Sex | 12 FS; 11 MC | 8 FS; 3 MC | P = .255 (Pearson chi‐square test) |
| Weight, kg; median (SD) | 6.10 (1.92) | 5.55 (1.38) | P = .356 (t‐test) |
| BCS; median (range) | 7 (4–9) | 6 (4–9) | P = .228 (Wilcoxon rank sum test) |
BCS, body condition score; MC, male castrated; FS, female spayed.
No cats were removed from the study before day 77. All cats enrolled were included in the safety analysis. The flow of participants through the study is illustrated in Figure 1. One cat (in the 0.4 mg/kg NV‐02 group) was determined to have severe renal dysfunction at day 77 (see AEs), and the data for this cat were removed from the efficacy analysis.
Figure 1.
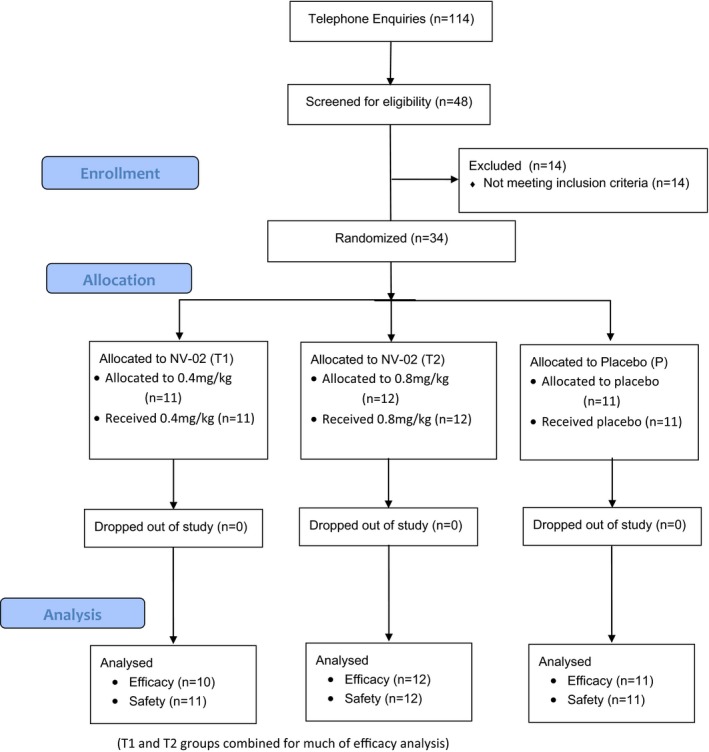
Diagram illustrating the flow of cases through the study.
Accelerometry (activity)
There was no statistical difference between activity in week 1 and week 2 (Wilcoxon signed‐rank paired test, P = .768), and thus, the combination of week 1 and week 2 (Wk1/2) data was used as the baseline period to give the maximum amount of baseline data.
No significant differences between changes in activity in the T1 and T2 groups were found, so T1 and T2 were pooled to examine the difference between treatment and placebo for each week of the study. Using the mean of Wk1/2 activity as the baseline and the change in activity expressed as a percentage of Wk1/2 activity, there was a significant Week × Week effect (P = .0096), a significant effect of treatment (P = .0258), and a significant Treatment × Week × Week effect (P = .017). Significant treatment effects (increased activity in the treatment group compared with placebo) were found for week 4 (P = .035), week 5 (P = .007), week 6 (P = .006), week 7 (P = .007), and week 8 (P = .018). The percentage change in activity over time for each of the groups is illustrated in Figure 2, and the mean percentage changes in activity for each group and for each week are tabulated in Table 5.
Figure 2.
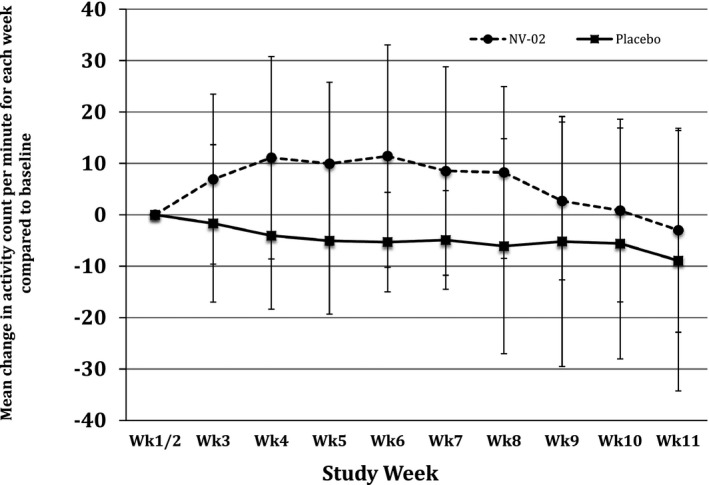
Plot of percentage change from baseline (average of weeks 1 and 2 activity, before the antibody or placebo being administered) in mean weekly activity counts (originally expressed as average activity count per minute over the week) by group (treatment/placebo), for each week of study.
Table 5.
Mean (± SD) percentage change from baseline for mean activity counts for each week of the study, for each group and the combined treatment group. Baseline activity was defined as the mean activity count per minute over the first 2 weeks of the study for each cat, before treatment
| Weeks of study | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| Weeks after treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Placebo | −1.7 (15.3) | −4.0 (14.3) | −5.0 (14.2) | −5.3 (9.7) | −4.9 (9.6) | −6.1 (20.9) | −5.2 (24.3) | −5.6 (22.5) | 8.9 (25.3) |
| T1 (NV‐02 0.4 mg/kg) | 11.5 (21.1) | 15.4 (24.7) | 14.9 (18.1) | 15.3 (22.1) | 5.1 (26.9) | 7.8 (18.4) | −0.6 (17.4) | 4.5 (19.2) | 2.8 (20.2) |
| T2 (NV‐02 0.8 mg/kg) | 3.1 (11.1) | 7.5 (14.4) | 5.8 (13.0) | 8.2 (21.6) | 11.4 (13.2) | 8.6 (16.0) | 5.4 (13.6) | −2.2 (16.7) | 3.1 (20.4) |
| T1 and T2 combined | 6.9 (16.5) | 11.1 (19.7) | 9.9 (15.9) | 11.4 (21.6) | 8.5 (20.3) | 8.2 (16.7) | 2.7 (15.3) | 0.8 (17.8) | −3.0 (19.8) |
SD, standard deviation; “Weeks after treatment,” the number of weeks following the administration of NV‐02 or placebo.
Owner Assessments
After analysis of the activity data, the treatment groups were combined for the analysis of the subjective data. The CSOM scores improved (increased) significantly within both the combined treatment group and the placebo group at each assessment time point (compared with baseline, day 14), except the day 77 time point for the placebo group (P = .051; Table 6). At day 35 (3 weeks after the administration of NV‐02 or placebo), there was a significant difference between the groups for the change in CSOM scores, with the treatment group improving significantly more than the placebo group (P = .035). There were no significant differences between the groups in terms of degree of improvement at day 56 or day 77.
Table 6.
Summary of medians (range) and statistical comparisons for changes in CSOM scores (from day 14) at day 35 (3 weeks following treatment), day 56 (6 weeks after treatment) and day 77 (9 weeks following treatment). Larger values indicate greater improvement
| Within‐Group Change (Wilcoxon Signed Rank) | Within‐Group Change (Wilcoxon Signed Rank) | Within‐Group Change (Wilcoxon Signed Rank) | ||||
|---|---|---|---|---|---|---|
| Assessment time | Day 35 | Day 56 | Day 77 | |||
| T1 and T2 combined | 5.5 (−2 to +10) | <0.0001 | 5 (0 to +11) | <0.0001 | 2.5 (−1 to +9) | <0.0001 |
| Placebo | 2 (−2 to +7) | 0.023 | 4 (−7 to +9) | 0.022 | 2 (−4 to +9) | 0.051 |
| Treatment‐placebo comparison (Wilcoxon rank sum) | 0.035 | 0.466 | 0.673 |
At day 35 (3 weeks after treatment), the improvement in the placebo group equated to a 22% decrease in disability/pain and the improvement in the treatment group equated to a 55% decrease in disability/pain. The effect size (95% CI) at day 35 for treatment over placebo was 0.74 (−0.02 to 1.5).
Using a cutoff of 2 units improvement in the CSOM scale, there were no significant differences between the groups with regard to success/failure, but more successes were observed in the treatment groups compared with the placebo group (Table 7). Using these criteria for success/failure, the caregiver placebo response over the first 3 weeks was 60%.
Table 7.
Success‐Failure summary for CSOM scores in the placebo and treatment groups
| D35 | D56 | D77 | |
|---|---|---|---|
| T1 (NV‐02 0.4 mg/kg) | 90% | 90% | 60% |
| T2 (NV‐02 0.8 mg/kg) | 75% | 92% | 75% |
| T1 and T2 combined | 82% | 91% | 68% |
| Placebo | 56% | 64% | 64% |
| Combined treatment‐placebo comparison (Pearson chi‐square) | 0.097 | 0.056 | 0.794 |
The %FMPIposs scores improved (increased) significantly within both the combined treatment group and the placebo group at each assessment time point (compared to baseline, day 14; Table 8). No significant differences were observed between the groups in terms of degree of improvement at any time point.
Table 8.
Summary of medians (range) and statistical comparisons for change in percent possible FMPI scores (from day 14 and based on questions 1–17) at day 35 (3 weeks following treatment), day 56 (6 weeks following treatment), and day 77 (9 weeks following treatment). Larger values indicate greater improvement
| Within‐Group Change (Wilcoxon Signed Rank) | Within‐Group Change (Wilcoxon Signed Rank) | Within‐Group Change (Wilcoxon Signed Rank) | ||||
|---|---|---|---|---|---|---|
| Assessment Time | Day 35 | Day 56 | Day 77 | |||
| T1 and T2 combined | 16 (1.3 to 36) | <0.0001 | 16 (2.6 to 34.5) | <0.0001 | 14.5 (−1.2 to +35.3) | <0.0001 |
| Placebo | 7.4 (0 to 26.6) | 0.002 | 10.6 (−9.4 to +27.8) | 0.010 | 14.4 (2.4 to 35.7) | 0.001 |
| Tx‐Placebo comparison (Wilcoxon rank sum) | 0.061 | 0.127 | 0.456 |
The FMPI scores for questions 18 and 19 improved significantly within both the combined treatment group and the placebo group at each assessment time point (compared with baseline, day 14). Greater improvement occurred in the treatment group compared with the placebo group, and significantly so, at day 35 for question 18 (“pain over the last 3 weeks”; P = .024) but not question 19 (“pain today”; P = .05).
Owners were able to identify cats that had received the anti‐NGF analgesic treatment more successfully than they were able to identify cats that had received placebo, with 83% of owners able to identify correctly that their cat had received NV‐02 versus only 45% being able to identify correctly that their cat had received placebo (P = .013). The expected result, due to a complete guess, would be 50% correct.
Safety and Adverse Events
No cats showed any signs of an allergic response to the injection of NV‐02.
Adverse events were reported or discovered in 6 cats. One cat (NV‐02, 0.8 mg/kg) started scratching at the collar, and this collar was removed for 4 days and replaced with a different collar. Triple antibiotic ointment and a small amount of hydrocortisone were applied on 2 occasions during the time the collar was not being worn. Two cats (NV‐02, 0.8 mg/kg and placebo) had similar increases in serum creatinine concentration detected on day 77 (2.0 mg/dL, up from 1.7 mg/dL; and 2.1 mg/dL up from 1.5 mg/dL, respectively). Repeated laboratory testing 2–3 months later showed stable but increased serum creatinine concentrations. The owner of 1 cat (NV‐02, 0.4 mg/kg) reported that the cat's preexisting mild asthma appeared to worsen with more frequent “squeaking.” An albuterol inhaler was prescribed and the cat appeared to improve and remained on the study. The preexisting asthma was not definitively diagnosed before the study. One cat (NV‐02, 0.8 mg/kg) vomited after the evening meal for 3 days after the injection of NV‐02. Given our experiences in several similar studies of therapeutics for DJD‐associated pain in cats, we did not consider any of these to be related to NV‐02 administration.
A SAE was reported in 1 cat that received NV‐02, 0.4 mg/kg, but the event was deemed not be associated with treatment. The cat was staged as IRIS stage 2 chronic kidney disease before the study. Half‐way through the study, the owner reported that a preexisting foot deformity and thickened area was painful, but evaluation (examination, fine needle aspiration of a thickened area, and radiography) did not identify a cause or show any change from the start of the study. The deformity or mass had been present as the cat was a kitten, was of unknown cause, and had not changed over time. On the day 77 visit, the owner reported that the cat had been hiding more and showing decreased appetite progressively over the past 10 days. The cat had lost 0.72 kg body weight since the start of the study and showed azotemia, hyperglobulinemia, and leukocytosis with a left shift on the day 77 laboratory results. The owner elected euthanasia rather than treatment. Full necropsy and a thorough investigation of the kidneys did not identify any probable link to NV‐02. Data were included for safety evaluation, but omitted from efficacy analysis. Necropsy identified multifocal, mild, chronic, glomerulopathy and lymphoplasmacytic interstitial nephritis, and focal, chronic, renal cortical atrophy and fibrosis (infarct), chronic lymphohistiocytic cholecystitis, and choledochitis considered to be due to congenital malformation of the biliary tree; a colonic mast cell tumor; a focal pulmonary adenocarcinoma; a focal, well‐differentiated fibrosarcoma on the hindlimb (known preexisting hindlimb mass that had not changed historically); moderate, multifocal, chronic, lymphohistiocytic pancreatitis with dissecting fibrosis and acinar loss; and multifocal, lymphoplasmacytic duodenitis. Electron microscopy of the renal tissues did not identify any evidence of immune complex glomerulonephritis. No association was found between treatment and the AE.
At day 0, there were no differences between the treatment and placebo groups for any blood chemistry, hematology, or urinalysis variables.
At day 77, total protein concentration was significantly higher in the treatment group (mean ± SD, 7.6 ± 2.2 g/dL versus 7.1 ± 1.7 g/dL; P = .024; reference range, 6.4–8.2 g/dL). Serum globulin concentration was significantly higher in the treatment group (mean ±SD, 4.2 ± 2.6 g/dL versus 3.7 ± 1.98 g/dL; reference range, 2.9–4.8 g/dL).
When change within groups was evaluated, there were no significant changes within the treatment or placebo groups over time for any variable. In total, 3 cats had serum creatinine concentrations that increased from within to above the reference range (detailed above).
Discussion
The results of our study showed a clear positive treatment effect associated with NV‐02, a felinized anti‐NGF antibody, in cats with DJD‐associated pain and mobility impairment. The beneficial effect was seen for objectively measured activity, and also, despite a large caregiver placebo effect, for owner‐assessed subjective measures.
Objectively measured activity showed an increase in activity between 2 and 6 weeks after treatment with NV‐02. In a recent study of meloxicam (blinded, placebo‐controlled; 2‐week baseline, 3‐week treatment)6 conducted by our group, the mean increase in activity compared to baseline period was 5.97% over the first 3‐week period of daily treatment with meloxicam (0.035 mg/kg daily) compared with 2.65% increase over the same time period for cats treated with placebo. The percentage increase in activity from baseline in the treatment group found in the present study (9.3% over the first 3 weeks) compares very favorably with the 0.035 mg/kg meloxicam study.
The meloxicam study was a full cross‐over study, and if both periods of meloxicam treatment are included, the mean increase in activity above baseline (where baseline was the period just before each treatment) for a 3‐week treatment period was 9.45%, and the difference between treatment and placebo phases for changes in activity was 10.62%, favoring meloxicam. As noted above, in the current NV‐02 study, the mean increase in activity from baseline in the treatment group was 9.3% and the difference between placebo‐treated and NV‐02‐treated cats over the first 3 weeks after treatment was 12.9%. This comparison shows that a single injection of NV‐02 produces increases in activity in cats that is the same or greater than the increase in activity produced by daily administration of 0.035 mg/kg of meloxicam. There are no other placebo‐controlled studies measuring activity in client‐owned cats with which to compare the current study.
Despite a high caregiver placebo response (60% over the first 3 weeks after treatment, using success/failure criteria), the owner‐completed client‐specific subjective assessment system (CSOM) clearly detected a treatment effect, with a significant difference between total scores 3 weeks after treatment and also a significant difference for change in CSOM scores over the first 3 weeks after treatment. The effect size (CI) based on CSOM was 0.74 (−0.02 to 1.50), which compares with −0.35 (−0.98 to 0.28) seen with 0.035 mg/kg of meloxicam.6 The FMPI scores showed similar trends, but the delineation between placebo and treatment groups was less obvious. The FMPI has undergone evaluation in several studies and was found to be readable and reliable,12, 16 but it did not show responsiveness in 1 study using relatively small numbers of cats.17 The FMPI did, however, show some responsiveness and criterion validity (using activity monitors) in a larger study using meloxicam.6, 7 The CSOM has detected treatment effects over placebo, but only in our most recent, larger NSAID study using meloxicam,6, 7 and a small pilot study using highly impaired cats.8 More work is needed to develop owner assessment outcomes or study designs that can robustly distinguish treatment from placebo and show criterion validity. One of the frustrations in the development of owner‐completed assessment tools is that it is not known if the analgesics (NSAIDs thus far) being used in the development of the subjective tools actually work. We believe that the efficacy seen in our current study was such that it may be the best currently available treatment with which to refine the owner assessment tools; however, the effect of differences in administration routes (oral versus injectable) and frequency of administration (once versus daily) must be taken into account.
In our study, the duration of effect appeared to be about 6 weeks, based on objectively measured activity. This is similar to the duration of efficacy of 0.2 mg/kg IV in dogs18 of at least 4 weeks, although in the study with dogs, assessments were not performed beyond 4 weeks. In studies of humans, duration of efficacy appears to be somewhat dose dependent,10 with 100 and 200 μg/kg showing efficacy for up to 8 weeks, although the efficacy at 8 weeks appeared to have decreased compared with that at 4 weeks.19 Reported duration of efficacy depends somewhat on the design of the study and at what intervals outcome measures are collected. We designed this study such that measures of activity would be collected beyond the expected duration of action, giving us the ability to report on the duration of efficacy. We believe that the potential impact in veterinary medicine of an injection lasting approximately 6 weeks for the control of long‐term pain in the cat is very positive and clinically relevant.
Toxicity is an obvious concern, especially for novel therapeutics. In studies of humans treated with anti‐NGF antibodies, reported AEs appear to be generally mild, but, as with efficacy, dose related.10, 20 Most AEs that seem to be related to NGF antibodies appear to be related to transient changes in sensation.20 In our study with dogs, no evidence of neurologic AEs was seen17 although the study was not powered to evaluate AEs. Subsequently, we have found no effect on sensation in dogs using quantitative sensory threshold (QST) testing (unpublished observations). In this study, no neurologic AEs were seen. In the 1 cat that suffered a decrease in renal function, we found no evidence of any relationship with the monoclonal antibody treatment, given the current body of knowledge. There are no reports of renal toxicity related to anti‐NGF treatment in any species, but this will obviously remain a concern in cats given the high prevalence of chronic kidney disease in cats.3 Larger studies, including repeated administrations of treatment, are required to fully assess the safety of NV‐02 in cats. Since the preparation of this report, USAN have adopted the non‐proprietary name frunevetmab for anti‐NGF mAB NV‐02.
Conclusions
Positive treatment effects in cats with DJD‐associated pain and mobility impairment were seen with the administration of a single SC injection of NV‐02, a felinized anti‐NGF antibody. These positive effects lasted up to 6 weeks, measured by objective activity evaluation. NV‐02 appears to offer great promise as a treatment for long‐term pain alleviation in cats with chronic DJD‐associated pain. Further clinical studies are warranted.
Acknowledgments
The authors acknowledge Lyndy Harden of the NCSU‐CVM Clinical Studies Core for performing quality control on the data. The authors thank Dr Rachel E. Cianciolo, International Veterinary Renal Pathology Service, for the electron microscopy evaluation of the kidney in one case.
Conflict of Interest Declaration: ME Gruen received funding support from the National Institutes of Health Ruth L. Kirschstein National Research Service Award (T32OD011130). BDX Lascelles is a paid consultant for Nexvet. DP Gearing is an employee of Nexvet. The study was supported by Nexvet Biopharma.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work was conducted at North Carolina State University, Comparative Pain Research Program, College of Veterinary Medicine.
Footnote
Actical®, Philips Respironics, Bend, Oregon, USA
References
- 1. Slingerland LI, Hazewinkel HAW, Meij BP, et al. Cross‐sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet J 2011;187:304–309. [DOI] [PubMed] [Google Scholar]
- 2. Lascelles BDX, Henry JB, Brown J, et al. Cross‐sectional study of the prevalence of radiographic degenerative joint disease in domesticated cats. Vet Surg 2010;39:535–544. [DOI] [PubMed] [Google Scholar]
- 3. Marino CL, Lascelles BDX, Vaden SL, et al. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J Feline Med Surg 2013;16:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gunew M, Menrath V, Marshall R. Long‐term safety, efficacy and palatability of oral meloxicam at 0.01–0.03 mg/kg for treatment of osteoarthritic pain in cats. J Feline Med Surg 2008;10:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gowan RA, Lingard AE, Johnston L, et al. Retrospective case–control study of the effects of long‐term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011;13:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gruen ME, Griffith EH, Thomson AE, et al. Criterion validation testing of clinical metrology instruments for measuring degenerative joint disease associated mobility impairment in cats. PLoS ONE 2015;10:e0131839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gruen ME, Griffith E, Thomson A, et al. Detection of clinically relevant pain relief in cats with degenerative joint disease associated pain. J Vet Intern Med 2014;28:346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lascelles BDX, Hansen BD, Roe S, et al. Evaluation of client‐specific outcome measures and activity monitoring to measure pain relief in cats with osteoarthritis. J Vet Intern Med 2007;21:410–416. [DOI] [PubMed] [Google Scholar]
- 9. Seidel MF, Wise BL, Lane NE. Nerve growth factor: An update on the science and therapy. Osteoarthritis Cartilage 2013;21:1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schnitzer TJ, Marks JA. A systematic review of the efficacy and general safety of antibodies to NGF in the treatment of OA of the hip or knee. Osteoarthritis Cartilage 2015;23(Suppl 1):S8–S17. [DOI] [PubMed] [Google Scholar]
- 11. Gearing DP, Huebner M, Virtue ER, et al. In vitro and in vivo characterization of a fully felinized therapeutic anti‐nerve growth factor monoclonal antibody for the treatment of pain in cats J Vet Intern Med 2016. DOI: 10.1111/jvim.13985 [E‐pub ahead of Print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gruen ME, Jiamachello KN, Thomson A, Lascelles BDX. Clinical trials involving cats: What factors affect owner participation? J Feline Med Surg 2014;16:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zamprogno H, Hansen BD, Bondell HD, et al. Item generation and design testing of a questionnaire to assess degenerative joint disease‐associated pain in cats. Am J Vet Res 2010;71:1417–1424. [DOI] [PubMed] [Google Scholar]
- 14. Lascelles BDX, Dong Y‐H, Marcellin‐Little DJ, et al. Relationship of orthopedic examination, goniometric measurements, and radiographic signs of degenerative joint disease in cats. BMC Vet Res 2012;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lascelles BDX, Hansen BD, Thomson A, et al. Evaluation of a digitally integrated accelerometer‐based activity monitor for the measurement of activity in cats. Vet Anaesth Analg 2008;35: 173–183. [DOI] [PubMed] [Google Scholar]
- 16. Benito J, DePuy V, Hardie E, et al. Reliability and discriminatory testing of a client‐based metrology instrument, feline musculoskeletal pain index (FMPI) for the evaluation of degenerative joint disease‐associated pain in cats. Vet J 2013;196:368–373. [DOI] [PubMed] [Google Scholar]
- 17. Benito J, Hansen B, DePuy V, et al. Feline musculoskeletal pain index: Responsiveness and testing of criterion validity. J Vet Intern Med 2013;27:474–482. [DOI] [PubMed] [Google Scholar]
- 18. Lascelles BDX, Knazovicky D, Case B, et al. A canine‐specific anti‐nerve growth factor antibody alleviates pain and improves mobility and function in dogs with degenerative joint disease‐associated pain. BMC Vet Res 2015;11:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lane NE, Schnitzer TJ, Birbara CA, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med 2010;363:1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bannwarth B, Kostine M. Targeting Nerve Growth Factor (NGF) for pain management: What does the future hold for NGF antagonists? Drugs 2014;74:619–626. [DOI] [PubMed] [Google Scholar]


