Abstract
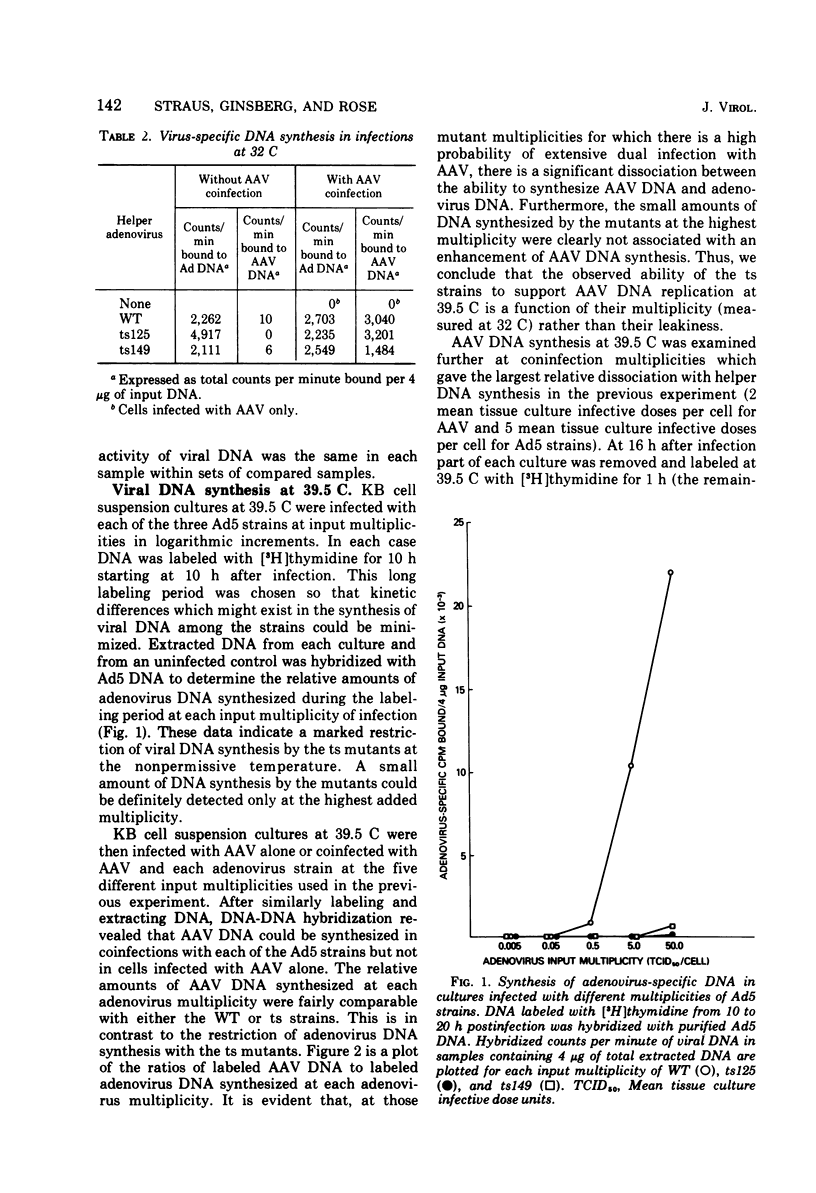
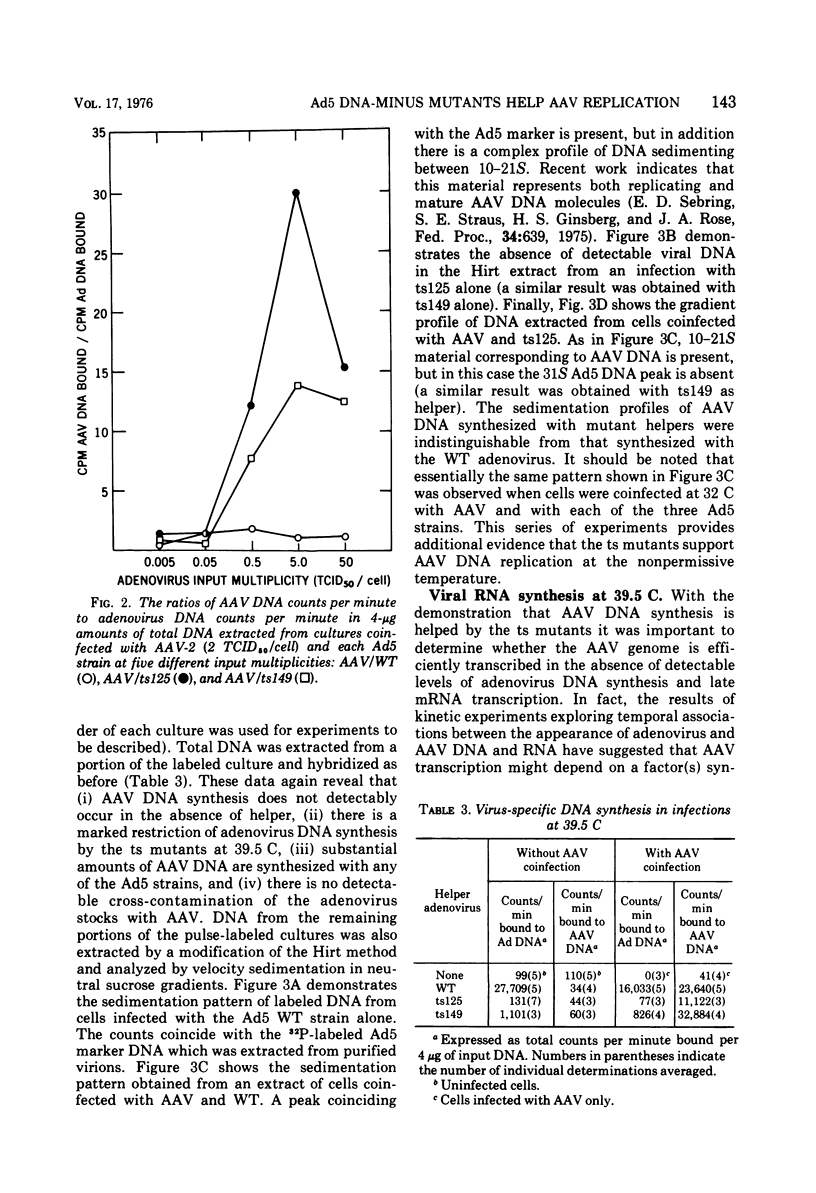
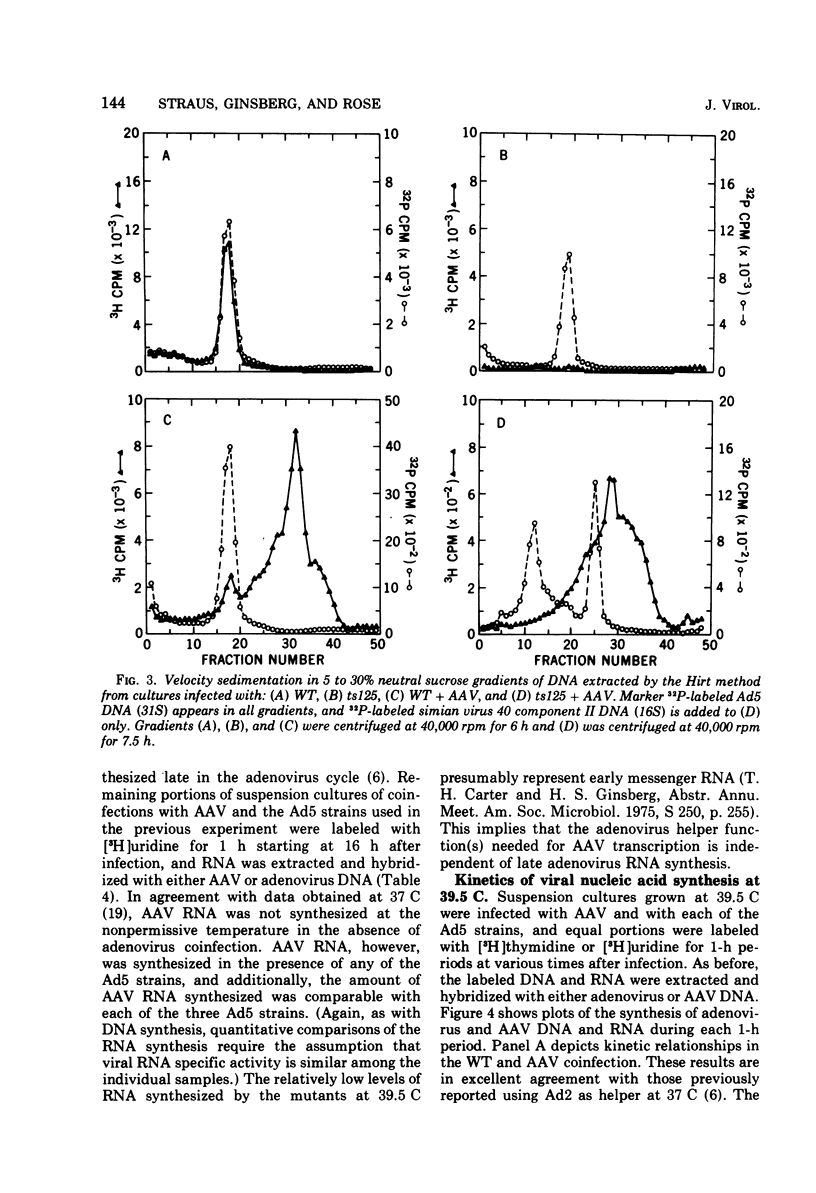
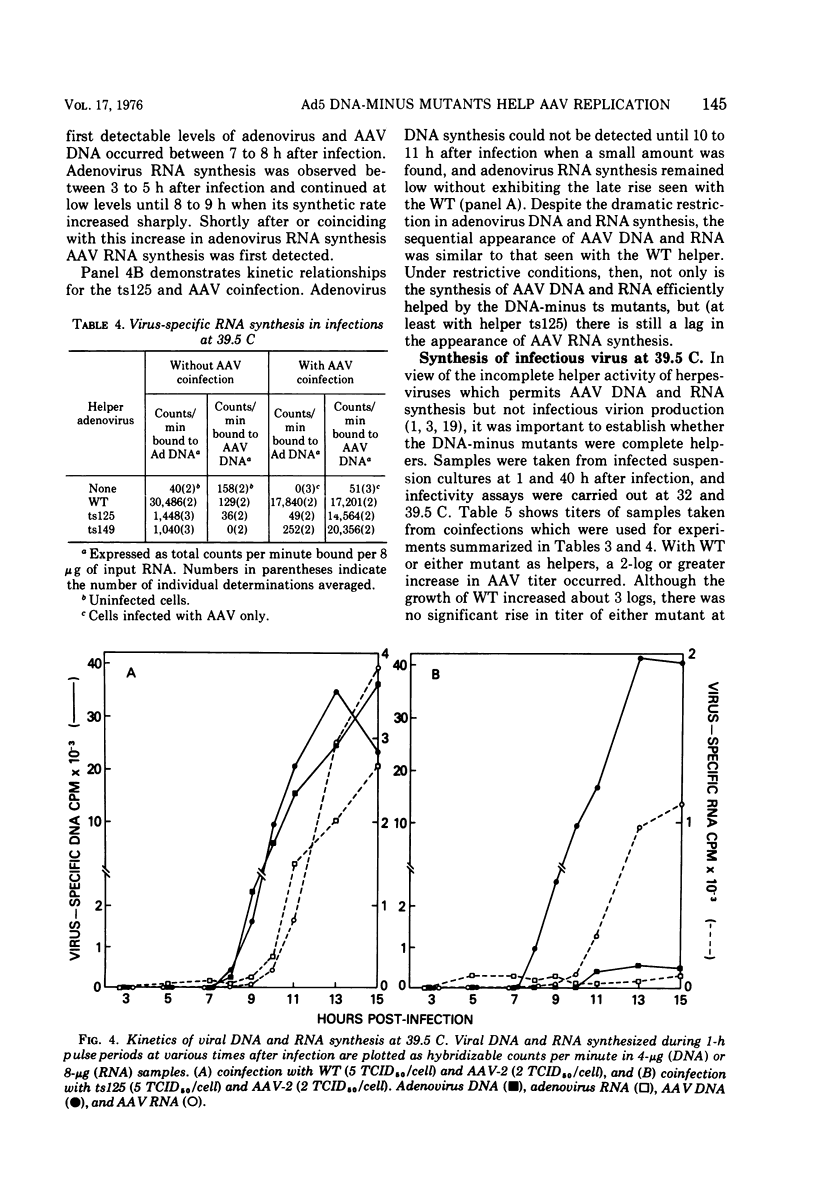
Efficient potentiation of adenovirus-associated viruses (AAV) replication occurs in coinfections with either of two DNA-minus temperature-sensitive mutants of adenovirus type 5 (Ad5), ts125 and ts149. The helper activity of these mutants does not result from leakiness. At the nonpermissive temperature (39.5 C) there was little or no detectable adenovirus DNA synthesis, and only a relatively low level of adenovirus transcription was observed. However, the synthesis of AAV DNA and RNA and the yield of infectious AAV were comparable in amounts to those found when wild-type Ad5 was the helper. Furthermore, an apparent lag in the initiation of AAV transcription after the onset of AAV DNA synthesis was seen in coinfections with both wild type or ts125. These findings strongly suggest that the adenovirus factor(s) required for AAV multiplication is produced early in the adenovirus cycle. In addition, it is likely that if AAV multiplication is linked to adenovirus DNA replication, this requirement does not include all factors directly needed for adenovirus DNA synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison R. W. The role of herpesviruses in adenovirus-associated virus replication in vitro. Virology. 1970 Sep;42(1):155–162. doi: 10.1016/0042-6822(70)90248-5. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., McClanahan M. S. Adenovirus-associated viruses: enhancement by human herpesviruses. Proc Soc Exp Biol Med. 1970 Sep;134(4):952–954. doi: 10.3181/00379727-134-34919. [DOI] [PubMed] [Google Scholar]
- Boucher D. W., Melnick J. L., Mayor H. D. Nonencapsidated infectious DNA of adeno-satellite virus in cells coinfected with herpesvirus. Science. 1971 Sep 24;173(4003):1243–1245. doi: 10.1126/science.173.4003.1243. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Koczot F. J., Garrison J., Rose J. A., Dolin R. Separate helper functions provided by adenovirus for adenovirus-associated virus multiplication. Nat New Biol. 1973 Jul 18;244(133):71–73. doi: 10.1038/newbio244071a0. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Adenovirus-associated virus multiplication. 8. Analysis of in vivo transcription induced by complete or partial helper viruses. J Virol. 1972 Jul;10(1):9–16. doi: 10.1128/jvi.10.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Berry K. W., Rose J. A. A unique form of terminal redundancy in adenovirus DNA molecules. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2391–2395. doi: 10.1073/pnas.69.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Suzuki E. Adeno-associated satellite virus growth supported by a temperature-sensitive mutant of human adenovirus. J Gen Virol. 1970 Dec;9(3):243–245. doi: 10.1099/0022-1317-9-3-243. [DOI] [PubMed] [Google Scholar]
- Koczot F. J., Carter B. J., Garon C. F., Rose J. A. Self-complementarity of terminal sequences within plus or minus strands of adenovirus-associated virus DNA. Proc Natl Acad Sci U S A. 1973 Jan;70(1):215–219. doi: 10.1073/pnas.70.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledinko N. Temperature-sensitive mutants of adenovirus type 12 defective in viral DNA synthesis. J Virol. 1974 Sep;14(3):457–468. doi: 10.1128/jvi.14.3.457-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm U., Doerfler W. Temperature-sensitive mutants of human adenovirus type 12. Virology. 1971 Sep;45(3):827–829. doi: 10.1016/0042-6822(71)90206-6. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Ratner J. Analysis of adeno-associated satellite virus DNA. Biochim Biophys Acta. 1973 Mar 19;299(2):189–195. doi: 10.1016/0005-2787(73)90341-9. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Koczot F., Shatkin A. J. Genetic relatedness studies with adenovirus-associated viruses. J Virol. 1968 Oct;2(10):999–1005. doi: 10.1128/jvi.2.10.999-1005.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VI. Base compostion of the deoxyribonucleic acid strand species and strand-specific in vivo transcription. J Virol. 1971 Nov;8(5):771–777. doi: 10.1128/jvi.8.5.771-777.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VII. Helper requirement for viral deoxyribonucleic acid and ribonucleic acid synthesis. J Virol. 1972 Jul;10(1):1–8. doi: 10.1128/jvi.10.1.1-8.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel W. C., Newman C., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5--serology. J Gen Virol. 1972 Dec;17(3):265–279. doi: 10.1099/0022-1317-17-3-265. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H. A temperature-sensitive mutant of adenovirus 31, defective in viral deoxyribonucleic acid replication. Virology. 1971 Feb;43(2):488–494. doi: 10.1016/0042-6822(71)90320-5. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmaar S. O., Cohen J. A. A quantitative assay for DNA-DNA hybrids using membrane filters. Biochem Biophys Res Commun. 1966 Aug 23;24(4):554–558. doi: 10.1016/0006-291x(66)90356-1. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Adenovirus-2 DNA contains an inverted terminal repetition. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3054–3057. doi: 10.1073/pnas.69.10.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]



