Abstract
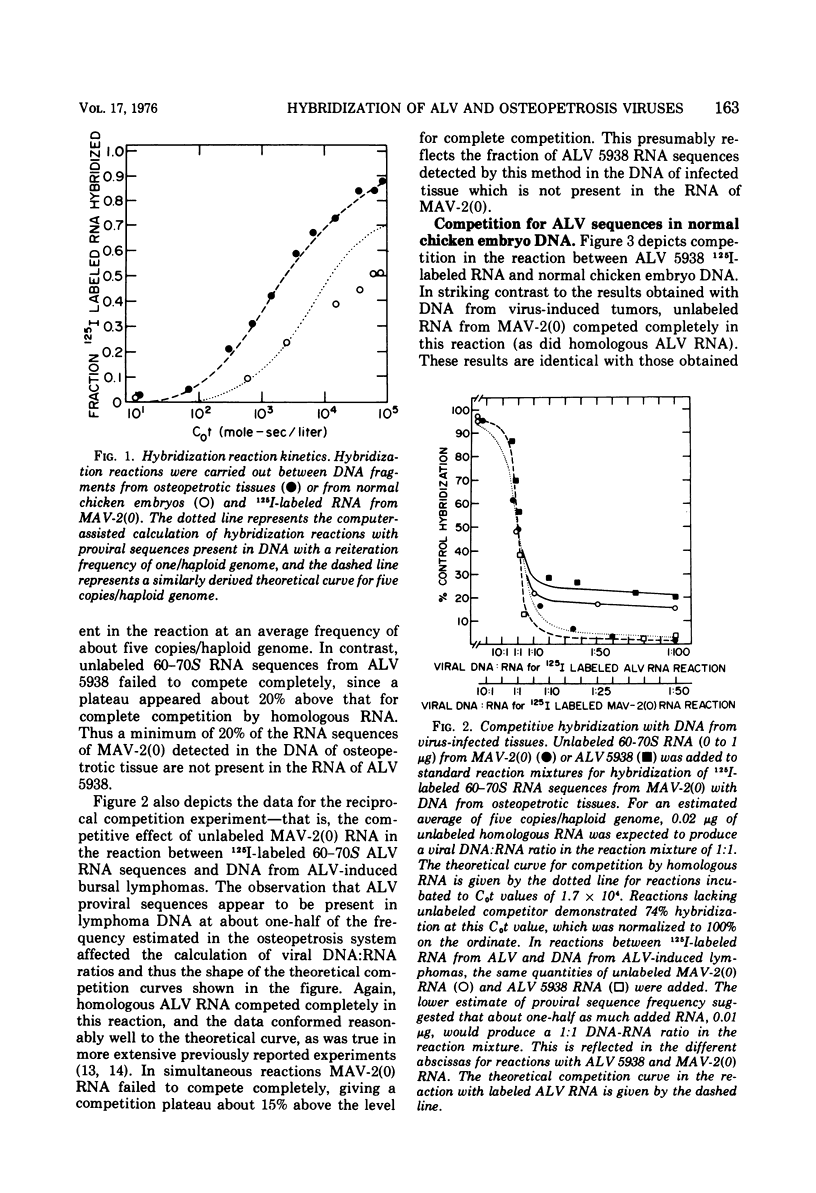
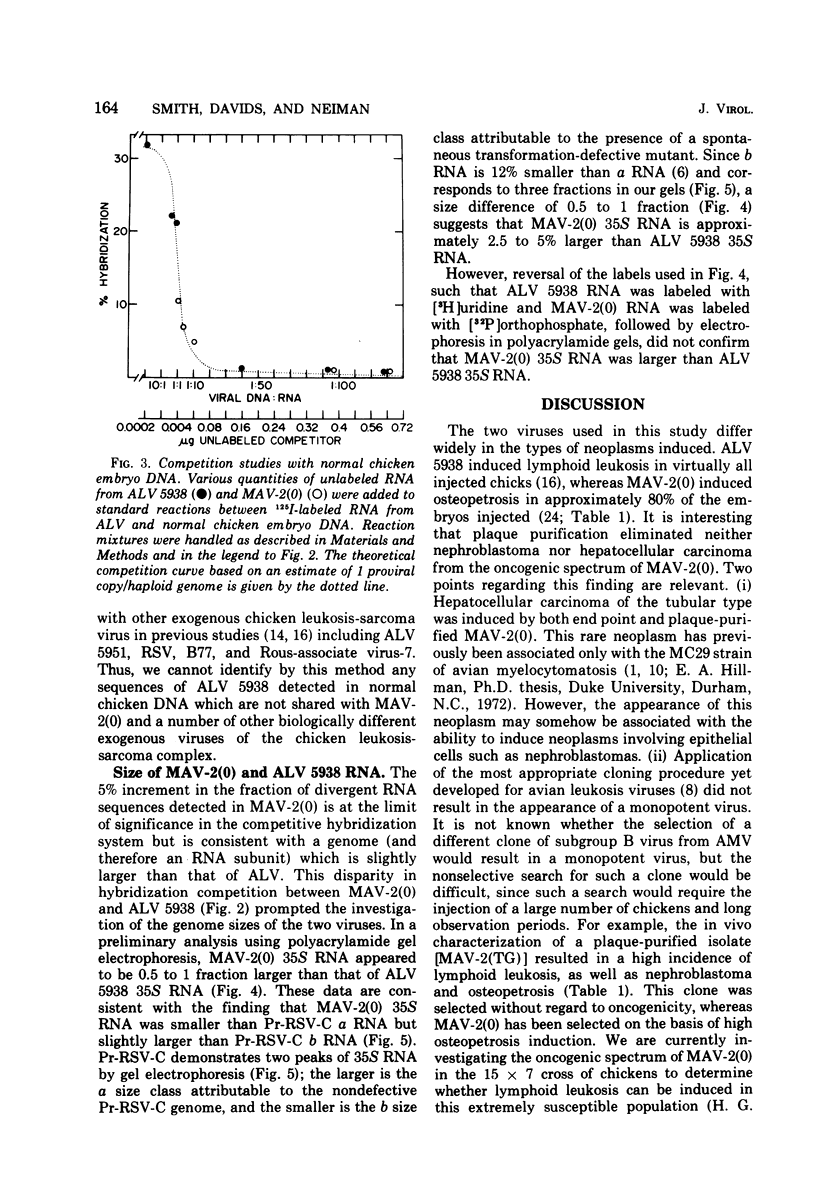
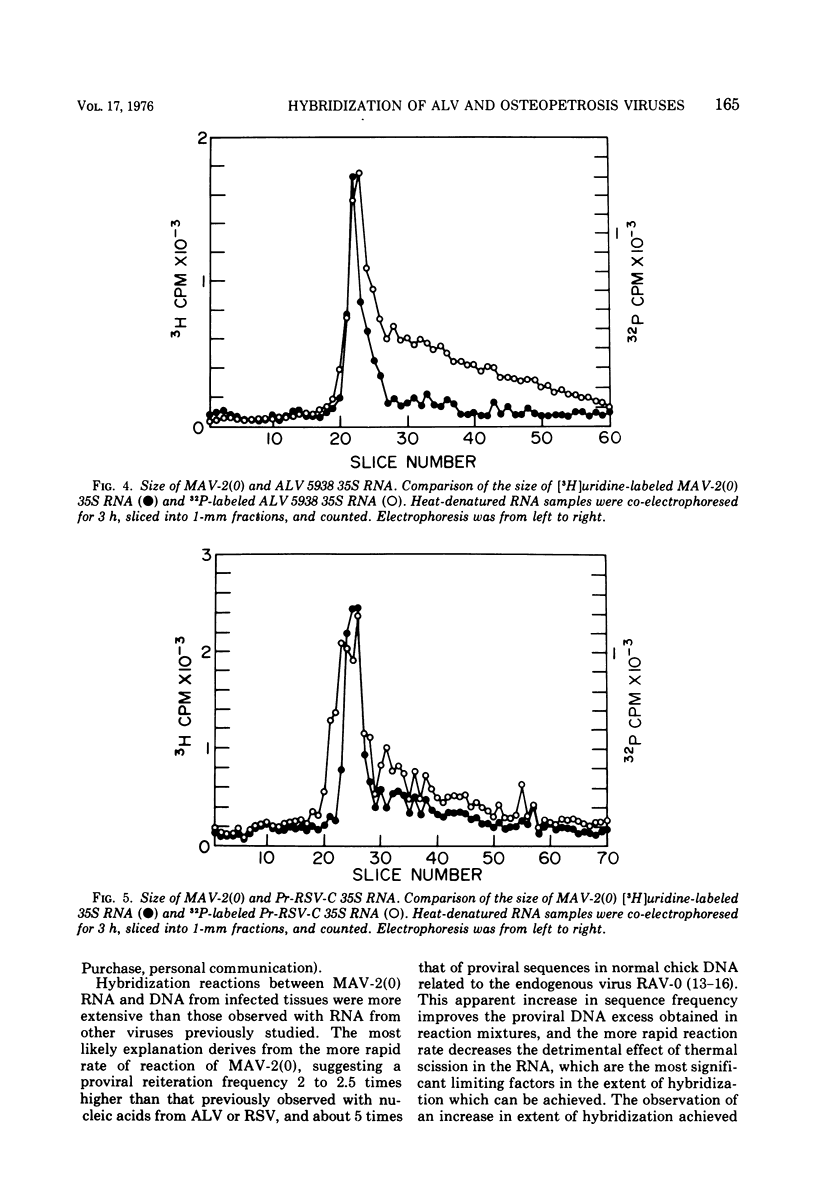
Myeloblastosis-associated virus (MAV)-2(0), a virus which was derived from avian myeloblastosis virus and induced a high incidence of osteopetrosis, was compared with avian lymphomatosis virus 5938, a recent field isolate which induced a high incidence of lymphomatosis. The following information was obtained. (i) MAV-2(0) induced osteopetrosis, nephroblastoma, and a very low incidence of hepatocellular carcinoma. No difference was seen in the oncogenic spectrum of end point and plaque-purified MAV-2(0). (ii) 125I-labeled RNA sequences from MAV-2(0) formed hybrids with DNA extracted from osteopetrotic bone at a rate suggesting five proviral copies per haploid cell genome. The extent of hybridization of MAV-2(0) RNA with DNA from osteopetrotic tissue was more extensive (87%) than was observed in reactions with DNA from uninfected chicken embryos (52%). (iii) Competition of unlabeled viral RNA in hybridization reactions between the radioactive RNA from the two viruses and their respective proviral sequences present in tumor tissues showed that 15 to 20% of the viral sequences detected in these reactions were unshared. In contrast, no differences were detected in competition analyses of RNA sequences from the two viruses detected in DNA of normal chicken cells. (iv) MAV-2(0) 35S RNA was indistinguishable in size from avian lymphomatosis virus 5938 35S RNA by polyacrylamide gel electrophoresis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURMESTER B. R., WALTER W. G., GROSS M. A., FONTES A. K. The oncogenic spectrum of two pure strains of avian leukosis. J Natl Cancer Inst. 1959 Aug;23:277–291. [PubMed] [Google Scholar]
- Beard J. W., Hillman E. A., Beard D., Lapis K., Heine U. Neoplastic response of the avian liver to host infection with strain Mc29 leukosis verus. Cancer Res. 1975 Jul;35(7):1603–1627. [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R. W., Stone M. P., Joklik W. K. Separation of single-stranded ribonucleic acids by acrylamide-agarose-urea gel electrophoresis. Anal Biochem. 1974 Jun;59(2):599–609. doi: 10.1016/0003-2697(74)90313-3. [DOI] [PubMed] [Google Scholar]
- Graf T. A plaque assay for avian RNA tumor viruses. Virology. 1972 Nov;50(2):567–578. doi: 10.1016/0042-6822(72)90408-4. [DOI] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Renaturation of bacteriophage phiX174 DNA-RNA hybrid: RNA length effect and nucleation rate constant. J Mol Biol. 1973 Jul 15;77(4):495–500. doi: 10.1016/0022-2836(73)90218-0. [DOI] [PubMed] [Google Scholar]
- Lapis K., Beard D., Beard J. W. Transplantation of hepatomas induced in the avian liver by MC29 leukosis virus. Cancer Res. 1975 Jan;35(1):132–138. [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. The interaction of oligodeoxynucleotides with denatured DNA. Biochim Biophys Acta. 1967 Nov 21;149(1):180–189. doi: 10.1016/0005-2787(67)90700-9. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of endogenous leukosis virus nucleotide sequences in the DNA of normal avian embryos by RNA-DNA hybridization. Virology. 1973 May;53(1):196–203. doi: 10.1016/0042-6822(73)90478-9. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Purchase H. G., Okazaki W. Chicken leukosis virus genome sequences in DNA from normal chick cells and virus-induced bursal lymphomas. Cell. 1975 Apr;4(4):311–319. doi: 10.1016/0092-8674(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., McMillin C., MacDonnell D. Nucleotide sequence relationships of avian RNA tumor viruses: measurement of the deletion in a transformation-defective mutant of Rous sarcoma virus. J Virol. 1974 Apr;13(4):837–846. doi: 10.1128/jvi.13.4.837-846.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P. E., Wright S. E., Purchase H. G. Studies of the interrelationship of chicken leukosis virus and host cell genomes by RNA-DNA hybridzation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):875–883. doi: 10.1101/sqb.1974.039.01.102. [DOI] [PubMed] [Google Scholar]
- Ogura H., Gelderblom H., Bauer H. Isolation of avian nephroblastoma virus from avian myeloblastosis virus by the infectious DNA technique. Intervirology. 1974;4(2):69–76. doi: 10.1159/000149845. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Pitkanen A., Rubin H. The nucleic acid of the Bryan strain of Rous sarcoma virus: purification of the virus and isolation of the nucleic acid. Proc Natl Acad Sci U S A. 1965 Jul;54(1):137–144. doi: 10.1073/pnas.54.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A., Evans R. Acquisition of new DNA sequences after infection of chicken cells with avian myeloblastosis virus. J Virol. 1974 Feb;13(2):331–339. doi: 10.1128/jvi.13.2.331-339.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E., Bernstein E. H. Production and purification of large amounts of Rous sarcoma virus. Appl Microbiol. 1973 Mar;25(3):346–353. doi: 10.1128/am.25.3.346-353.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. E. High specific infectivity avian RNA tumor viruses. Virology. 1974 Aug;60(2):543–547. doi: 10.1016/0042-6822(74)90348-1. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Moscovici C. The oncogenic effects of nontransforming viruses from avian myeloblastosis virus. Cancer Res. 1969 Jul;29(7):1356–1366. [PubMed] [Google Scholar]
- Stone M. P., Smith R. E., Joklik W. K. 35S a and b RNA subunits of avian RNA tumor virus strains cloned and passaged in chick and duck cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):859–868. doi: 10.1101/sqb.1974.039.01.100. [DOI] [PubMed] [Google Scholar]
- Tereba A., McCarthy B. J. Hybridization of 125I-labeled ribonucleic acid. Biochemistry. 1973 Nov 6;12(23):4675–4679. doi: 10.1021/bi00747a020. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. E., Neiman P. E. Base-sequence relationships between avian ribonucleic acid endogenous and sarcoma viruses assayed by competitive ribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 26;13(7):1549–1554. doi: 10.1021/bi00704a035. [DOI] [PubMed] [Google Scholar]



