Abstract
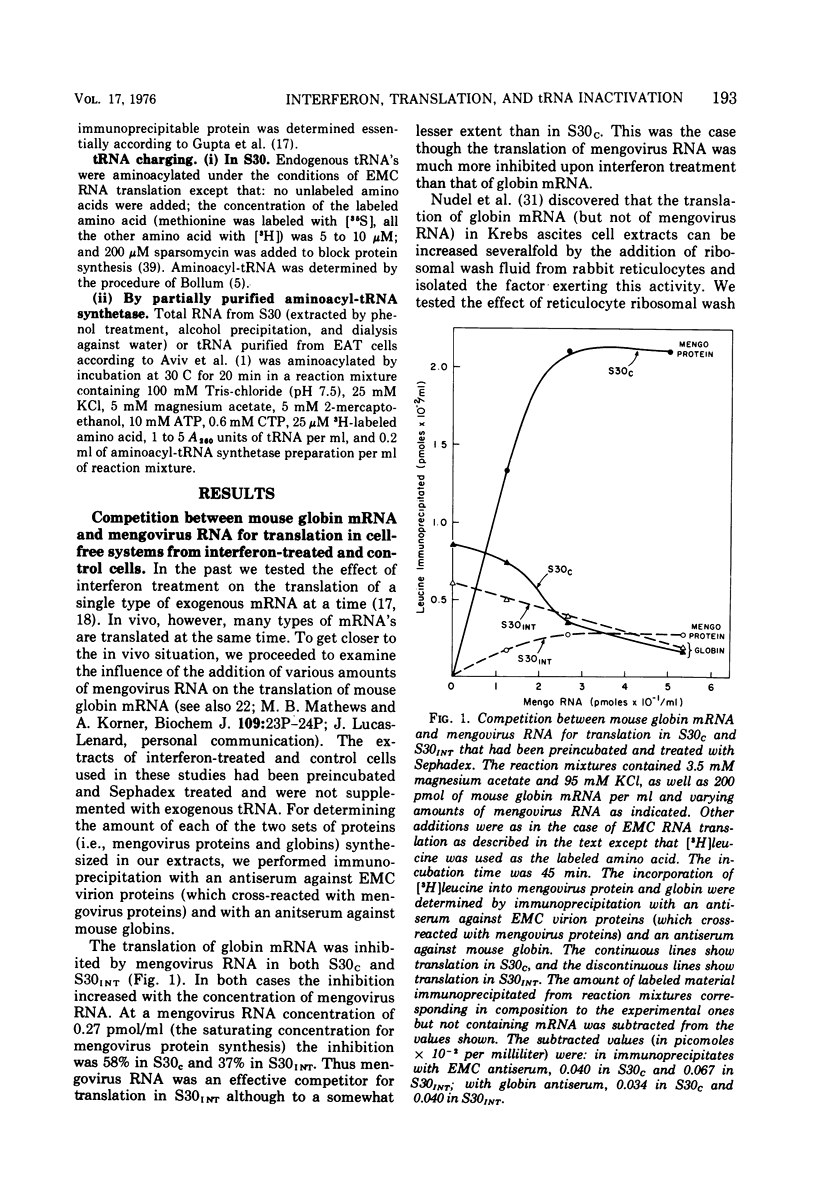
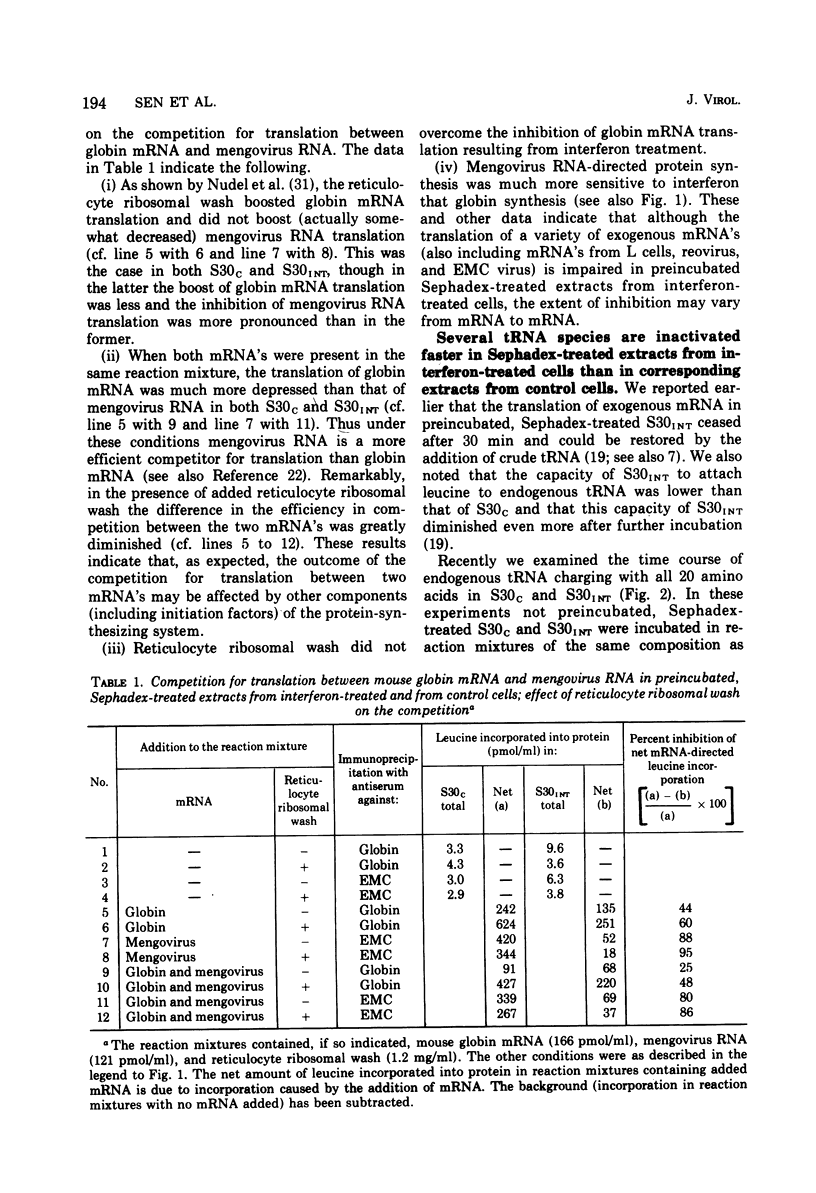
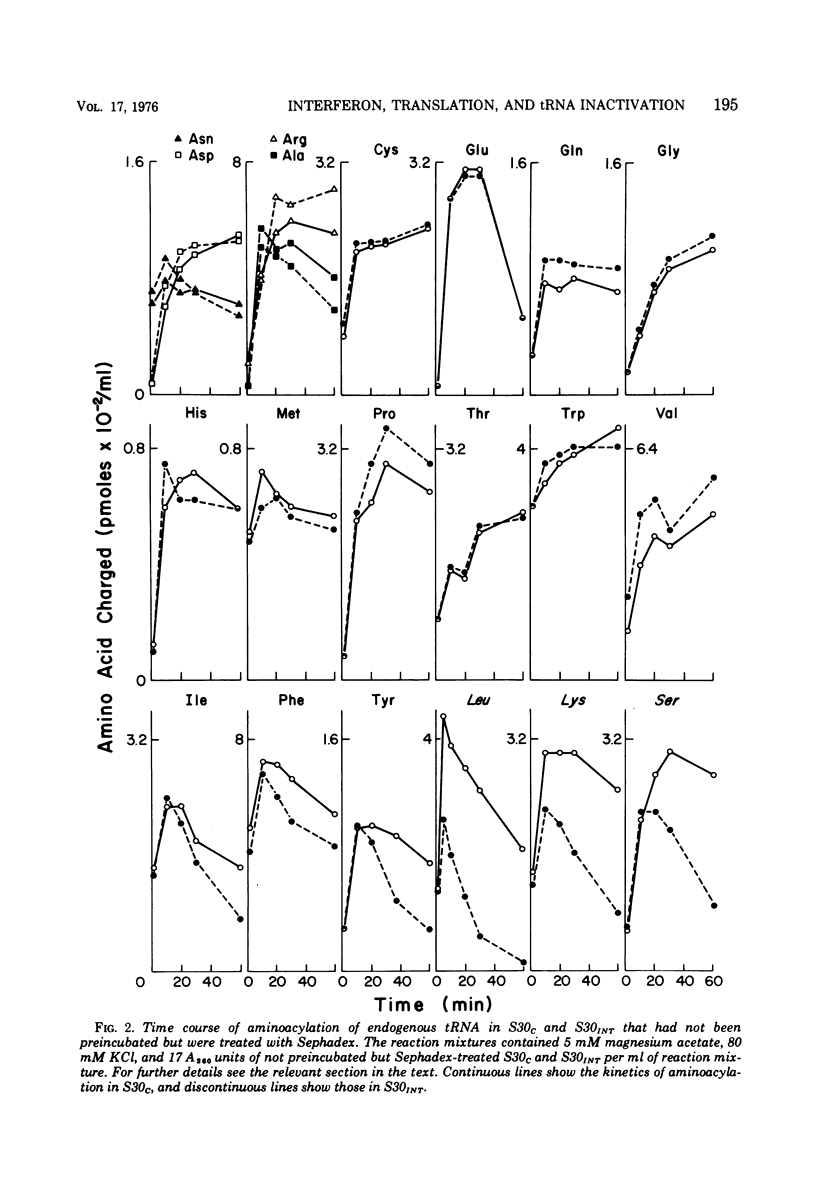
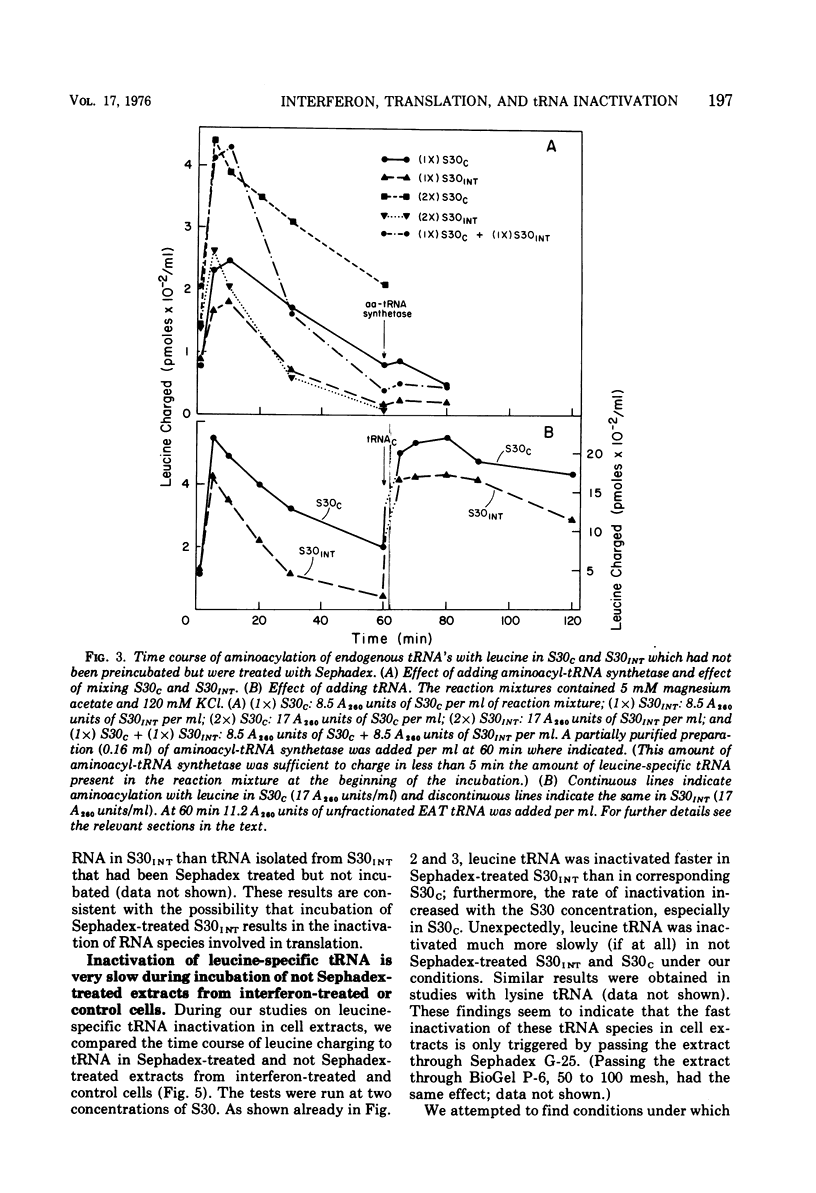
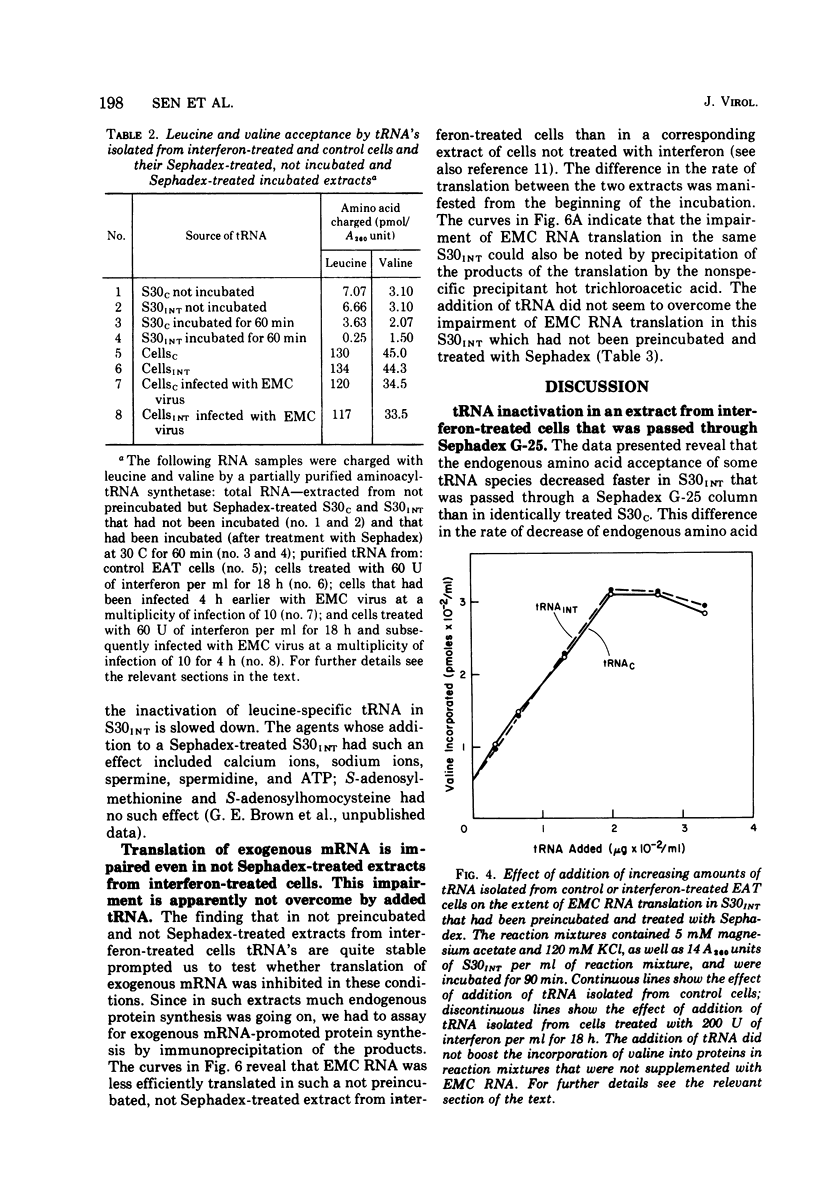
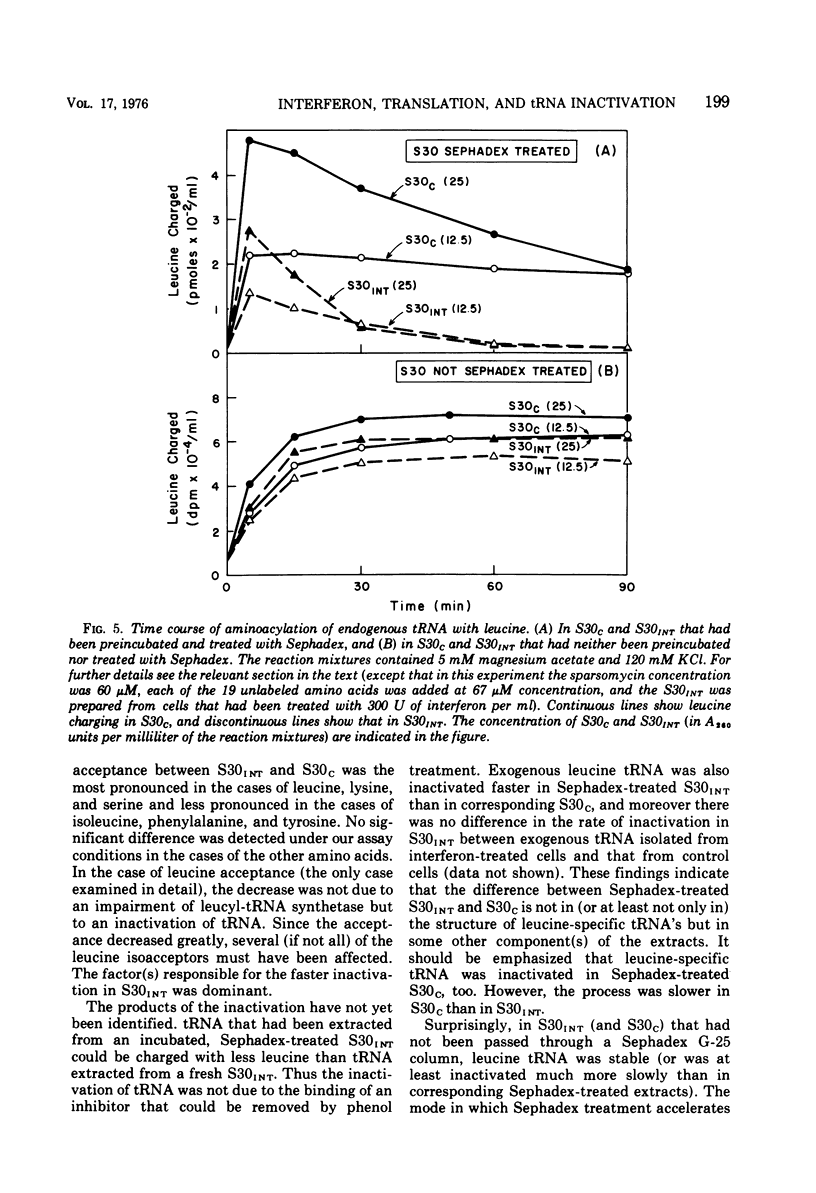
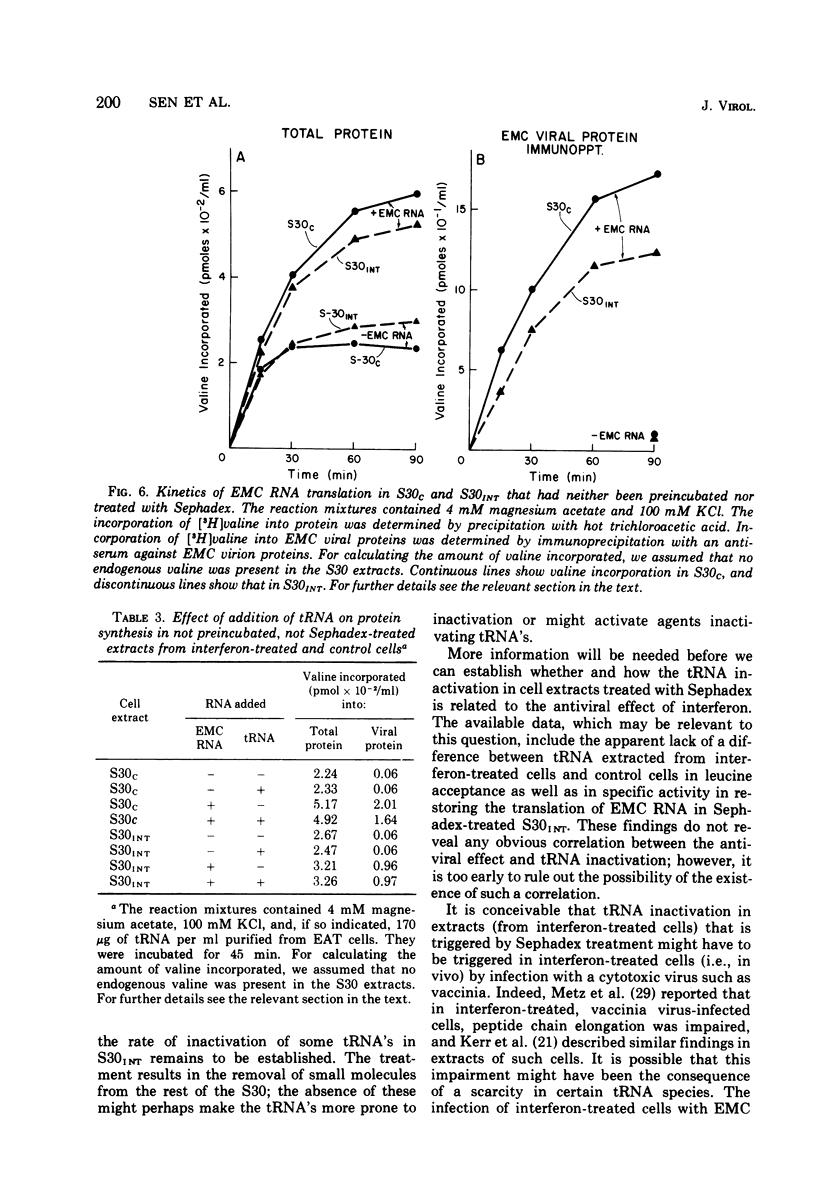
We reported earlier that in cell extracts that were prepared from interferon-treated Ehrlich ascites tumor cells and preincubated and passed through Sephadex G-25 (S30INT), the translation of exogenous mRNA (viral and host) was impaired and the impairment could be overcome to a large extent by adding a crude tRNA preparation from Ehrlich ascites tumor cells but not from Escherichia coli. We find now that the rate of inactivation of some tRNA's (especially those specific for leucine, lysine, and serine) but not those of many others is faster in S30INT than in corresponding extracts from control cells. This increased rate of tRNA inactivation may perhaps account for the need for added RNA to overcome at least partially the impairment of translation in S30INT. The relationship of the increased rate of tRNA inactivation to the antiviral effect of interferon is unclear. So far no significant difference has been detected in the amount of tRNA needed to overcome the impairment of encephalomyocarditis virus RNA translation in S30INT between tRNA from interferon-treated cells and tRNA from control cells. Furthermore, no difference was found in the rate of inactivation in S30INT between leucine-specific tRNA's from interferon-treated and from control cells. tRNA's specific for leucine and lysine were not inactivated (unless very slowly) during incubation under our conditions in an extract from interferon-treated (or from control) cells unless the extract had been passed through Sephadex G-25 or dialyzed. The translation of exogenous mRNA was, however, impaired in an extract from interferon-treated cells that had not been passed through Sephadex G-25. This impairment was apparently not overcome by added tRNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean W. J., Jr, Simpson R. W. Primary transcription of the influenza virus genome in permissive cells. Virology. 1973 Dec;56(2):646–651. doi: 10.1016/0042-6822(73)90067-6. [DOI] [PubMed] [Google Scholar]
- Bialy H. S., Colby C. Inhibition of early vaccinia virus ribonucleic acid synthesis in interferon-treated chicken embryo fibroblasts. J Virol. 1972 Feb;9(2):286–289. doi: 10.1128/jvi.9.2.286-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo G., Scheirer W., Suh M., Schultze B., Horak I., Jungwirth C. Protein synthesis in pox-infected cells treated with interferon. Virology. 1972 Oct;50(1):140–147. doi: 10.1016/0042-6822(72)90354-6. [DOI] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., Lebleu B., Zilberstein A., Berissi H., Revel M. Mechanism of the interferon-induced block of mRNA translation in mouse L cells: reversal of the block by transfer RNA. FEBS Lett. 1974 Apr 15;41(1):125–130. doi: 10.1016/0014-5793(74)80970-1. [DOI] [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Lebleu B., Revel M. Correlation between the antiviral effect of interferon treatment and the inhibition of in vitro mRNA translation in noninfected L cells. J Virol. 1973 Sep;12(3):421–430. doi: 10.1128/jvi.12.3.421-430.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Inhibition of arbovirus protein synthesis by interferon. J Virol. 1968 Oct;2(10):1081–1085. doi: 10.1128/jvi.2.10.1081-1085.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M., Metz D. H., Esteban R. M., Tovell D. R., Ball L. A., Kerr I. M. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol. 1972 Dec;10(6):1184–1198. doi: 10.1128/jvi.10.6.1184-1198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Miura K. A blocked structure at the 5' terminus of mRNA from cytoplasmic polyhedrosis virus. Nature. 1975 Jan 31;253(5490):374–375. doi: 10.1038/253374a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Muthukrishnan S., Shatkin A. J. Reovirus messenger RNA contains a methylated, blocked 5'-terminal structure: m-7G(5')ppp(5')G-MpCp-. Proc Natl Acad Sci U S A. 1975 Jan;72(1):362–366. doi: 10.1073/pnas.72.1.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J. Effect of interferon on synthesis of ssRNA in reovirus type 3-infected L cell cultures. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1228–1236. doi: 10.1016/0006-291x(72)90966-7. [DOI] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Inhibition of early viral ribonucleic acid synthesis in interferon-treated cells infected with frog polyhedral cytoplasmic deoxyribovirus. Virology. 1972 Dec;50(3):916–919. doi: 10.1016/0042-6822(72)90447-3. [DOI] [PubMed] [Google Scholar]
- Graziadei W. D., 3rd, Roy D., Konigsberg W., Lengyel P. Translation of reovirus messenger ribonucleic acids synthesized in vitro into reovirus proteins in a mouse L cell extract. Arch Biochem Biophys. 1973 Sep;158(1):266–275. doi: 10.1016/0003-9861(73)90621-8. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Graziadei W. D., 3rd, Weideli H., Sopori M. L., Lengyel P. Selective inhibition of viral protein accumulation in interferon-treated cells; nondiscriminate inhibition of the translation of added viral and cellular messenger RNAs in their extracts. Virology. 1974 Jan;57(1):49–63. doi: 10.1016/0042-6822(74)90107-x. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Inhibition of protein synthesis directed by added viral and cellular messenger RNAs in extracts of interferon-treated Ehrlich ascites tumor cells. Location and dominance of the inhibitor(s). Biochem Biophys Res Commun. 1973 Sep 18;54(2):777–783. doi: 10.1016/0006-291x(73)91491-5. [DOI] [PubMed] [Google Scholar]
- Gupta S. L., Sopori M. L., Lengyel P. Release of the inhibition of messenger RNA translation in extracts of interferon-treated Ehrlich ascites tumor cells by added transfer RNA. Biochem Biophys Res Commun. 1974 Apr 8;57(3):763–770. doi: 10.1016/0006-291x(74)90612-3. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Friedman R. M., Brown R. E., Ball L. A., Brown J. C. Inhibition of Protein Synthesis in Cell-Free Systems from Interferon-Treated, Infected Cells: Further Characterization and Effect of Formylmethionyl-tRNA(F). J Virol. 1974 Jan;13(1):9–21. doi: 10.1128/jvi.13.1.9-21.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. B., Carter W. A. Molecular basis of the action of interferon. J Mol Biol. 1968 Feb 14;31(3):561–577. doi: 10.1016/0022-2836(68)90428-2. [DOI] [PubMed] [Google Scholar]
- Lieberman D., Voloch Z., Aviv H., Nudel U., Revel M. Effects of interferon on hemoglobin synthesis and leukemia virus production in Friend cells. Mol Biol Rep. 1974 Dec;1(8):447–451. doi: 10.1007/BF00360670. [DOI] [PubMed] [Google Scholar]
- Manders E. K., Tilles J. G., Huang A. S. Interferon-mediated inhibition of virion-directed transcription. Virology. 1972 Aug;49(2):573–581. doi: 10.1016/0042-6822(72)90508-9. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Engelhardt D. L., Hunt J. M., Sekellick M. J. Interferon action: inhibition of vesicular stomatitis virus RNA synthesis induced by virion-bound polymerase. Science. 1971 Nov 5;174(4009):593–598. doi: 10.1126/science.174.4009.593. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Terry T. M., Levine S. Interferon action II. Membrane-bound alkaline ribonuclease activity in chick embryo cells manifesting interferon-mediated interference. Proc Natl Acad Sci U S A. 1975 Jan;72(1):182–186. doi: 10.1073/pnas.72.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz D. H., Esteban M., Danielescu G. The effect of interferon on the formation of virus polyribosomes in L cells infected with vaccinia virus. J Gen Virol. 1975 May;27(2):197–209. doi: 10.1099/0022-1317-27-2-197. [DOI] [PubMed] [Google Scholar]
- Metz D. H., Esteban M. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature. 1972 Aug 18;238(5364):385–388. doi: 10.1038/238385a0. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Stinebring W. R. Prolonged application of interferon and "aging" of human diploid fibroblasts. Proc Soc Exp Biol Med. 1971 May;137(1):191–195. doi: 10.3181/00379727-137-35541. [DOI] [PubMed] [Google Scholar]
- Nudel U., Lebleu B., Revel M. Discrimination between messenger ribonucleic acids by a mammalian translation initiation factor. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2139–2144. doi: 10.1073/pnas.70.7.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman M. N., Baron S., Black P. H., Takemoto K. K., Habel K., Rowe W. P. The effect of interferon on SV-40 T antigen production in SV-40-transformed cells. Virology. 1967 May;32(1):122–127. doi: 10.1016/0042-6822(67)90260-7. [DOI] [PubMed] [Google Scholar]
- Oxman M. N., Levin M. J. Interferon and transcription of early virus-specific RNA in cells infected with simian virus 40. Proc Natl Acad Sci U S A. 1971 Feb;68(2):299–302. doi: 10.1073/pnas.68.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik P., Flamand A., Bishop D. H. Effect of interferon upon the primary and secondary transcription of vesicular stomatitis and influenza viruses. J Virol. 1974 Nov;14(5):1169–1178. doi: 10.1128/jvi.14.5.1169-1178.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman F., Shatkin A. J., Perry R. P. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974 Nov;3(3):197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., Joklik W. K. A protein synthesizing system from interferon-treated cells that discriminates between cellular and viral messenger RNAs. Virology. 1974 Apr;58(2):476–491. doi: 10.1016/0042-6822(74)90082-8. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Lebleu B., Brown G. E., Rebello M. A., Furuichi Y., Morgan M., Shatkin A. J., Lengyel P. Inhibition of reovirus messenger RNA methylation in extracts of interferon-treated Ehrlich ascites tumor cells. Biochem Biophys Res Commun. 1975 Jul 8;65(1):427–434. doi: 10.1016/s0006-291x(75)80111-2. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Smith A. E., Wigle D. T. A rapid assay for the initiation of protein synthesis in extracts of animal cells. Eur J Biochem. 1973 Jun 15;35(3):566–573. doi: 10.1111/j.1432-1033.1973.tb02874.x. [DOI] [PubMed] [Google Scholar]
- Sonnabend J. A., Martin E. M., Mécs E., Fantes K. H. The effect of interferon on the synthesis and activity of an RNA polymerase isolated from chick cells infected with Semliki forest virus. J Gen Virol. 1967 Jan;1(1):41–48. doi: 10.1099/0022-1317-1-1-41. [DOI] [PubMed] [Google Scholar]
- Soreq H., Nudel U., Salomon R., Revel M., Littauer U. Z. In vitro translation of polyadenylic acid-free rabbit globin messenger RNA. J Mol Biol. 1974 Sep 5;88(1):233–245. doi: 10.1016/0022-2836(74)90307-6. [DOI] [PubMed] [Google Scholar]
- Van Ventrooij W. J., Henshaw E. C., Hirsch C. A. Nutritional effects on the polyribosome distribution and rate of protein synthesis in Ehrlich ascites tumor cells in culture. J Biol Chem. 1970 Nov 25;245(22):5947–5953. [PubMed] [Google Scholar]
- Vassef A., Beaud G., Paucker K., Lengyel P. Interferon assay based on the inhibition of double-stranded reovirus RNA accumulation in mouse L cells. J Gen Virol. 1973 Apr;19(1):81–87. doi: 10.1099/0022-1317-19-1-81. [DOI] [PubMed] [Google Scholar]
- Vassef A., Spencer C., Gelehrter T. D., Lengyel P. Selectivity of interferon action: hormonal induction of tyrosine aminotransferase in rat hepatoma cells is much less sensitive to interferon than the replication of vesicular stomatitis virus or reovirus. Biochim Biophys Acta. 1974 Jun 14;353(1):115–120. doi: 10.1016/0005-2787(74)90102-6. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylation of newly synthesized viral messenger RNA by an enzyme in vaccinia virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3014–3018. doi: 10.1073/pnas.71.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]



