Abstract
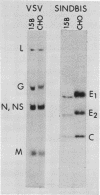
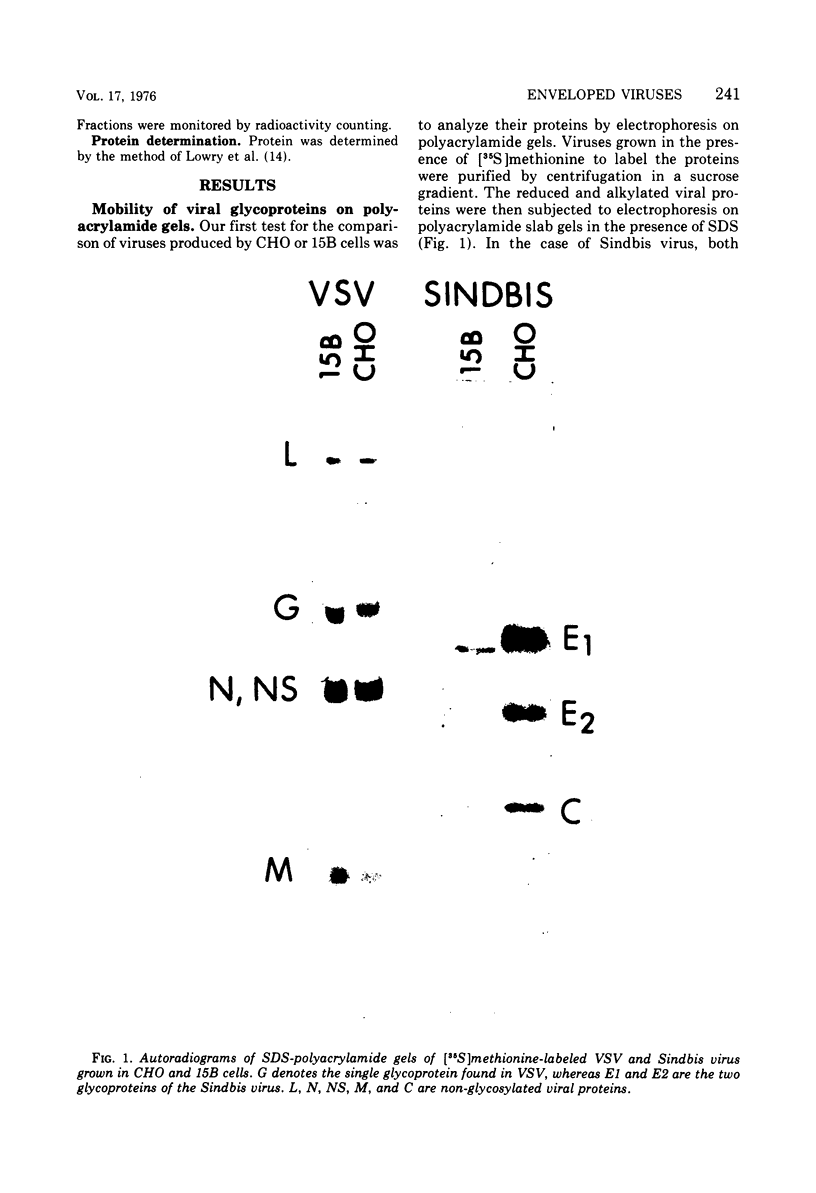
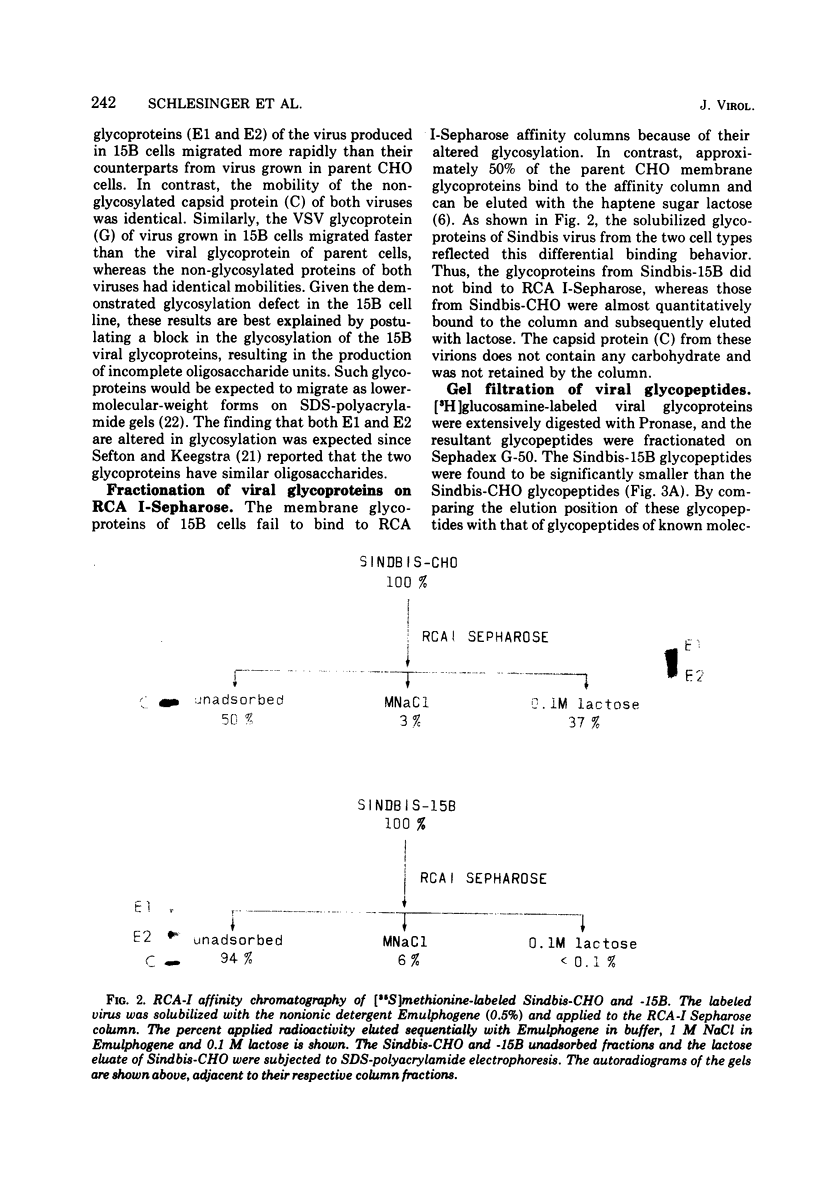
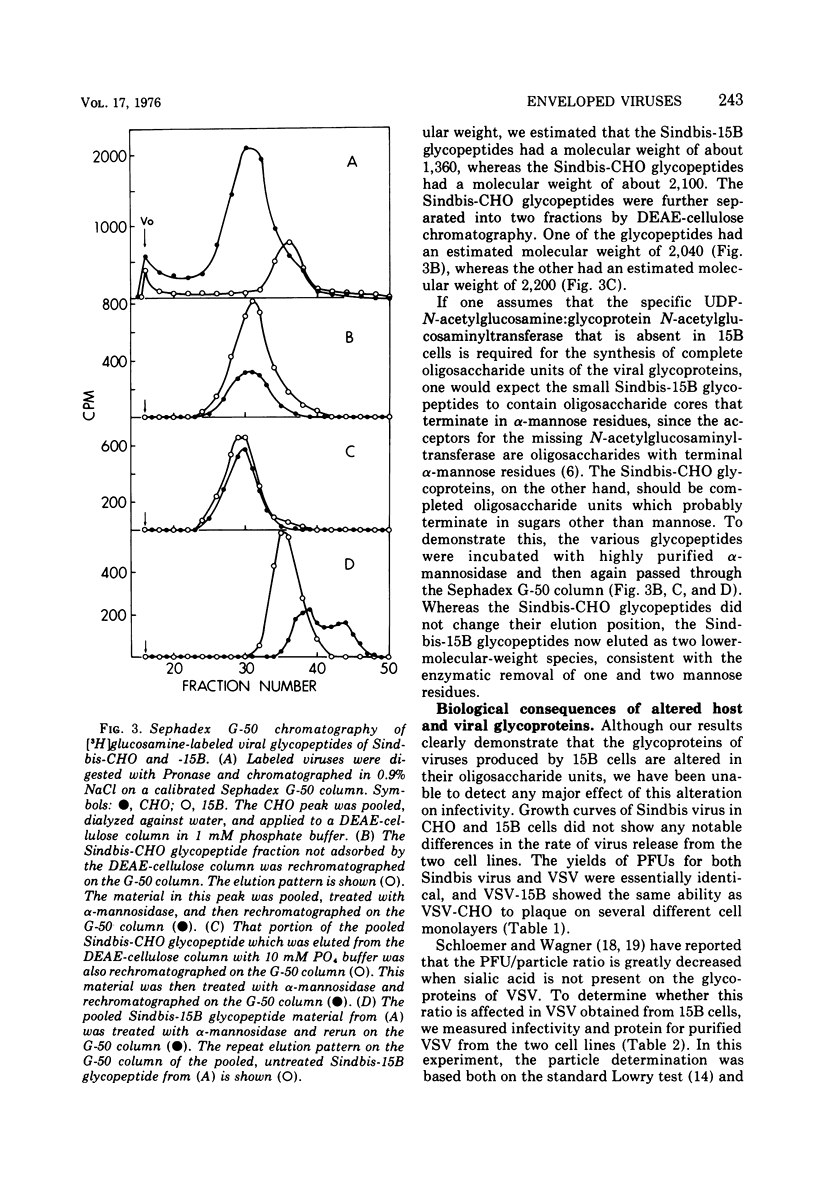
Sindbis and vesicular stomatitis viruses were grown in a line (termed 15B) of Chinese hamster ovary (CHO) cells that is deficient in a specific UDP-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase. Both viruses replicated normally in the cell line, but the glycoproteins of the released virus migrated faster on sodium dodecyl sulfate-polyacrylamide gels than did glycoproteins of virus grown in parent CHO cells. Digestion of the viral glycoproteins with Pronase followed by gel filtration demonstrated that the glycopeptides of Sindbis-15B virus were much smaller than the glycopeptides of Sindbis-CHO virus. In addition, Sindbis-15B viral glycopeptides but not Sindbis-CHO viral glycopeptides contained terminal α-mannose residues as shown by their susceptibility to α-mannosidase digestion. These findings demonstrate that the oligosaccharide units of the glycoproteins of vesicular stomatitis and Sindbis viruses are altered when the viruses are grown in 15B cells. We conclude that the N-acetylglucosaminyltransferase that is missing in 15B cells normally participates in the biosynthesis of the oligosaccharide units of the viral glycoproteins, and in the absence of this enzyme incomplete oligosaccharide chains are produced. Viruses released from 15B cells appear to retain full infectivity; Sindbis-15B virus, however, showed a significant decrease in hemagglutination titer compared with that of Sindbis-CHO virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Duda E., Schlesinger M. J. Alterations in Sindbis viral enbelope proteins by treating BHK cells with glucosamine. J Virol. 1975 Feb;15(2):416–419. doi: 10.1128/jvi.15.2.416-419.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison J. R., Holland J. J. Carbohydrate composition of the membrane glycoprotein of vesicular stomatitis virus grown in four mammalian cell lines. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4011–4014. doi: 10.1073/pnas.71.10.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger J., Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine:glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975 May 10;250(9):3303–3309. [PubMed] [Google Scholar]
- Gottlieb C., Skinner A. M., Kornfeld S. Isolation of a clone of Chinese hamster ovary cells deficient in plant lectin-binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1078–1082. doi: 10.1073/pnas.71.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza G., Schmidt M. F., Scholtissek C. Effect of 2-deoxy-D-glucose on the multiplication of Semliki Forest virus and the reversal of the block by mannose. Virology. 1973 Jul;54(1):179–189. doi: 10.1016/0042-6822(73)90127-x. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. The effect of enzymes on structural and biological properties of Semliki forest virus. J Gen Virol. 1974 May;23(2):129–143. doi: 10.1099/0022-1317-23-2-129. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenard J., Compans R. W. The membrane structure of lipid-containing viruses. Biochim Biophys Acta. 1974 Apr 8;344(1):51–94. doi: 10.1016/0304-4157(74)90008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Vesicular stomatitis virus envelope glycoprotein alterations induced by host cell transformation. Cell. 1974 May;2(1):63–70. doi: 10.1016/0092-8674(74)90009-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Mosquito cells infected with vesicular stomatitis virus yield unsialylated virions of low infectivity. J Virol. 1975 Apr;15(4):1029–1032. doi: 10.1128/jvi.15.4.1029-1032.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Sialoglycoprotein of vesicular stomatitis virus: role of the neuraminic acid in infection. J Virol. 1974 Aug;14(2):270–281. doi: 10.1128/jvi.14.2.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Klenk H. D. Inhibition of glycosylation of the influenza virus hemagglutinin. J Virol. 1974 Nov;14(5):1023–1034. doi: 10.1128/jvi.14.5.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Weiss B., Schlesinger S. Defective interfering passages of Sindbis virus: chemical composition, biological activity, and mode of interference. J Virol. 1973 Oct;12(4):862–871. doi: 10.1128/jvi.12.4.862-871.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]